INTRODUCTION
Major depressive disorder (MDD) is a mood disorder prevalent worldwide and affects quality of life. An estimated 3.8% of people worldwide have experienced depression, including 5% of adults (6% among women and 4% among women) and 5.7% of the population over 60 years. Approximately 280 million people worldwide have MDD, according to the Institute of Health Metrics and Evaluation report in 2023. In the U.S., the number of adults suffering from MDD increased from 13.7 to 17.5 million, with the prevalence rate growing from 6.8% in 2005 to 7.1% in 2018 [1]. The treatment strategies for patients with MDD are remarkably complex due to the wide variability in disease presentation and the heterogeneity of treatment responses.
There are several causes of depression, such as neurotransmitter imbalance, genetic defects, or chronic stressful situations [2]. The neurochemical pathology of depression is still unclear, but whatever the trigger, it is alleged that the underlying biological mechanism of depression is a serotonin 5-hydroxytryptamine (5-HT) deficiency [3].
Long-term ongoing stress or chronic stress exposure (CRS) can lead to a risk of MDD. Previous studies have indicated that CRS induces glucocorticoid secretion, reducing the release of 5-HT from the brain [4]. In addition, numerous research reports have demonstrated a close link between brain oxidative stress pathways and the occurrence of depression [5,6]. However, a variety of currently available antidepressants and antistress agents have a delay in the onset of clinical action and produce unwanted side effects [7]. To solve this challenge, effective novel therapeutic agents need to be developed to address stress and depression.
Numerous published reports showed that fruit residuals, such as peels or seeds, are an important source of safe constituents and bioactive components with many health benefits [8]. Most bioactive phytochemicals of fruit residuals are secondary metabolites, including polyphenols and flavonoids, which have antioxidative properties, playing a vital role in treating or preventing many diseases, including MDD [9]. For example, the methanolic extract of Ananas comosus Linn banana peel [10] and the extract of banana peel [11] has an antidepressant effect, possibly via their antioxidant mechanism. As a result, many researchers continue to investigate the antidepressant properties of natural products or fruit waste against chronic stress-induced depression using a variety of animal studies [12].
A previous study revealed that the extract from Lychee Litchi chinensis Sonn. (LC) peel has a strong antioxidant potential [13]. LC peel contains several phytochemicals, such as ascorbic acid, gallic acid, cyanidin 3-rutinoside, cyanidin 3-glucoside, quercetin-3-rutinoside, quercetin-3-glucoside, procyanidin B2 and epicatechin [14]. In addition, the seed and peel of this plant are said to have pharmacological properties such as antiviral, anti-inflammatory, anticoagulant, anticancer, antifungal, antihyperlipidemic, antidiabetic, and cardio- and hepatoprotective effects [15].
Nevertheless, we have found no scientific evidence to support the effect of LC peel extract on chronic stress-induced depression-like behavior until now. Thus, this study has been undertaken to evaluate the potential antidepressant activity of LC peel extract in chronic restraint stress (CRS)-induced depressive behavior in rats.
MATERIALS AND METHODS
Plant materials and the preparation of lychee (LC) peel extract
Fresh ripe LC fruits were harvested from Amphoe Ma Chai, Phayao Province, Thailand, in January 2022. The LC fruits were peeled, and the peels were washed three times under running tap water, cut into small pieces, and then dried using a laboratory hot air oven at 40°C. Dried samples of the LC peel were blended and macerated in a mixture of distilled water and 90% ethanol for 72 hours. The LC peel extract was then separated and concentrated under a vacuum in a rotary evaporator at 60°C and kept in amber glass at 4°C until required. The maceration method used in the extraction process gave an extraction yield of 15.23% (w/w).
Phytochemical screening of the LC peel extract
A small portion of the ethanolic LC peel extract was analyzed for various phytoconstituents using the following methods: the flavonoid test used sodium hydroxide (NaOH), the test for tannins used bromine water, the test for alkaloids used Wagner’s reagent, the test for phenols used ferric chloride solution, the carbohydrate test used Molisch’s test and the test for reducing sugars used Fehling’s reagent [16]. The total phenols content of the LC peel extract was measured using the Folin-Ciocalteu method, as reported by Lee et al. [17]. The concentration of total flavonoids in the LC extract was determined by the colorimetric procedures established by Shi et al. [18].
Animals
All experimental and animal care procedures were approved by the Institutional Animal Care and Use Committee of the University of Phayao (No. 640104037) and were performed in accordance with the principles of Laboratory Animal Care (National Institutes of Health publication No.85–23, revised 1985).
Young adult male Sprague-Dawley rats weighing 200–250 g were obtained from Nomura Siam International Co, Ltd., Thailand. A total of 50 rats were housed at a temperature of 20°C ± 2°C with a 12:12 hours light-dark cycle, fed a standard commercial pellet food, and allowed tap water ad libitum. After 7 days of habituation, the rats were randomly separated into the following groups: Group I = naïve control (non-stress); Group II = vehicle (distilled water) + stress; Group III = fluoxetine 20 mg/kg + stress; Group IV = LC peel extract 150 mg/kg + stress; Group V = LC peel extract 300 mg/kg + stress. In this study, distilled water was used for dissolving the extract, and various doses of the extract were chosen based on its antioxidant capacity in vitro from our preliminary studies. In addition, our study revealed no death or signs of toxicity were observed in rats treated with LC peel extract at a dose of 3,000 mg/kg (data not shown). By keeping 1/10th of (300 mg/kg) dose as the highest, the doses of 150 and 300 mg/kg of the extract was chosen as working doses to evaluate its antidepressant-like effects in rats. All substances were orally administered to the CRS rats once daily at 7.00 a.m. for 56 days—oral administration of the extract, fluoxetine, and vehicle to rodents using the gavage technique. In addition, the total volume for oral administration of the extract and vehicle does not exceed 1.0 ml (the recommended maximum is 10 ml/kg).
CRS-induced depression
Except for the naïve control group, each rat was exposed to chronic stress daily for 56 days in the form of 6 hours of restraint (08.00 a.m.–2.00 p.m.) in an acrylic cylindrical animal restrainer (20 cm long × 7 cm internal diameter) with air holes for breathing. The length of the restraint apparatus was adjusted to tightly fit the size of the rat’s body. After being restrained, the rat was removed from the restrainer, put in its cage, and given free access to food and water.
Behavioral assessment
To investigate the antidepressant effect of the LC peel extract, the following behavioral tests were carried out: the open field test (OFT), the tail suspension test (TST), and the forced swimming test (FST). The behavioral tests were conducted on days 1, 14, 28, 42, and 56 of treatment after the CRS. All scheduled behavioral testing was conducted at an interval within 1 hour after oral administration of all substances [19].
The open field test
The OFT is a popular model used to screen exploratory behavior and locomotor activity in rodents [20]. The test was undertaken 30 minutes after the oral administration of the LC peel extract or vehicle. The apparatus consisted of plain lexiglas (L: 30, W: 30 and H: 20 cm). An individual rat was placed in the center of the apparatus and stereotypical behaviors were recorded for 5 minutes, including (1) grooming: the number of times the rat “cleaned” itself by wiping, body and pubis licking, and scratching; (2) rearing: the number of times the rat stood on its hind legs in the maze. After each trial, the apparatus was wiped clean with a 70% alcohol solution to avoid possible bias due to the smell left by previous rats.
The tail suspension test
The TST is a widely accepted test to assess antidepressant activity in rodents. This test was conducted following a previously described method [21]. Each rodent was hung by the tail from a horizontal bar (approximately 50 cm above the floor). The total duration of immobility of each rat during the test (5 minutes) was recorded. In this case, “immobility” was defined as the period when the rat stopped struggling and movement was absent for ≥ 1 second.
The forced swimming test
The FST is one of the most frequently used pharmacological models to determine antidepressant activity. The FST protocol employed here was modified following Porsolt et al.’s method [22]. Each rat was placed in a vertical clear glass cylinder (22 cm in diameter, 40 cm in height) containing water at 25°C to a height of 20 cm for 6 minutes. After a habituation period of 1 minute, the duration of immobility or despair was recorded for 5 minutes. The rat was considered immobile when it tried to maintain its head above water without struggling. The freezing behavior or the immobility time of each rat was recorded using a stopwatch.
Plasma corticosterone (CORT) level determination
On day 57, before the rats were sacrificed, cardiac punctures from the posterior vena cava were collected to determine the plasma CORT level. Each blood specimen was centrifuged for 15 minutes at 1,000 g at 8°C and kept in a −80°C ultra-low temperature freezer before analysis. The CORT level was determined using an ELISA kit (Biovalue Inc., China), following the manufacturer’s instructions. The absorbance of each well was read at 450 nm wavelengths using a microplate reader.
Determination of brain serotonin 5-HT levels
After decapitation, the brain of each rat was immediately removed and placed on an ice-cold plastic Petri dish. Each brain was separated into the right and left halves. The right half of the brain was used to determine the oxidative stress parameters and antioxidant enzyme activity. The left half of the brain was used to measure the 5-HT concentration.
To determine the concentration of 5-HT, the frozen brain tissue was homogenized in a cold solution of HCl-butanol and centrifuged at 10,000 rpm for 10 minutes. The 5-HT level in the rat brain tissue was estimated according to the fluorometric method described by Ciarlone [23]. The fluorescence was measured using a spectrofluorometer (model RF-5301PC; Shimadzu, Japan) at 300–400 nm.
Determination of brain lipid peroxidation malondialdehyde (MDA) and superoxide dismutase (SOD) enzyme activity
MDA, an important marker of lipid peroxidation, was estimated according to the method of Ohkawa et al. [24]. In brief, tissue from the right half of the rat’s brain was homogenized in Tris–HCl buffer (pH 7) and centrifuged for 10 minutes at 5,000 g at 4°C. The resulting supernatant was removed from a new test tube and placed in a boiling water bath for 15 minutes. The optical density of the pink supernatant was measured at 532 nm using a plate reader and the MDA concentration was then calculated from the following formula:
MDA (nmol/ml) = (SA/SV) × DF = C.
where SA is the amount of MDA in the sample (nmol), as determined from the standard curve; SV is the sample volume (ml) or amount (mg) added to the well plate; DF is the sample dilution factor (DF = 1 for undiluted samples); C is the concentration of MDA in the sample.
The brain SOD activity was determined using a commercially available SOD Assay Kit (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany), following the manufacturer’s protocol, and calculated using the following formula:
SOD activity = (A – B) – (C – D) × 10, (A – B)
where A is the absorbance value of the No SOD control; B is the absorbance value of the blank; C is the absorbance value of the sample; D is the absorbance value of the No XO control (xanthine oxidase working solution).
Statistical analysis
All results were presented as the mean ± standard error of the mean. The statistical significance between different groups was analyzed by one-way analysis of variance (ANOVA) in GraphPad Prism version 5, followed by Tukey’s post hoc test. In all analyses, a value of p < 0.05 was considered statistically signi?cant.
RESULTS
Interestingly, the preliminary phytochemical analysis of the crude ethanolic extract of LC peel indicated the presence of phenols, flavonoids, tannins, alkaloids, and carbohydrates. The total phenol content and total flavonoid content of the LC peel extract were 126.95 mg of gallic acid equivalents per gram of dry-weight LC peel extract and 90.05 mg quercetin equivalents per gram of dry-weight LC peel extract, respectively, as shown in Table 1.
The in vivo study showed that the daily oral administration of LC peel extract for 56 days did not result in death or signs of toxicity in any of the rats. The extract of LC fruit peel presented antidepressant activity in behavior animal models of depression. One-way ANOVA of the immobility times in TST and FST revealed significant differences. Tukey’s post hoc test showed that the vehicle + stress group had a markedly induced immobility time in the TST and FST compared with the naïve control group (p < 0.001). Our results indicate that the despair behavior observed following restraint stress immobilization was reversed by LC peel extract supplementation. The groups treated with a high dose of LC peel extract (300 mg/kg) or the synthetic antidepressant drug fluoxetine (20 mg/kg) had significantly reduced immobility times in both the TST and FST compared with the vehicle + stress group (p < 0.001 and p < 0.01, respectively). However, the LC extract (300 mg/kg) had no significant effect on this parameter compared to the naïve control group. The results are shown in Figures 1A and B.
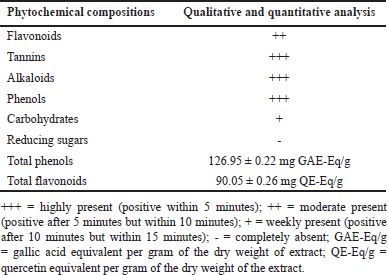 | Table 1. Phytochemical compositions analysis of LC peel extract. [Click here to view] |
To avoid a false-positive result, we also determined locomotor behaviors to check the motor-stimulating activities of the LC peel extract. No significant differences were observed in the locomotor activities of rats treated with LC peel extract compared to the vehicle + stress group. These results are summarized in Table 2.
Plasma CORT was determined at the end of the experiment using the ELISA technique. As depicted in Figure 2A, increased plasma CORT levels were observed in the vehicle + stress rats compared to the naïve control rats (p < 0.001). However, this increase was significantly ameliorated by treatment with 300 mg/kg LC peel extract or fluoxetine (all p < 0.05). Reduced brain 5-HT levels were observed in the vehicle + stress-treated rats compared to the naïve control rats (p < 0.001). Tukey’s post hoc analysis confirmed that the high dose of LC peel extract and the fluoxetine 20 mg/kg both significantly elevated the brain 5-HT levels in stressed rats (p < 0.001) compared to the vehicle + stress group. Unfortunately, the extract showed no significant changes in this parameter when compared with the naïve control group, as shown in Figure 2B.
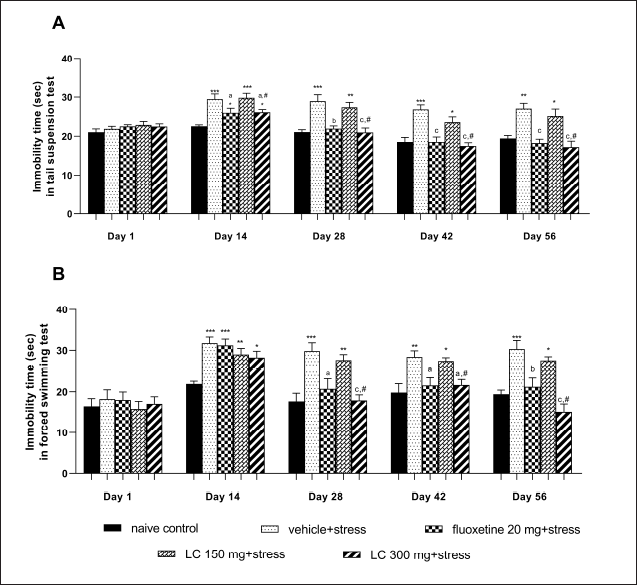 | Figure 1. Effect of LC peel extract on immobility time in the TST (A) and the FST (B) of chronically stressed rats. Data were expressed as mean ± SEM (n = 10). *p < 0.05, **p < 0.01, and ***p < 0.001 versus naïve control group; ap < 0.05, bp < 0.01, and cp < 0.001 versus vehicle + stress-treated group; #p < 0.01 versus LC 150 mg + stress-treated group. [Click here to view] |
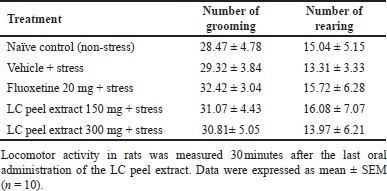 | Table 2. Effect of LC peel extract in the OFT in rats. [Click here to view] |
Excessive free radicals are associated with chronic restrain stress-induced oxidative stress status in the brain. Our results supported this finding, as shown in Figures 3A and B. A significant increase in MDA level was observed in the vehicle + stress group compared to the naïve control rats (p < 0.01). In contrast, the SOD antioxidant enzyme activity significantly decreased in the vehicle + stress group (p < 0.001). Again, only higher doses of LC peel extract and fluoxetine significantly attenuated the MDA level in the brain (p < 0.05) and increased the SOD activity compared to the vehicle + stress group (p < 0.001). However, the highest dose of the extract had no significant effect on this parameter when compared with the naïve control group.
DISCUSSION
In the present study, we have demonstrated that the daily oral supplementation of LC peel extract for 56 days could relieve signs of depression in a rat model of chronic stress. Our results showed that a 300 mg/kg dose of LC peel extract reduced the immobility time in both the FST and TST, significantly attenuated the depletion of brain 5-HT levels and decreased the level of plasma CORT, while oxidative markers were significantly decreased in the rat brain.
A vast amount of scientific evidence indicates that CRS plays a vital role in developing depressive illness disorders. Stress has been found to induce the activation of neuroendocrine systems via the limbic-hypothalamic-pituitary adrenal (HPA) axis, leading to the elevation of glucocorticoid production [25], which in turn impairs the neurotransmitters in the cortical brain region, resulting in depression-like symptoms [26]. Previous studies suggest that patients with depression often have low concentrations of 5-HT in their brains [27]. Consistent with this suggestion, the currently available antidepressant drugs focus on enhancing concentrations of monoamine neurotransmitters, especially 5-HT, by the selective serotonin reuptake inhibitors [28], which exert action by inhibiting the reuptake of the serotonin neurotransmitter, leading to increased serotonin signaling [29].
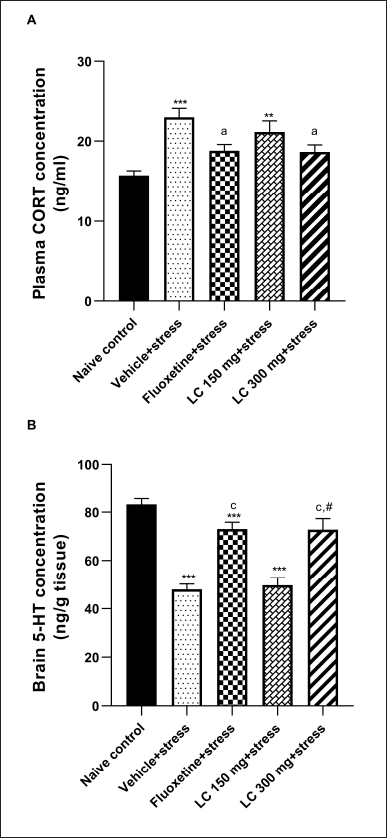 | Figure 2. Effect of LC peel extract on plasma CORT levels (A) and the brain serotonin (5-HT) concentrations (B) of chronically stressed rats. Data were expressed as mean ± SEM (n = 10). **p < 0.01, and ***p < 0.001 versus naïve control group; ap < 0.05 and cp < 0.001 versus vehicle + stress-treated group; #p < 0.01 versus LC 150 mg + stress-treated group. [Click here to view] |
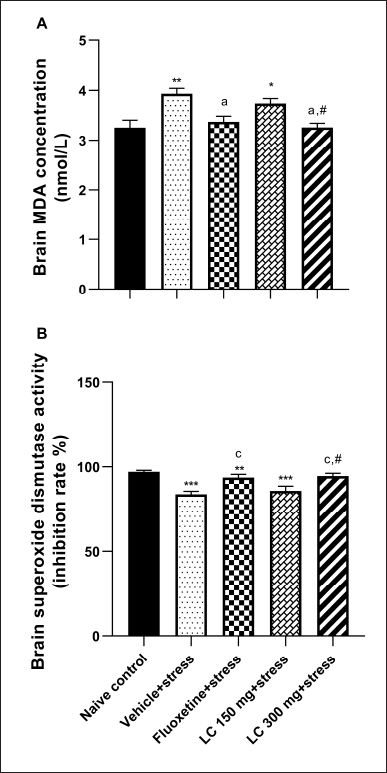 | Figure 3. Effect of LC peel extract on brain lipid peroxidation (A) and SOD activity (B) of chronically stressed rats. Data were expressed as mean ± SEM (n = 10). *p < 0.05, **p < 0.01, and ***p < 0.001 versus naïve control group; ap < 0.05 and cp < 0.001 versus vehicle + stress-treated group; #p < 0.01 versus LC 150 mg + stress-treated group. [Click here to view] |
The restraint stress or immobilization method is a validated experimental animal model of depressive-type behavior, which is mainly used to study the pathogenesis and screening of potential therapeutic antidepression agents [30]. In this paradigm, the succession of CRS exposure increases plasma adrenocorticotropic hormone and CORT concentrations, which in turn affects serotonergic transmission [31].
Immobility is interpreted as behavioral despair or a sign of depression [32]. The TST and FST are the most used tools for evaluating the effectiveness of several antidepressant agents and are also used to infer “depression-like” behavior in rodents [33]. Studies have shown that the induction of immobility in both the TST and FST is associated with a decrease in serotonergic function [34]. Supporting these findings, the present results reveal that the rise in plasma CORT concentration and reduced brain 5-HT levels in the vehicle plus restraint stress rats leads to a depressed-despair or hopelessness, as evidenced by the significantly increased despair time in both the FST and TST.
To date, the true mechanisms underlying chronic stress-induced depressive behavior are still not clearly understood. In addition to the neuroendocrine systems via the limbic-HPA axis and monoamine hypothesis, the oxidative stress hypothesis has received growing attention in recent years [35]. Long-term stress exposure is a contributing factor to MDD, which can induce oxidative damage, leading to neuroprogression in depressive symptoms [36]. The concentration of different oxidative stress biomarkers, such as SOD and MDA, are found to be changed in depressive illness [37].
MDA is a product of lipid peroxidation and has been widely used as a biomarker of oxidative stress-related medical conditions, including depressive disorder. It has been reported that MDA levels are increased in patients with depression [38]. SOD is an antioxidant enzyme that acts as a reactive oxygen species scavenger. SOD activity was found to be decreased both in vivo [39] and in vitro [40] in cases of major depression.
Taking all the above data into consideration, our results are in agreement with previous reports showing that when rats are kept in a vehicle with exposure to CRS, there is an increase in plasma concentrations of CORT and brain MDA levels, accompanied by lower levels of SOD antioxidant enzyme, which results in the reduction of brain 5-HT levels, leading to depressed-despair or hopelessness, as evidenced by the significantly increased immobility time in both the FST and TST. Furthermore, this study found that the highest oral dose of LC peel extract (300 mg/kg) successfully ameliorated depressive behavior in CRS-treated rats, as observed by a significant decrease in immobility time in both behavioral despair tests. No differences in locomotor behavior were observed among the groups, indicating that the antidepressant effect of the LC peel extract was specific and not a false-positive result. These depression-like behavioral improvements were paralleled by biochemical alterations, including lower serum CORT and brain MDA levels, accompanied by higher brain SOD enzyme activity, indicating that the antioxidant properties of LC peel extract may be correlated with alterations of the HPA axis activity, leading to decreased serum CORT levels. However, there is only the highest dose of LC peel extract ameliorated depressive symptoms by attenuation of the oxidative stress and the concentration of CORT. This phenomenon may be related to an insufficient concentration of bioactive constituents from a low dose of LC peel extract, which may not have reached the plateau phase of treatment.
Several studies have reported the positive results of using fruit peel extract to prevent and treat depression. For example, Samad et al. [11] reported that male mice administered banana peel extract showed a decreased immobility time in the FST, suggesting antidepressant-like effects. Furthermore, Syeda et al. [41] showed that Punica granatum peel extract administered to mice produced significant antidepressant-like behavior in the FST, and its effect was found to be comparable to imipramine treatment. However, there is limited evidence on the neurobehavioural effects of LC peel. Therefore, one strand of this preliminary study will indicate that the ethanolic extract of LC peel possesses antidepressant activity. Curiously, even with a different phytochemical composition, the level of total oxidants among these fruits peel was enough to ensure the potential neuropsychological effects, being a possible supportive treatment for depressive disorder.
Interestingly, the antidepressant action in terms of the decreased immobility time caused by the oral supplementation of LC peel extract at a dose of 300 mg/kg was similar to that caused by fluoxetine treatment, indicating that LC peel extract exerts its antidepression-like effects through the modulation of the serotonergic system. Similarly, clinical studies have found that depressed patients treated with fluoxetine show a lower plasma cortisol level than untreated (control) depressed patients [42]. In addition, previous studies have confirmed that fluoxetine activates both mineralocorticoid and glucocorticoid receptors, which in turn reduces HPA axis activity [43], resulting in a decrease in plasma cortisol levels.
The present study showed that the peels of LC, which are usually discarded as waste, are a potential source of various phytochemical constituents. These phytochemicals have great antioxidant potential and free radical scavenging activity against oxidative stress. However, further investigation is required to identify and analyze the bioactive compounds of the LC peel extract responsible for the antidepressant-like activity and to establish the molecular mechanisms of action, as well as carry out a toxicological evaluation.
CONCLUSION
To summarise our study, treatment with LC peel extract significantly prevented depressed-despair activity in the CRS model. The possible mechanism of the antidepressant effect of the LC peel extract occurs via its antioxidant properties, resulting in decreased CORT levels and improved levels of brain 5-HT neurotransmitters. LC peel extract should be considered as a potential candidate for treatment strategies for major depression. The peel of LC fruit can therefore be used as an ingredient for the generation of healthy food products rather than being discharged to the environment.
AUTHORS’ CONTRIBUTIONS
Wathita Phachonpai: literature search, conceptualization, methodology, investigation, data collection, statistical analysis, writing original draft, review, and editing. Watcharaporn Preedapirom: literature search, experimental studies. Wuthiyan Kitipong: experimental studies. Tongun Terdthai: investigation, statistical analysis. All authors approved the final version for publication and agreed to submit it to this journal.
FINANCIAL SUPPORT
This research was supported by the Division of Research Administration (DRA) of the University of Phayao, Thailand (Grant no. RSPGUP 65/14).
CONFLICTS OF INTEREST
All authors declare that there are no financial or any other conflicts of interest in this research.
ETHICAL APPROVALS
All procedures and protocols in this experiment were approved by the ethical committee of the Institutional Animal Care of the University of Phayao, Thailand (approval number: 640104037).
DATA AVAILABILITY
All results data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Greenberg PE, Fournier AA, Sisitsky T, Mark S, Berman R, Koenigsberg SH, et al. The economic burden of adults with major depressive disorder in the United States (2010 and 2018). Pharmacoeconomics. 2021;39(6):653–65.
2. aan het Rot M, Mathew SJ, Charney DS. Neurobiological mechanisms in major depressive disorder. CMAJ. 2009;180(3):305–13.
3. Yohn CN, Gergues MM, Samuels BA. The role of 5-HT receptors in depression. Mol Brain. 2017;10(1):28.
4. Bambico FR, Nguyen NT, Gobbi G. Decline in serotonergic firing activity and desensitization of 5-HT1A autoreceptors after chronic unpredictable stress. Eur Neuropsychopharmacol. 2009;19(3):215–8.
5. Ng F, Berk M, Dean O, Bush AI. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int J Neuropsychopharmacol. 2008;11(6):851–76.
6. Anderson G, Maes M. Oxidative/nitrosative stress and immuno-inflammatory pathways in depression: treatment implications. Curr Pharm Des. 2014;20(23):3812–47.
7. Bet PM, Hugtenburg JG, Penninx BW, Hoogendijk WJ. Side effects of antidepressants during long-term use in a naturalistic setting. Eur Neuropsychopharmacol. 2013;23(11):1443–51.
8. Fidelis M, de Moura C, Kabbas Junior T, Pap N, Mattila P, Mäkinen S, et al. Fruit seeds as sources of bioactive compounds: sustainable production of high value-added ingredients from by-products within circular economy. Molecules. 2019;24(21):3854.
9. Thériault M, Caillet S, Kermasha S, Lacroix M. Antioxidant, antiradical and antimutagenic activities of phenolic compounds present in maple products. Food Chem. 2009;98(3):490–501.
10. Kafeel H. Antidepressant activity on methanolic extract of Ananas Comosus linn peel (MeACP) by using forced swim and tail suspension apparatus in mice. Sci Int. 2016;28(3):2525–31.
11. Samad N, Muneer A, Ullah N, Zaman A, Ayaz MM, Ahmad I. Banana fruit pulp and peel involved in antianxiety and antidepressant effects while invigorate memory performance in male mice: possible role of potential antioxidants. Pak J Pharm Sci. 2017;30(3(Suppl.):989–5.
12. Xing H, Zhang K, Zhang R, Shi H, Bi K, Chen X. Antidepressant-like effect of the water extract of the fixed combination of gardenia jasminoides, citrus aurantium and Magnolia officinalis in a rat model of chronic unpredictable mild stress. Phytomedicine. 2015;22(13):1178–85.
13. Duan X, Jiang Y, Su X, Zhang ZY, Shi J. Antioxidant properties of anthocyanins extracted from litchi (Litchi chinenesis sonn.) fruit pericarp tissues in relation to their role in the pericarp browning. Food Chem. 2007;101(4):1365–71.
14. Jiang G, Lin S, Wen L, Jiang Y, Zhao M, Chen F, et al. Identification of a novel phenolic compound in litchi (Litchi chinensis Sonn.) pericarp and bioactivity evaluation. Food Chem. 2013;136(2):563–8.
15. Bhoopat L, Srichairatanakool S, Kanjanapothi D, Taesotikul T, Thananchai H, Bhoopat T. Hepatoprotective effects of lychee (Litchi chinensis Sonn.): a combination of antioxidant and anti-apoptotic activities. J Ethnopharmacol. 2011;136(1):55–66.
16. Auwal MS, Saka S, Mairiga IA, Sanda KA, Shuaibu A, Ibrahim A. Preliminary phytochemical and elemental analysis of aqueous and fractionated pod extracts of Acacia nilotica (Thorn mimosa). Vet Res Forum. 2014;5(2):95–100.
17. Lee YH, Choo C, Watawana MI, Jayawardena N, Waisundara VY. An appraisal of eighteen commonly consumed edible plants as functional food based on their antioxidant and starch hydrolase inhibitory activities. J Sci Food Agric. 2015;95(14):2956–64.
18. Shi JY, Zou XB, Zhao JW, Mel H, Wang KL, Wang X, et al. Determination of total flavonoids content in fresh Ginkgo biloba leaf with different colors using near infrared spectroscopy. Spectrochim Acta A Mol Biomol Spectrosc. 2012;94:271–6.
19. Chomchuen, S, Singharachai C, Ruangrungsi N, Towiwat P. Antipyretic effect of the ethanolic extract of Ficus racemosa root in rats. J Health Res. 2018; 24(1):23–8.
20. Asano Y. Characteristics of open field behavior of wistar and sprague-dawley rats. Jikken Dobutsu. 1986;35(4):505–8.
21. Cavanagh HM, Wilkinson JM. Biological activities of lavender essential oil. Phytother Res. 2002;16(4):301–8.
22. Porsolt RD, Bertin A, Jalfre M. “Behavioural despair” in rats and mice: strain differences and the effects of imipramine. Eur J Pharmacol. 1978;51(3):291–4.
23. Ciarlone AE. Further modification of a fluoromertric method for analyzing brain amines. Microchem J. 1978;23(1):9–12.
24. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–8.
25. Papadimitriou A, Priftis KN. Regulation of the hypothalamic-pituitary-adrenal axis. Neuroimmunomodulation. 2009;16(5):265–71.
26. Palazidou E. The neurobiology of depression. Br Med Bull. 2012;101:127–45.
27. Asberg M, Thorén P, Träskman L, Bertilsson L, Ringberger V. “Serotonin depression”--a biochemical subgroup within the affective disorders? Science. 1976;191(4226):478–80.
28. Jakobsen JC, Katakam KK, Schou A, Hellmuth SG, Stallknecht SE, Leth-Møller K, et al. Selective serotonin reuptake inhibitors versus placebo in patients with major depressive disorder. A systematic review with meta-analysis and trial sequential analysis. BMC Psychiatry. 2017;17(1):58.
29. Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301(5634):805–9.
30. Wang Q, Timberlake MA, Prall K, Dwivedi Y. The recent progress in animal models of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2017l;77:99–109.
31. Laaris N, Haj-Dahmane S, Hamon M, Lanfumey L. Glucocorticoid receptor-mediated inhibition by corticosterone of 5-HT1A autoreceptor functioning in the rat dorsal raphe nucleus. Neuropharmacology. 1995;34(9):1201–10.
32. Holmes PV. Rodent models of depression: reexamining validity without anthropomorphic inference. Crit Rev Neurobiol. 2003;15(2):143–74.
33. Castagné V, Moser P, Roux S, Porsolt RD. Rodent models of depression: forced swim and tail suspension behavioral despair tests in rats and mice. Curr Protoc Neurosci. 2011;8:Unit 8.10A.
34. Dang H, Chen Y, Liu X, Wang Q, Wang L, Jia W, et al. Antidepressant effects of ginseng total saponins in the forced swimming test and chronic mild stress models of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(8):1417–24.
35. Samarghandian S, Azimi-Nezhad M, Samini F. Preventive effect of safranal against oxidative damage in aged male rat brain. Exp Anim. 2015;64(1):65–71.
36. Tsuboi H, Tatsumi A, Yamamoto K, Kobayashi F, Shimoi K, Kinae N. Possible connections among job stress, depressive symptoms, lipid modulation and antioxidants. J Affect Disord. 2006;91(1):63–70.
37. Ozcan ME, Gulec M, Ozerol E, Polat R, Akyol O. Antioxidant enzyme activities and oxidative stress in affective disorders. Int Clin Psychopharmacol. 2004;19(2):89–95.
38. Sarandol A, Sarandol E, Eker SS, Erdinc S, Vatansever E, Kirli S. Major depressive disorder is accompanied with oxidative stress: short-term antidepressant treatment does not alter oxidative-antioxidative systems. Hum Psychopharmacol. 2007;22(2):67–73.
39. Stefanescu C, Ciobica A. The relevance of oxidative stress status in first episode and recurrent depression. J Affect Disord. 2012;143(1-3):34–8.
40. Kurhe Y, Radhakrishnan M, Gupta D, Devadoss T. QCM-4 a novel 5-HT3 antagonist attenuates the behavioral and biochemical alterations on chronic unpredictable mild stress model of depression in Swiss albino mice. J Pharm Pharmacol. 2014;66(1):122–32.
41. Syeda SF, Mohsin M. Evaluations of antidepressant activity of Punica granatum peel extract in albino mice. IJBCP. 2020;9(3):449–53.
42. Piwowarska J, Chimiak A, Matsumoto H, Dzikliska A, Radziwo-Zaleska M, Szelenberger W, et al. Serum cortisol concentration in patients with major depression after treatment with fluoxetine. Psychiatry Res. 2012;198(3):407–11.
43. Pariante CM, Thomas SA, Lovestone S, Makoff A, Kerwin RW. Do antidepressants regulate how cortisol affects the brain? Psychoneuroendocrinology. 2004;29(4):423–7.