INTRODUCTION
Kidneys are essential for the maintenance of homeostasis as well as other regulatory, excretory, and endocrine processes in our body. A decline in kidney function as seen in chronic kidney disease (CKD) patients can, therefore, result in several complications like hyperkalemia, hyperphosphatemia, metabolic acidosis, volume overload, nausea, vomiting, anorexia, anemia, osteomalacia, and malnutrition [1]. These complications, along with the high rate of coincidence of several diseases like hypertension, diabetes mellitus, ischemic heart disease, and dyslipidemia makes the pharmacotherapy of CKD complicated, owing to multiple drug use [2]. CKD patients are therefore highly vulnerable to potential drug-drug interactions (pDDIs). Similarly, when numerous prescribers are involved in treating the same patients, the number of prescribed medicines may rise, thereby increasing the risk of pDDIs [3,4].
Drug-drug interactions (DDIs) are usually preventable, but, if they happen, they could lead to ineffective therapeutic responses, significant morbidity, mortality, and serious adverse events, which will ultimately increase the burden on the duration of hospitalization and treatment cost [5]. Studies have shown that around 11% of patients develop symptoms linked with DDIs, accounting for approximately 3% of all hospitalizations worldwide [6]. In a tertiary health facility, the incidence of clinically significant DDIs might reach 54%, with an average of 1.7 interactions per patient [7].
With prior knowledge about the characteristics of the administered drugs, it is now possible to predict pDDIs in a patient using computational techniques. Though several pDDI checking websites are available, they often do not consider patient-specific characteristics for the prediction of pDDIs. Whereas, in the development of pDDI prediction models, several determining factors such as age and sex of the patient, chemical characteristics of drugs, number of drugs used, creatinine clearance, glomerular filtration rate (GFR), renal and hepatic status, and presence of other diseases are considered to obtain more accurate outcomes, specific to the study population [8,9]. Prediction models use the characteristics of a patient to estimate the probability of an outcome within a definite time period and identify the best set of predictors of that particular outcome [10]. Not much research work has been conducted for pDDI prediction in CKD patients. Most of the previously published literature used a logistic regression approach to identify the risk factors associated with the occurrence of pDDIs [5,11], whereas in this study we have developed and statistically validated a multivariable Poisson regression model to identify the risk factors linked to the number of pDDIs. With the vast variability in etiology, clinical presentation, and treatment of diseases among patients, it is more reasonable to select multiple predictors [12]. Poisson regression is more appropriate to analyze the factors associated with the number of events occurring in a given period of time as the number of pDDIs occurring during the period of treatment may follow a Poisson distribution.
This study could allow an early prediction and assessment of pDDIs in a patient and assist the clinician to choose therapeutic alternatives, make dosage adjustments, and perform the needful interventions either by reducing the number of drugs or reducing the frequency of administration, which will ultimately improve the therapeutic outcome, minimize the adverse effects, and reduce the economic burden on the patient. It may also improve the quality of prescribing and serve as a foundation for evidence-based medicine. The aim of this study was to determine the number and types of pDDIs in CKD patients and to identify the independent risk factors associated with an increased number of pDDIs using Poisson regression analysis to develop a prediction model.
MATERIALS AND METHODS
Study design and data collection
This retrospective observational study was carried out at the nephrology department of a tertiary care teaching hospital in South India. A total of 392 hospitalized patients with CKD having the International Classification of Diseases code numbers ranging from N18.1 to N18.9, who were between 18 to 60 years of age, were selected by consecutive sampling and included in the study. Patients discharged against medical advice and those with incomplete files were excluded. They were selected sequentially in order of appearance based on their convenient accessibility during this process, which ended when the required number of patients had been reached. All their relevant demographic and clinical data were collected from the patient files obtained from the Medical Records Department (MRD) of the hospital and documented in a case record form. Data collection was performed from January 2022 to March 2022 and the medical records of CKD patients admitted for conservative treatment between September 2021 to March 2022, for a period of 6 months were screened.
The data were gathered in two stages. In the first stage, three independent researchers analyzed the medication chart of every participant enrolled in the study for the number of drugs prescribed per patient, number of therapeutic subgroups (second level Anatomical Therapeutic Chemical/ATC classification) prescribed, number of prescribers, age, gender, weight, CKD stage, serum creatinine, creatinine clearance, estimated glomerular filtration rate (eGFR), comorbid conditions, dialysis status, and other relevant factors. The patients were classified into different stages of CKD based on their eGFR values, according to the Kidney Disease: Improving Global Outcomes guidelines. Stage 1 was defined as renal damage with normal or relatively high GFR (≥90 ml/minute/1.73 m2) followed by stage 2 which was defined as renal damage with a mild reduction in GFR (60–89 ml/minute/1.73 m2). Stage 3 was divided into 3a and 3b, where 3a was a mild to moderate reduction in GFR (45–59 ml/minute/1.73 m2) and 3b was a moderate to severe reduction in GFR (30–44 ml/minute/1.73 m2). Stage 4 was defined as a severe reduction in GFR (15–29 ml/minute/1.73 m2), and finally, stage 5 was defined as an end-stage renal disease with GFR lesser than 15 ml/minute/1.73 m2 [13].
In the second stage, all prescriptions were analyzed for pDDIs by utilizing the Micromedex® Drug-Reax® System. Depending on the severity of the outcome and the quality of documentation, drug interactions were classified as contraindicated, major, moderate, or minor. A minor interaction was defined as the one having limited clinical consequences, and which may not necessitate a significant change in medication while a moderate interaction was characterized as the one that may worsen the patient’s condition, needing alternate therapy, extra care, or longer hospitalization. Similarly, a major interaction was defined as the one that is generally life-threatening and requires immediate medical attention, and finally, the drugs that should not be administered together at any cost were considered as contraindications [14].
Predictor identification
According to previously published literature, patient characteristics that include quantitative variables like the number of drugs prescribed per patient, number of therapeutic subgroups prescribed, number of prescribers, creatinine clearance, eGFR, and number of comorbidities, as well as categorical variables like gender, age groups, CKD stages, dialysis status, and the major comorbid conditions may play an important role in precipitating pDDIs in CKD patients [3,4,7]. As a result, all these characteristics have been collected as probable predictors of pDDIs.
Statistical analysis
The sample size for the study was derived based on the sample size calculation for identifying risk factors using regression models by considering the incidence of severe pDDIs in CKD patients from previously published data, which was found to be 16.8% [5], and detecting a risk factor with an odds ratio (OR) of 1.5 or more at 5% level of significance and 80% power. Based on the calculation, 392 patients were needed to be recruited for this study.
Descriptive statistics were used to describe the characteristics of the patient population, with quantitative variables expressed as mean ± standard deviation (SD) for normally distributed data and median with interquartile range (IQR) for non-normally distributed data, and categorical variables expressed as frequency and percentage. Data were entered and analyzed using IBM Statistical Package for the Social Sciences software v20.0. Poisson regression analysis was used to identify the independent factors associated with the number of pDDIs. All the predictors were initially selected based on previously known clinical considerations and assessed individually by univariate analysis, after which those with a statistical significance level less than 0.25 (p < 0.25) were taken together and screened by multivariate analysis until all the insignificant predictors were eliminated. During the initial screening by univariate analysis, a slightly relaxed significance level (p < 0.25) was chosen to prevent the exclusion of potentially important variables, and care was taken to ensure that there were an adequate number of events (at least 10 events) per independent variable. The identified risk factors were expressed in terms of OR, with a significance level less than 0.05 (p < 0.05), and a confidence interval set to 95%. Using intercept (β0) and coefficients (β) of variables having statistical significance, the prediction model was developed. The statistical validity of the developed model was assessed by the Pearson chi-square test, omnibus test, and residual analysis.
RESULTS
Demographic and clinical characteristics of the study population
The medical records of 392 CKD patients were screened and included in the study. Table 1 summarizes the demographic and clinical characteristics of the patients. The mean age of this study population was found to be 45.97 ± 10.32 (Mean ± SD) and males accounted for 74.40% (N = 293) of the entire population. It was observed that the majority of CKD patients, 87.0% (N = 341), belonged to stage-5.
Characteristics of the pDDIs
Out of 392 patients, 89.79% (N = 352) had shown pDDIs. A total of 2,054 pDDIs of different severities had been observed with a mean of 5.18 ± 4.91 (N = 2,030) pDDIs per patient. Figure 1 illustrates the distribution of pDDIs based on severity.
The most common classes of drugs involved in pDDIs were cardiovascular drugs, antidiabetic drugs, antimicrobial agents, respiratory drugs, gastrointestinal drugs, and vitamins/minerals. Table 2 elucidates a detailed description of the most frequently prescribed drug combinations which were associated with pDDIs.
Identification of independent risk factors associated with the number of pDDIs and development of a prediction model by Poisson regression analysis
Univariate Poisson regression analysis had initially revealed that the significant variables (p < 0.25) associated with the number of pDDIs were the age groups of 31-60 years, CKD stages 3a, 3b, and 5, male gender, creatinine clearance, dialysis, ischemic heart disease, liver diseases, hypertension, diabetes mellitus, hypothyroidism, congestive heart failure, number of therapeutic subgroups, number of comorbidities, number of drugs, and number of prescribers.
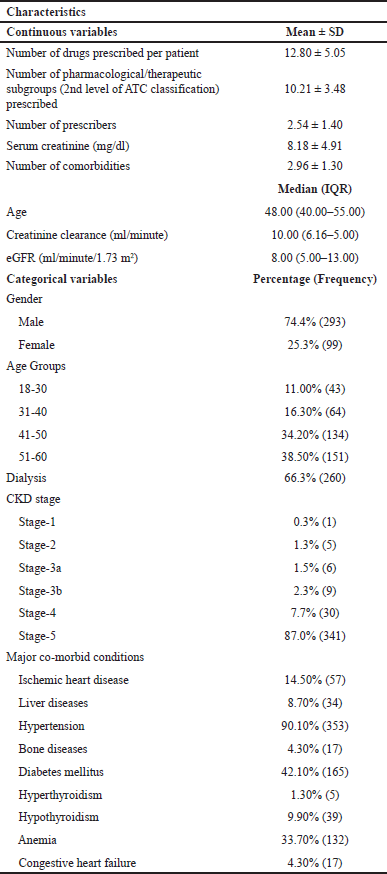 | Table 1. Demographic and clinical characteristics of the study population. [Click here to view] |
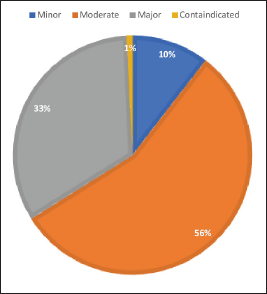 | Figure 1. Distribution of pDDIs based on the severity. [Click here to view] |
For developing the pDDI prediction model, these variables were analyzed together by multivariate Poisson regression until all the insignificant variables were removed. The results of this final regression analysis are shown in Table 3, where it can be observed that male patients (95% CI = 1.171–1.461, p < 0.001) were associated with an increase in the number of pDDIs by 1.308 times when compared to females. Similarly, the presence of comorbidities like ischemic heart disease (95% CI = 1.050–1.314, p = 0.005), hypertension (95% CI = 1.464–2.087, p < 0.001), diabetes mellitus (95% CI=1.248–1.498, p < 0.001), and congestive heart failure (95% CI = 1.186–1.698, p < 0.001) had increased the number of pDDIs by 1.175, 1.747, 1.368, and 1.419 times, respectively. On the other hand, it was observed that the presence of liver diseases (95% CI = 0.622–0.872, p < 0.001) was associated with a reduction in the number of pDDIs by 0.737 times. Furthermore, the number of pDDIs was also raised by 1.070 times and 1.327 times for a unit increase in the number of drugs (95% CI = 1.052–1.089, p < 0.001) and the number of therapeutic subgroups (95% CI = 1.021–1.076, p < 0.001) prescribed per patient, respectively.
A Poisson regression model was created to predict the number of pDDIs with respect to the various independent contributing factors. The following equation was derived based on the coefficients of independent risk factors and the constant or intercept (β0), which was found to be −0.753.
log (no.of pDDIs)
= – 0.753+0.268 (male gender)
+0.161 (presence of ischemic heart disease)
–0.305 (presence of liver diseases)
+0.558 (presence of hypertension)
+0.313 (presence of diabetes mellitus)
+0.350 (presence of congestive heart failure)
+0.068 (no.of drugs prescribed)
+0.047 (no.of therapeutic subgroups prescribed)
Statistical validation of the developed model
The performance of the developed model was statistically evaluated. Figure 2 shows a scatter plot of the predicted value of the mean response on the x-axis against standardized Pearson residual values on the y-axis. It reveals that the developed regression model shows a relatively equal distribution of points above and below the horizontal line at residuals = 0, indicating that the linearity and equal-variance assumptions of the regression model are not violated.
The Pearson chi-square goodness of fit test value for the developed model was found to be 1.899, which indicates a slight overdispersion. However, due to the small sample size of our study, this value is unlikely to affect the assumption of equidispersion seriously. In the omnibus test, the p-value was found to be less than 0.001, indicating that the developed model is statistically significant, where all the independent variables collectively improve the model over the intercept-only model.
DISCUSSION
In this study, pDDIs were seen in 89.79% of the patients, similar to the findings of a study performed in the medicine outpatient department of an Indian tertiary care hospital [15]. Most of the interactions were found to be of moderate severity which is in concordance with the findings of another Indian study by Rama et al. [16] and a Palestinian study by Al-Ramahi et al. [17]. The percentage of contraindications in our study was found to be 0.7% which was very much similar to three other studies [17–19]. However, our frequencies of major and minor pDDIs were slightly contrasting from other published literature, which may be due to variations in the study design, prescribing patterns, sample size, and databases used to check for drug interactions.
The most common classes of drugs involved in pDDIs were cardiovascular drugs, antidiabetic drugs, antimicrobial agents, respiratory drugs, gastrointestinal drugs, and vitamins/minerals which is consistent with the aforementioned study conducted in the same hospital setting by Rama et al. [16]. Similar to our study, they also identified that clonidine/metoprolol and insulin/metoprolol were the most frequently interacting drugs in the admitted CKD patients. A case report by Handler [20] describes the development of severe sinus bradycardia, with slurred speech, and visual blurriness in a 65-year-old woman following the introduction of clonidine to longstanding metoprolol therapy. Apart from that, a randomized controlled trial involving patients with type 2 diabetes mellitus found that vascular insulin sensitivity had deteriorated when insulin infusion was co-administered with metoprolol but remained unchanged in the case of carvedilol [21]. In the study conducted by Al-Ramahi et al. [17], aspirin/furosemide and aspirin/calcium preparations were found to interact most often. Interaction between aspirin and furosemide was suspected to cause grade-1 renal parenchymal disease in a 60-year-old female who was concomitantly receiving both drugs for 6 days [22]. A prospective interventional study in Italy showed that concurrent administration of aspirin with calcium carbonate, magnesium hydroxide, and aluminum significantly impaired aspirin absorption, with calcium carbonate causing the greatest decrease in the plasma concentration of aspirin out of all [23]. Furthermore, Sgnaolin et al. [24] identified iron supplements/calcium preparations as one of the most frequently interacting drugs, while Okoro and Farate et al. [19] identified iron supplements/pantoprazole, both of which are consistent with our findings. Ganipisetti et al. [25] described the case of a pregnant woman who received an excessive dose of calcium carbonate with iron supplements and developed severe iron deficiency anemia due to poor iron absorption, requiring two units of packed red blood cells and parenteral iron therapy. A prospective study conducted in New Jersey showed that people with iron deficiency are more likely to have a suboptimal response to ferrous sulfate when given with proton-pump inhibitors, necessitating intravenous iron supplementation or a longer period of oral iron treatment [26]. However, a higher frequency of interaction between insulin and oral antidiabetics with furosemide was not found in most of the studies.
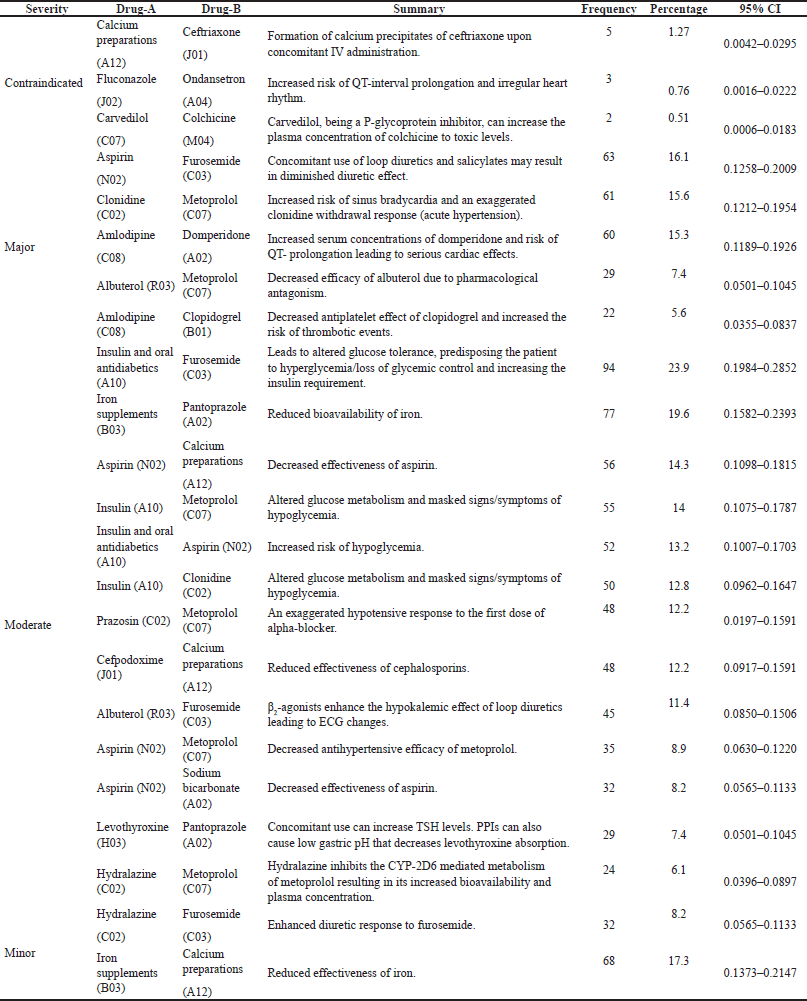 | Table 2. Pairs of frequently interacting drugs with their pharmacological/therapeutic subgroups (second level of ATC classification). [Click here to view] |
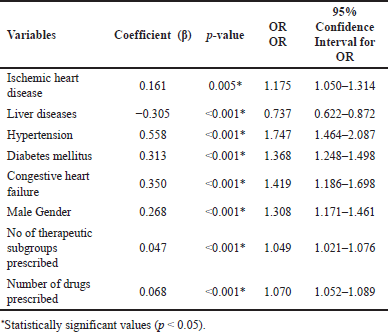 | Table 3. Characteristics of final multivariate Poisson regression model for predicting the number of pDDIs. [Click here to view] |
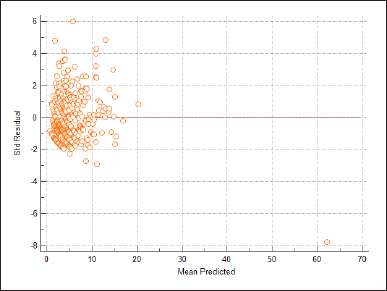 | Figure 2. Scatter plot of the predicted value of the mean response versus standardized Pearson residual values. [Click here to view] |
The results of the developed regression model showed that male gender, comorbidities like ischemic heart disease, hypertension, diabetes mellitus, and congestive heart failure, a higher number of therapeutic subgroups, and drugs per prescription were shown to significantly increase the number of pDDIs. While the presence of liver diseases was associated with a decrease in the number of pDDIs. The number of pDDIs increased in direct proportion to the number of drugs and therapeutic subgroups. An increase in the number of drugs prescribed can be attributed to a number of factors, including the presence of multiple comorbidities that necessitate multiple medications, the involvement of several physicians from different specialties, and physicians working in different shifts, which causes frequent changes in the therapeutic regimen, resulting in patients’ treatment plans not being reviewed together [7,27]. Supporting our finding, about a decade ago Delafuente [28] demonstrated that if patients are on more than six medications, the number of pDDIs increases from 39% to 100% when compared to when they are on 2–3 medications. Our findings are also consistent with those of other studies conducted in similar hospital settings [15,17,29–31].
In our study, the male gender showed higher pDDIs as there were predominantly more male CKD patients. However, this contrasts with some studies which show that more than half of the renal patients were females and with higher pDDIs than males [32], while another study’s findings are parallel to ours [33]. These discrepancies in the results are incomprehensible and could be mainly due to the differences in sampling and unequal distribution of both genders.
Ischemic heart disease, liver disease, hypertension, diabetes mellitus, and congestive heart failure were found to be the significant factors associated with an increased number of pDDIs in accordance with an Indian prospective cohort study [34]. A high frequency of pDDIs was observed in patients taking anti-hypertensive medications in our study, accounting for 90.1% of the study sample. Patients with hypertension typically require the use of more than one anti-hypertensive and a multidrug regimen is frequently used due to several associated comorbidities. This subsequently increases the chances of polypharmacy and thus the number of pDDIs, as shown in two studies from Iowa [35] and Telangana [36]. Cardiovascular diseases like ischemic heart disease and congestive cardiac failure are prevalent in increasing the number of pDDIs. Cardiac diseases lead to an increase in the number of drugs and the number of potentially interacting drug pairs per prescription [34]. The number of pDDIs in patients with congestive cardiac failure was documented in a study by Herrlinger and Klotz [37], who found that more than 90% of interactions were either moderate or major in severity. Furthermore, patients with diabetes showed a high number of pDDIs, which could be attributed to the substantial increase in the number of antidiabetic drugs and the risk of possible interactions that can cause an imbalance in glucose homeostasis [38].
It was an interesting observation that the presence of liver diseases was linked to a lower number of pDDIs. This remains unexplained; however, it could be due to the lower number of interacting drug pairs prescribed to patients with liver function impairment in our study.
The high frequency of pDDIs observed per patient and identification of the associated risk factors may serve as an alert to healthcare professionals in the hospital setting and can assist the clinician in choosing therapeutic alternatives, making dosage adjustments, and performing the necessary interventions by reducing the number of drugs or the frequency of administration. A clinical pharmacist’s role is to ensure that medications are properly screened for interactions along with identifying harmful combinations to avoid and manage the adverse effects. According to an Italian study, being aware of the pDDIs caused by digoxin and other drugs resulted in fewer interactions [39]. This could ultimately improve the therapeutic outcome, minimize the adverse effects, establish rational drug use, and reduce the economic burden on the patient.
There were various limitations to this study that should be considered. First and foremost, the study was conducted retrospectively, because of which the outcomes of the pDDIs could not be assessed and preventive interventions could not be taken. Since all the information was acquired only from medical records without any direct involvement of the patient, only a limited number of predictors could be included. Any information on a person’s social background, smoking habits, alcohol intake, and so on could not be collected, resulting in information bias. Furthermore, data on the use of non-prescription drugs could not be collected, which might also significantly contribute to pDDIs. The sample size was calculated based on a closely related study with a similar population resulting in a small sample size and convenient sampling. This may make extrapolating study findings to the wider population problematic. Moreover, patients over the age of 60 had to be excluded from the study to avoid any bias in the model due to overfitting, since the number of admitted patients in that category was less than 10 and all relevant demographic details were unavailable. In the developed prediction model, it was observed that more points of the scatter plot were above the reference line than below. This indicates that the developed model slightly overpredicts the number of pDDIs and there is a modest difference in the predicted and actual values. Future directives could be taken to externally validate the prediction model in a broader population and to expand the sample size, which would aid in the development of a stronger model and produce more exact findings.
CONCLUSION
Our study revealed that factors like male gender, comorbid conditions like ischemic heart disease, hypertension, diabetes mellitus, and congestive heart failure, a higher number of therapeutic subgroups, and drugs per prescription were shown to significantly increase the number of pDDIs. While the presence of liver diseases was associated with a decrease in the number of pDDIs. Because it considers patient-specific clinical data, the developed model may produce more reliable and accurate outcomes than existing pDDI checking websites and could be useful for the early detection and prevention of pDDIs in CKD patients. We have also identified the most typical pairs of interacting drugs at the nephrology department of that hospital, that can assist the clinicians to avoid the simultaneous use of harmful drug combinations. Vigilant and continuous monitoring can aid in identifying pDDIs which may ultimately prevent morbidity and mortality of patients. It is the responsibility of the clinical pharmacist to detect and prevent undesirable interactions and provide necessary interventions in case of any adverse events.
AUTHORS’ CONTRIBUTIONS
Vijayanarayana Kunhikatta contributed to the selection, conceptualization, supervision, review, and editing of the study. Soumyajeet Paul, Ananya Rudra, and Suparna Bhattacharjee performed data collection, analysis, and writing the original draft. Girish Thunga and Ravindra Prabhu Attur were involved in the conceptualization, supervision, and review of this study.
ACKNOWLEDGMENTS
The authors are thankful to the Manipal Academy of Higher Education for providing facilities to conduct this study as well as the employees of the MRD of Kasturba Hospital, Manipal for their cooperation. The article was uploaded to a preprint server before submission to this journal; the web link is here: https://doi.org/10.21203/rs.3.rs-1752892/v1
FINANCIAL SUPPORT
This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CONFLICTS OF INTEREST
The authors report no conflicts of interest to declare. The listed authors are solely responsible for the originality and content of the manuscript. The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
ETHICAL APPROVAL
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Ethics Committee (IEC) of Kasturba Medical College, Manipal (Ethical Approval Number-IEC:624/2021 issued on January 8, 2022). The confidentiality of the patients was maintained.
DATA AVAILABILITY
Supplementary material on the equation for generalized linear modeling by Poisson regression is provided. The rest of the data and materials associated with this study are not deposited in any repositories. It will be available from the corresponding author upon the proper request.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
PERMISSION TO REPRODUCE MATERIAL FROM OTHER SOURCES
This study did not require permission as it did not reproduce material from other sources.
REFERENCES
1. Bastos MG, Carmo WD, Abrita RR, Almeida ED, Mafra D, Costa DD, et al. Doença renal crônica: problemas e soluções. J Bras Nefrol. 2004;26(4):202–15.
2. Thomas R, Kanso A, Sedor JR. Chronic kidney disease and its complications. Prim Care. 2008;35(2):329–44. doi: https://doi.org/10.1016/j.pop.2008.01.008
3. Busari AA, Oreagba IA, Oshikoya KA, Kayode MO, Olayemi SO. High risk of drug-drug interactions among hospitalized patients with kidney diseases at a Nigerian teaching hospital: a call for action. Niger Med J. 2019;60(6):317–25. doi: https://doi.org/10.4103/nmj.NMJ_2_19
4. Marquito AB, Fernandes NM, Colugnati FA, Paula RB. Identifying potential drug interactions in chronic kidney disease patients. J Bras Nefrol. 2014;36(1):26–34. doi: https://doi.org/10.5935/0101-2800.20140006
5. Venkatakrishnan K, von Moltke LL, Obach RS, Greenblatt DJ. Drug metabolism and drug interactions: application and clinical value of in vitro models. Curr Drug Metab. 2003;4(5):423–59.
6. Merlo J, Liedholm H, Lindblad U, Björck-Linné A, Fält J, Lindberg G, et al. Prescriptions with potential drug interaction dispensed at swedish pharmacies in January 1999: cross-sectional study. Br Med J. 2001;323:427–8.
7. Jankovi? SM, Pej?i? AV, Milosavljevi? MN, Opan?ina VD, Peši? NV, Nedeljkovi? TT, et al. Risk factors for potential drug-drug interactions in intensive care unit patients. J Crit Care. 2018;43:1–6. doi: https://doi.org/10.1016/j.jcrc.2017.08.021
8. Shipe ME, Deppen SA, Farjah F, Grogan EL. Developing prediction models for clinical use using logistic regression: an overview. J Thorac Dis. 2019;11(Suppl 4):S574–84. doi: https://doi.org/10.21037/jtd.2019.01.25
9. Yan C, Wang J, Lan W, Wu FX, Pan Y. Sdtrls: predicting drug-target interactions for complex diseases based on chemical substructures. Complexity. 2017;2017:10.
10. Moons KG, Kengne AP, Woodward M, Royston P, Vergouwe Y, Altman DG, et al. Risk prediction models: I. Development, internal validation, and assessing the incremental value of a new (bio)marker. Heart. 2012; 98:683–90.
11. Saleem A, Masood I, Khan TM. Clinical relevancy and determinants of potential drug–drug interactions in chronic kidney disease patients: results from a retrospective analysis. Integr Pharm Res Pract. 2017;6:71–7.
12. Moons KG, Royston P, Vergouwe Y, Grobbee DE, Altman DG. Prognosis and prognostic research: what, why, and how? BMJ. 2009;338:b375. doi: https://doi.org/10.1136/bmj.b375
13. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 2013;3:1–150.
14. Klasko RK. DRUG-REAX® System [Internet database]. Greenwood Village, CO: Thomson Micromedex. Available from: https://www.micromedexsolutions.com/micromedex2/librarian/CS/A0963B/ND_PR/evidencexpert/ND_P/evidencexpert/DUPLICATIONSHIELDSYNC/28E6E8/ND_PG/evidencexpert/ND_B/evidencexpert/ND_AppProduct/evidencexpert/ND_T/evidencexpert/PFActionId/evidencexpert.FindDrugInteractions?navitem=topInteractions&isToolPage=true
15. Shetty V, Chowta MN, Shenoy A, Kamath A, Kamath P. Evaluation of potential drug-drug interactions with medications prescribed to geriatric patients in a tertiary care hospital. J Aging Res. 2018;5728957. doi: https://doi.org/10.1155/2018/5728957
16. Rama M, Viswanathan G, Acharya LD, Attur RP, Reddy PN, Raghavan SV. Assessment of drug-drug interactions among renal failure patients of nephrology ward in a South Indian tertiary care hospital. Indian J Pharm Sci. 2012;74(1):63–8. doi: https://doi.org/10.4103/0250-474X.102545
17. Al-Ramahi R, Raddad AR, Rashed AO, Bsharat A, Abu-Ghazaleh D, Yasin E, et al. Evaluation of potential drug-drug interactions among Palestinian hemodialysis patients. BMC Nephrol. 2016;17:96.
18. Fasipe OJ, Akhideno PE, Nwaiwu O, Adelosoye AA. Assessment of prescribed medications and pattern of distribution for potential drug–drug interactions among chronic kidney disease patients attending the nephrology clinic of Lagos University Teaching Hospital in sub-Saharan West Africa. Clin Pharmacol. 2017;9:125–32.
19. Okoro RN, Farate VT. Evaluation of potential drug–drug interactions among patients with chronic kidney disease in northeastern Nigeria. Afr J Nephrol. 2019;22(1):77–81. doi: https://doi.org/10.21804/22-1-3577
20. Handler J. Adverse effects using combined rate-slowing antihypertensive agents. J Clin Hypertens (Greenwich). 2011;13(7):529–32. doi: https://doi.org/10.1111/j.1751-7176.2011.00486.x
21. Kveiborg B, Hermann TS, Major-Pedersen A, Christiansen B, Rask-Madsen C, Raunsø J, et al. Metoprolol compared to carvedilol deteriorates insulin-stimulated endothelial function in patients with type 2 diabetes—a randomized study. Cardiovasc Diabetol. 2010;9:21. doi: https://doi.org/10.1186/1475-2840-9-21
22. Reddy GK, Kuriakose NG. Adverse drug interaction between aspirin and furosemide: a case report. Asian J Pharm Clin Res. 2019;12(10):6–8. doi: https://doi.org/10.22159/ajpcr.2019.v12i10.34626.
23. Gaspari F, Viganò G, Locatelli M, Remuzzi G. Influence of antacid administrations on aspirin absorption in patients with chronic renal failure on maintenance hemodialysis. Am J Kidney Dis. 1988;11(4):338–42. doi: https://doi.org/10.1016/s0272-6386(88)80140-9
24. Sgnaolin V, Sgnaoli V, Engroff P, DeCarli GA, Figueiredo AE. Avaliação dos medicamentos utilizados e possíveis interações medicamentosas em doentes renais crônicos. Sci Med (Porto Alegre). 2014;24:329–5.
25. Ganipisetti VM, Khaira H, Dowlatshahi S, Bollimunta P, Mangat S, Sinsheimer E. Presence Saint Francis Hospital, Evanston, IL. An unusual cause of iron deficencyanemia: a case of pica for antacids in a pregnant women. J Gen Intern Med. 2014;29(Suppl 1):1–545. doi: https://doi.org/10.1007/s11606-014-2834-9
26. Ajmera AV, Shastri GS, Gajera MJ, Judge TA. Suboptimal response to ferrous sulfate in iron-deficient patients taking omeprazole. Am J Ther. 2012;19(3):185–9. doi: https://doi.org/10.1097/MJT.0b013e3181f9f6d2
27. Venturini CD, Engroff P, Ely LS, Zago LF, Schroeter G, Gomes I, et al. Gender differences, polypharmacy, and potential pharmacological interactions in the elderly. Clinics (São Paulo). 2011;66:1867–72.
28. Delafuente JC. Understanding and preventing drug interactions in elderly patients. Crit Rev Oncol Hematol. 2003;48(2):133–43. doi: https://doi.org/10.1016/j.critrevonc.2003.04.004.
29. Olumuyiwa JF, Akinwumi AA, Ademola OA, Oluwole BA, Ibiene EO. Prevalence and pattern of potential drug-drug interactions among chronic kidney disease patients in south-western Nigeria. Niger Postgrad Med J. 2017;24:88–92.
30. Hedge S, Udaykumar P, Manjuprasad MS. Potential drug interactions in chronic kidney disease patients—a cross sectional study. Int J Recent Trends Sci Technol. 2015;16:56–60.
31. Ren W, Liu Y, Zhang J, Fang Z, Fang H, Gong Y, et al. Prevalence of potential drug-drug interactions in outpatients of a general hospital in China: a retrospective investigation. Int J Clin Pharm. 2020;42(4):1190–96. doi: https://doi.org/10.1007/s11096-020-01068-3
32. Cruciol-Souza JM, Thomson JC. Prevalence of potential drug-drug interactions and its associated factors in a Brazilian teaching hospital. J Pharm Pharm Sci. 2006;9:427–33.
33. Kabakama C, Pydimarri R, Ponnusankar S. A prospective study on assessment of clinically potential drug-drug interactions in hospital and community pharmacy prescriptions. Af J Pharm Pharmacol. 2021;15:118–25.
34. Kashyap M, D’Cruz S, Sachdev A, Tiwari P. Drug-drug interactions and their predictors: Results from Indian elderly inpatients. Pharm Pract (Granada). 2013 Oct;11(4):191–5. doi: https://doi.org/10.4321/s1886-36552013000400003.
35. Carter BL, Lund BC, Hayase N, Chrischilles E. The extent of potential antihypertensive drug interactions in a Medicaid population. Am J Hypertens. 2002;15(11):953–7. doi: https://doi.org/10.1016/s0895-7061(02)03026-1.
36. Sivva D, Mateti UV, Neerati VM, Thiruthopu NS, Martha S. Assessment of drug-drug interactions in hypertensive patients at a superspeciality hospital. Avicenna J Med. 2015;5(2):29–35. doi: https://doi.org/10.4103/2231-0770.154194
37. Herrlinger C, Klotz U. Drug metabolism and drug interactions in the elderly. Best Pract Res Clin Gastroenterol. 2001;15(6):897–918. doi: https://doi.org/10.1053/bega.2001.0249
38. Manjusha S, Amit M, Ronak S. A study on prescribing pattern and potential drug-drug interactions in type 2 diabetes mellitus inpatients. Indian J Pharm Pract. 2014;7(1):1–12.
39. Magro L, Conforti A, Del Zotti F, Leone R, Iorio ML, Meneghelli I, et al. Identification of the severe potential DDIs by using an Italian general practitioner database. Eur J Clin Pharmacol. 2008;64:303–9.