INTRODUCTION
P-glycoprotein (P-gp) is one of the ATP-binding cassette (ABC) transporters and acts as an efflux pump [1]. Encoded by the ABCB1 gene, it is found on the apical surface of epithelial tissues in various organs including the brain, gastrointestinal tract, liver, and kidney, and plays roles in the absorption, distribution, metabolism, and elimination of drugs and xenobiotics from cells [2]. In cancer cells, P-gp overexpression frequently occurs, resulting in decreased intracellular concentrations of chemotherapy drugs leading to drug resistance and treatment failure [3]. Some food or medicinal substances can inhibit the function of P-gp, causing drug-drug or food/herb-drug interactions [4–6], which might benefit the therapeutic effects by increasing the accumulation of anti-cancer drugs, especially P-gp substrates, and decreasing multidrug resistance of cancer cells [7,8].
A number of reports showed the effects of chemical constituents of herbal medicines and food supplements, such as benzophenones, flavonoids, lignans, naphthoquinones, and xanthones, on P-gp activity [9–15]. For example, long-term use of Ginkgo biloba extracts altered the pharmacokinetics of a beta-blocker, talinolol, in healthy volunteers because flavonoids in the plant extract could inhibit P-gp function [11]. Several flavonoids, i.e., hesperidin, naringin, and quercetin, commonly found in fruits, vegetables, and herbal medicine, were studied in silico on their possible interference with P-gp function by interacting with the ATP-binding site on nucleotide-binding domain 1 (NBD1) [14]. St. John’s wort (Hypericum perforatum), widely used for its antidepressant effect, could increase P-gp expression and its drug efflux function in peripheral blood lymphocytes of healthy volunteers [16]. On the other hand, a number of food ingredients, including piperine, capsaicin, and sesamin, could both increase the mRNA expression and inhibit the function of P-gp in vinblastine-resistant colon carcinoma (LS-180V) cells [17].
Garcinia plants (family Clusiaceae) are distributed in Asia, America, Australia, tropical and southern Africa. In Thailand, asam gelugur (G. atroviridis), cowa mangosteen (G. cowa), mangosteen (G. mangostana), and ma-dan (G. schomburgkiana) have been utilized as medicinal and edible plants [18]. They have been used in traditional medicine to treat cough, constipation, menstrual disorders, and diabetes. These plants contain flavonoids, polyisoprenylated benzophenones, and xanthones which displayed anti-inflammatory, antifungal, antioxidant, anti-human immunodeficiency virus, antilipidemic, and cytotoxic activities [19]. Guttiferone K and oblongifolin C are two polyisoprenylated benzophenones isolated from the branches, wood, and bark of G. schomburgkiana [18,20–22]. They were also found in other Garcinia species, such as the fruits of G. yunnanensis [7] and G. cambogia [23]. Although both compounds exhibited remarkable anti-cancer properties by inducing apoptosis and autophagy in vitro and in vivo studies [18,24,25]. Their effect on the P-gp function has not been investigated. Oblongifolin C has been shown to enhance the chemosensitivity of gemcitabine-resistant pancreatic cancer to the drug [26]. In a previous study, both compounds could increase the P-gp expression in Caco-2 cells [27]. Both compounds might be able to interact with P-gp function, and the present study aimed to first demonstrate the effect of guttiferone K and oblongifolin C on P-gp function in human colorectal adenocarcinoma (Caco-2) cells and utilize the computational prediction of P-gp substrate/inhibitor and molecular docking to elucidate their mechanism of action.
MATERIALS AND METHODS
Chemicals and reagents
Calcein acetoxymethyl ester (Calcein-AM), dimethyl sulfoxide (DMSO), Hank’s balanced salt solution (HBSS), non-essential amino acids, penicillin, streptomycin, Triton X-100, and verapamil were purchased from Sigma Chemical Co. (St. Louis, MO). Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), and L-glutamine from Gibco Life Technologies (Grand Island, NY). Guttiferone K and oblongifolin C (Fig. 1) were isolated from the wood of G. schomburgkiana as previously described [18]. Stock solutions of these two compounds and verapamil were prepared by dissolving in DMSO and stored at −20°C before use.
Cell culture
Caco-2 cell line American Type Culture Collection (ATCC®? HTB-37™?) was obtained from (ATCC, Rockville, MD). The cells were sub-cultured every 3 days to maintain at approximately 70% confluence and were grown in DMEM complete medium, supplemented with 10% FBS, 1% non-essential amino acids, 1% penicillin-streptomycin, and 2 mM L-glutamine, in a humidified incubator containing 5% CO2 at 37°C [28].
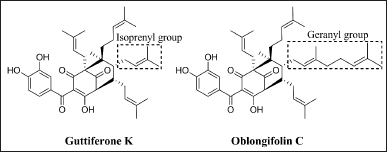 | Figure 1. Chemical structures of guttiferone K and oblongifolin C. [Click here to view] |
Determination of the P-gp function
The P-gp function was determined based on the accumulation of substrate-based fluorescent probes using the calceine-AM uptake assay [15]. Caco-2 cells (passage no. 51–65) were seeded at a density of 1.3 × 104 cells/cm2 in 24-well plates. Fresh medium was supplied to the cells for 1 day after seeding and changed every 2 days for 21 days. The cells were washed three times with HBSS and then pretreated with guttiferone K (1.25–20 μM), oblongifolin C (1.25–20 μM), or verapamil (100 μM) for 30 minutes at 37°C. Then, the P-gp substrate calcein-AM (0.4 μM) was added and the mixture was incubated for another 30 minutes as the pre/co-treatment condition. At this step, endogenous esterases were allowed to hydrolyze the non-fluorescent calcein-AM into fluorescent calcein within the cells. After this incubation period, the cells were washed with ice-cold phosphate-buffered saline to stop the reaction and lysed with 1% Triton X-100. The fluorescence intensity was measured with a microplate reader (Wallac 1420 VICTOR 3, PerkinElmer Inc., Hopkinton, MA) at wavelengths of 485 nm (excitation) and 535 nm (emission).
Prediction of P-gp substrate or P-gp inhibitor
The analysis of determining whether guttiferone K and oblongifolin C were P-gp substrate or P-gp inhibitor was performed computationally SwissADME and admetSAR programs [29,30]. Verapamil was used as a drug reference for P-gp substrate and inhibitor. The two-dimensional (2D) structures of compounds were drawn using ChemDraw 16 (PerkinElmer, Waltham, MA) and converted into SMILES files. These files were submitted through http://www.swissadme.ch/ and http://lmmd.ecust.edu.cn/admetsar2.
Molecular docking
Computational modelling was utilized to investigate protein-ligand interactions between NBD1 (amino acid positions 398–602) of P-gp (PDB; 4Q9H) and compounds including guttiferone K, oblongifolin C, and verapamil [14]. Water molecules were removed from the P-gp structure, and hydrogen atoms were added, followed by the addition of Gasteiger charge through AutoDoc Suite 4.2.6 (TSRI, La Jolla, CA). Three-dimensional (3D) structures of the compounds and P-gp were transformed into Protein Data Bank with partial charge Q and atom type T format files. The grid box was set at a dimension of 100 × 100 × 100 Å to cover the ATP-binding region at NBD1. Docking simulation was performed with a Lamarckian algorithm by setting default parameters at 50 times. The lowest binding energy (ΔG) and inhibition constant (Ki) were observed after docked simulation. The best ligand-protein docking was visualized as 2D and 3D intermolecular interactions using Discovery Studio 2021 Client (BIOVIA, San Diego, CA).
Statistical analysis
Data were expressed as the mean ± SEM of three independent experiments. Statistical analysis was performed using a one-way analysis of variance, followed by Dunnett’s test. p < 0.05 was considered statistically significant
RESULTS
Effect of guttiferone K and oblongifolin C on P-gp function
The effects of guttiferone K and oblongifolin C on P-gp function were evaluated in Caco-2 cells using calcein-AM uptake assay to determine the intracellular accumulation of the P-gp substrate calcein. The Caco-2 cell has been characterized as in vitro intestinal absorption model and used to identify the properties of chemicals to be a substrate or inhibitor in guidance of the U.S. Food and Drug Administration and the European Medicines Agency [31]. Under our experimental condition, Caco-2 cells treated with the known P-gp inhibitor verapamil (100 μM) accumulated calcein at approximately three folds higher than in the control (Fig. 2). Both benzophenone-treated groups also displayed inhibition of P-gp function in a dose-dependent manner. Although at a minimum concentration of 1.25 μM both guttiferone K and oblongifolin C could not inhibit P-gp activity, significant inhibitory effect on P-gp function could be observed at higher concentrations of oblongifolin C and guttiferone K (2.5 and 10 μM, respectively). At similar concentrations of between 2.5–20 μM, oblongifolin C appeared to exhibit stronger inhibitory action on P-gp than guttiferone K. Guttiferone K, at 5, 10, and 20 μM, was about half as active as oblongifolin C. At the highest concentration of oblongifolin C (20 μM), it was able to increase the accumulation of calcein within Caco-2 cells to approximately 2 folds higher than that by verapamil.
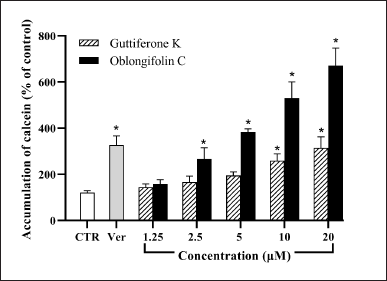 | Figure 2. Effect of guttiferone K and oblongifolin C against P-gp activity in Caco-2 cells. Verapamil (Ver) at concentration of 100 μM was used as positive control. *p < 0.05 compared to control (CTR). [Click here to view] |
Predicting properties of guttiferone K and oblongifolin C as P-gp substrate and P-gp inhibitor
The properties of guttiferone K and oblongifolin C as P-gp substrate/inhibitor was predicted computationally by SwissADME and admetSAR programs [29,30] to define their role against P-gp function. Our results (Table 1) showed that both of them could be P-gp substrates and P-gp inhibitors, similar to verapamil.
Molecular docking of guttiferone K and oblongifolin C to ATP-binding region on NBD1 of P-gp
The interaction between the P-gp inhibitor and the domains of ATP-binding region on NBD1 of P-gp plays critical roles in the inference of drug efflux action [14]. In this study, the interactions of guttiferone K, oblongifolin C, and verapamil with the amino acid residues of the ATP-binding site within NBD1 were examined in silico [32]. As shown in Table 2, the binding free energy (ΔG) to the ATP-binding site on NBD1 of both guttiferone K (−6.87 kcal/mol) and oblongifolin C (−6.79 kcal/mol) indicated their greater affinity than verapamil (−5.46 kcal/mol). Similarly, both benzophenones displayed lower inhibitory constants (Ki) than verapamil by approximately 9–10 folds. All compounds could interact with the amino acid residues within the ATP-binding site on NBD1 via several intermolecular forces, such as hydrogen bond, alkyl-alkyl, pi-alkyl, pi-sigma, and Van der Waals interactions (Fig. 3 and Table 2). The best ligand was oblongifolin C, which could bind to the largest number of amino acids when compared to others by conventional hydrogen bonds (GLU472 and PRO473), alkyl-alkyl (VAL433, LEU439, and VAL468), pi-sigma (VAL474), and Van der Waals forces (GLN434, GLN437, SER470, GLN471, LEU475, GLU522, and LYS532). Guttiferone K also bounded to amino acids of NBD1 through alkyl-alkyl (CYS427), pi-alkyl (VAL403 and ILE405), pi-pi T-shaped (TYR397), and Van der Waals interactions (AGR400, GLN404, GLY428, GLN434, SER430, THR431, and TYP440), whereas verapamil bound to the lowest number of amino acid residues within the same binding site via alkyl-alkyl interaction (VAL468) and Van der Waals forces (GLN437, SER470, and GLU472).
 | Table 1. Predicted properties of guttiferone K, oblongifolin C, and verapamil as P-gp substrate and P-gp inhibitor through SwissADME and admetSAR programs. [Click here to view] |
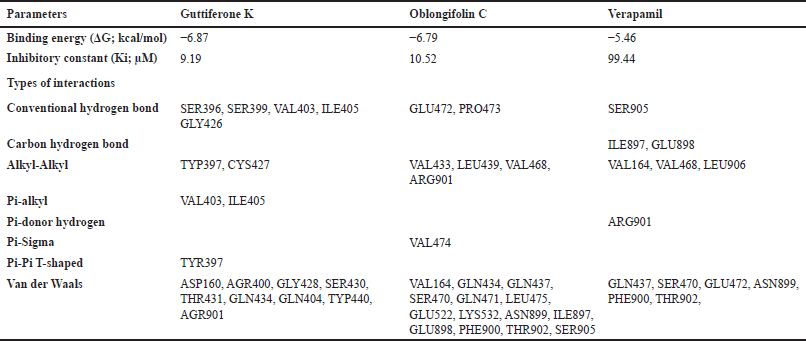 | Table 2. Molecular interactions of guttiferone K, oblongifolin C, and verapamil with the ATP-binding region on NBD1 of P-gp. [Click here to view] |
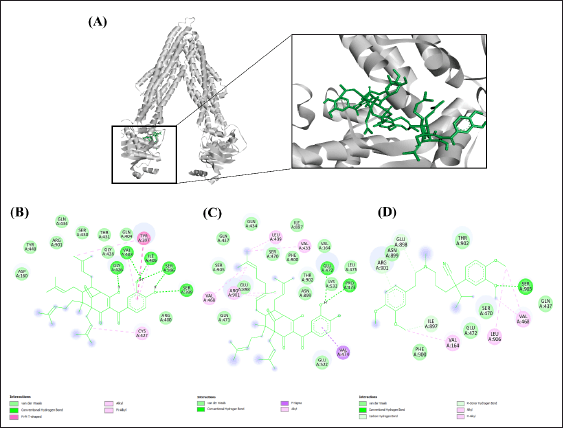 | Figure 3. The 3D interaction between NBD1 of (A) P-gp and compounds. (B) The 2D interaction among guttiferone K, (C) oblongifolin C, and (D) verapamil and ATP binding site at NBD1 of P-gp. [Click here to view] |
DISCUSSION
The polyprenylated benzophenones guttiferone K and oblongifolin C are major chemical constituents of the edible fruits of several Garcinia species, some of which are employed as traditional medicine [7,23,33]. They are also found in the bark and twigs of G. schomburgkiana which are utilized in traditional Thai medicine [21]. The plant extracts rich in each compound or both of them displayed anticancer activities in various cell and animal models [18,20–22]. These benzophenones showed high potential as chemotherapeutic agents in the future [7,24,34]. Nevertheless, their effects on cellular transport proteins such as P-gp need to be further investigated.
In this study, we observed inhibition of P-gp function by both guttiferone K and oblongifolin C. At the same concentration of 20 μM, oblongifolin C could inhibit P-gp more potently than guttiferone K by approximately three folds. Both benzophenones possess the same core structures, but with a difference in one of their isoprenylated substituents: while guttiferone K has four isoprenyl groups, one of these substituents in oblongifolin C is replaced by a geranyl group (Fig. 1). Hence, it is noticeably more hydrophobic than guttiferone K, which corresponds to their logarithm of n-octanol-water partition coefficient (log P) values of 8.2 and 7.1, respectively [24]. A similar result has been observed with geranylated chrysin (8-C-geranylchrysin), which showed a higher hydrophobicity index, log P value, and binding affinity than its isoprenylated analog [8-C-(3,3-dimethylallyl)-chrysin] [35]. Alkylation could increase the permeability of benzophenones through the cell membrane and their binding to the substrate binding site and NDBs of P-gp [36]. Therefore, more hydrophobic moiety such as a geranyl group would be an important factor to differentiate the permeability and binding affinity to P-gp of these two benzophenones.
P-gp function can be altered directly by its substrate or inhibitor. Predicting which compounds would be P-gp substrate or P-gp inhibitor is very difficult in in vitro and in vivo experiments [37,38]. The computational SwissADME and admetSAR programs are good tools to predict substrate, non-substrate, or inhibitors of P-gp based on their chemical structures. For drug discovery, these programs are also recommended for pre-screening pharmacokinetic parameters and correlating with in vitro and in vivo investigations [29,30]. In this study, both programs were utilized in order to determine the action of guttiferone K and oblongifolin C on P-gp, in comparison with verapamil, a well-known substrate and inhibitor of P-gp [39]. Our computational result indicated that, similar to verapamil, both polyprenylated benzophenones were substrates and inhibitors of P-gp, supporting previous results with benzophenone derivatives including norathyriol and benzophenone sulfonamide [36,40,41]. It is possible that these benzophenone substrates of P-gp could directly interfere with P-gp function and act as inhibitors.
Generally, P-gp function can be inhibited by several mechanisms such as blockage of the ATP binding site of NBDs in ATP hydrolysis [3,42,43]. In this study, the molecular docking experiment was used to suggest the mechanism which guttiferone K and oblogifolin C might act against NBD1 of P-gp at ATP binding. The formation of a complex structure between benzophenones and the ATP binding site of the NBD1 domain was more favorable than a verapamil-NBD1 complex, based on their observed ΔG and Ki constants. From the result of ligand-protein interaction, verapamil appeared to unfavorably bind with the ATP binding site within NBD1. Therefore, it likely interfered at the substrate binding site on the transmembrane (TMB) domains since it was a substrate of P-gp [3,43]. However, guttiferone K and oblongifolin C could readily interact with the ATP binding site within NBD1 of P-gp. These benzophenones might also interact with TMB domains because they were predicted to be P-gp substrates. Their effects on P-gp transport and ATPase activity should be further confirmed in in vitro study.
CONCLUSION
Two polyprenylated benzophenones, namely, guttiferone K and oblongifolin C, which are the chemical constituents of several Garcinia species (family Clusiaceae), were able to inhibit P-gp function in Caco-2 cells. Both compounds were predicted to be both P-gp substrates and inhibitors via SwissADME and admetSAR programs. Molecular docking analysis showed that they could bind to the ATP binding site of NBD1. Guttiferone K and oblongifolin C could thus cause herb-drug interactions when Garcinia plant extracts or fruits are co-administered or consumed with drugs that are P-gp substrates.
ACKNOWLEDGMENTS
The authors would like to thank the Pharmaceutical Research Instruments, Faculty of Pharmaceutical Sciences, Chulalongkorn University, for providing facilities.
AUTHOR CONTRIBUTIONS
Cherdsak Boonyong, Nonthalert Lernitikul, and Angkana Wongsakul: Concept and design, data acquisition, data analysis/interpretation, drafting manuscript, critical revision of manuscript, statistical analysis, supervision, and final approval. Rutt Suttisri: Material support, writing-review, and editing. Suree Jianmongkol: Material support and supervision. Chutichot Pattamadilok and Supotchanna Sitthigool: Material support. All data were generated in-house, and no paper mill was used. All authors agree to be accountable for all aspects of work ensuring integrity and accuracy.
FINANCIAL SUPPORT
There is no funding to report
CONFLICTS OF INTEREST
All authors declared that there is no conflict of interest.
ETHICAL APPROVAL
There are no animal or human subjects involved in this study.
DATA AVAILABILITY
This original manuscript and all data are available for only research purposes from principal investigators.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Juvale IIA, Hamid AAA, Halim KBA, Has ATC. P-glycoprotein: new insights into structure, physiological function, regulation and alterations in disease. Heliyon. 2022;8(6):e09777. doi: https://doi.org/10.1016/j.heliyon.2022.e09777
2. Heming CP, Muriithi W, Macharia LW, Filho PN, Moura-Neto V, Aran V. P-glycoprotein and cancer: what do we currently know?. Heliyon. 2022;8(10):e11171. doi: https://doi.org/10.1016/j.heliyon.2022.e11171
3. Karthika C, Sureshkumar R, Zehravi M, Akter R, Ali F, Ramproshad S, et al. Multidrug resistance of cancer cells and the vital role of P-glycoprotein. Life (Basel). 2022;12(6):1–20. doi: https://doi.org/10.3390/life12060897
4. Cao Y, Shi Y, Cai Y, Hong Z, Chai Y. The effects of traditional Chinese medicine on P-glycoprotein-mediated multidrug resistance and approaches for studying the herb-P-glycoprotein interactions. Drug Metab Dispos. 2020;48(10):972–9. doi: https://doi.org/10.1124/dmd.120.000050
5. Miyake T, Tsutsui H, Haraya K, Tachibana T, Morimoto K, Takehara S, et al. Quantitative prediction of P-glycoprotein-mediated drug-drug interactions and intestinal absorption using humanized mice. Br J Pharmacol. 2021;178(21):4335–51. doi: https://doi.org/10.1111/bph.15612
6. Patel KA, Bhatt MH, Hirani RV, Patel VA, Patel VN, Shah GB, et al. Assessment of potential drug-drug interactions among outpatients in a tertiary care hospital: focusing on the role of P-glycoprotein and CYP3A4 (retrospective observational study). Heliyon. 2022;8(11):e11278. doi: https://doi.org/10.1016/j.heliyon.2022.e11278
7. Li H, Meng XX, Zhang L, Zhang BJ, Liu XY, Fu WW, et al. Oblongifolin C and guttiferone K extracted from Garcinia yunnanensis fruit synergistically induce apoptosis in human colorectal cancer cells in vitro. Acta Pharmacol Sin. 2017;38(2):252–63. doi: https://doi.org/10.1038/aps.2016.101
8. Ye Q, Liu K, Shen Q, Li Q, Hao J, Han F, et al. Reversal of multidrug resistance in cancer by multi-functional flavonoids. Front Oncol. 2019;9:487. doi: https://doi.org/10.3389/fonc.2019.00487
9. Bai J, Zhao S, Fan X, Chen Y, Zou X, Hu M, et al. Inhibitory effects of flavonoids on P-glycoprotein in vitro and in vivo: food/herb-drug interactions and structure-activity relationships. Toxicol Appl Pharmacol. 2019;369:49–59. doi: https://doi.org/10.1016/j.taap.2019.02.010
10. Dewanjee S, Dua TK, Bhattacharjee N, Das A, Gangopadhyay M, Khanra R, et al. Natural products as alternative choices for P-glycoprotein (P-gp) inhibition. Molecules. 2017;22(6):1–93. doi: https://doi.org/10.3390/molecules22060871
11. Fan L, Tao GY, Wang G, Chen Y, Zhang W, He YJ, et al. Effects of Ginkgo biloba extract ingestion on the pharmacokinetics of talinolol in healthy Chinese volunteers. Ann Pharmacother. 2009;43(5):944–9. doi: https://doi.org/10.1345/aph.1L656
12. Silva V, Gil-Martins E, Silva B, Rocha-Pereira C, Sousa ME, Remião F, et al. Xanthones as P-glycoprotein modulators and their impact on drug bioavailability. Expert Opin Drug Metab Toxicol. 2021;17(4):441–8. doi: https://doi.org/10.1080/17425255.2021.1861247
13. Sukhaphirom N, Vardhanabhuti N, Chirdchupunseree H, Pramyothin P, Jianmongkol S. Phyllanthin and hypophyllanthin inhibit function of P-gp but not MRP2 in Caco-2 cells. J Pharm Pharmacol. 2013;65(2):292–9. doi: https://doi.org/10.1111/j.2042-7158.2012.01593.x
14. Wongrattanakamon P, Lee VS, Nimmanpipug P, Jiranusornkul S. Nucleotide-binding domain 1 modelling: a novel molecular docking approach for screening of P-glycoprotein inhibitory activity of bioflavonoids. CDC. 2016;2:10–6. doi: https://doi.org/10.1016/j.cdc.2016.06.001
15. Wongwanakul R, Vardhanabhuti N, Siripong P, Jianmongkol S. Effects of rhinacanthin-C on function and expression of drug efflux transporters in Caco-2 cells. Fitoterapia. 2013;89:80–5. doi: https://doi.org/10.1016/j.fitote.2013.05.019
16. Hennessy M, Kelleher D, Spiers JP, Barry M, Kavanagh P, Back D, et al. St John’s Wort increases expression of P-glycoprotein: implications for drug interactions. Br J Clin Pharmacol. 2002;53(1):75–82. doi: https://doi.org/10.1046/j.0306-5251.2001.01516.x
17. Okura T, Ibe M, Umegaki K, Shinozuka K, Yamada S. Effects of dietary ingredients on function and expression of P-glycoprotein in human intestinal epithelial cells. Biol Pharm Bull. 2010;33(2):255–9. doi: https://doi.org/10.1248/bpb.33.255
18. Mungmee C, Sitthigool S, Buakeaw A, Suttisri R. A new biphenyl and other constituents from the wood of Garcinia schomburgkiana. Nat Prod Res. 2013;27(21):1949–55. doi: https://doi.org/10.1080/14786419.2013.796469
19. Espirito Santo BLSD, Santana LF, Kato Junior WH, de Araújo FO, Bogo D, Freitas KC, et al. Medicinal potential of Garcinia species and their compounds. Molecules. 2020;5(19):1–30. doi: https://doi.org/10.3390/molecules25194513
20. Kaennakam S, Mudsing K, Rassamee K, Siripong P, Tip-Pyang S. Two new xanthones and cytotoxicity from the bark of Garcinia schomburgkiana. J Nat Med. 2019;73:257–61. doi: https://doi.org/ 10.1007/s11418-018-1240-8
21. Kaennakam S, Sukandar ER, Rassamee K, Siripong P, Tip-pyang S. Cytotoxic polyprenylated benzoylphloroglucinol derivatives from the branches of Garcinia schomburgkiana. Planta Med. 2023;89(5):508–15. doi: https://doi.org/10.1055/a-1841-0745
22. Kaennakam S, Sukandar ER, Juntagoot T, Siripong P, Tip-Pyang S. Four new xanthones and their cytotoxicity from the stems of Garcinia schomburgkiana. J Nat Med. 2021;75(4):871–6. doi: https://doi.org/10.1007/s11418-021-01527-9
23. Masullo M, Bassarello C, Suzuki H, Pizza C, Piacente S. Polyisoprenylated benzophenones and an unusual polyisoprenylated tetracyclic xanthone from the fruits of Garcinia cambogia. J Agric Food Chem. 2008;56(13):5205–10. doi: https://doi.org/ 10.1021/jf800416j
24. Bailly C, Vergoten G. Anticancer properties and mechanism of action of oblongifolin C, guttiferone K and related polyprenylated acylphloroglucinols. Nat Prod Bioprospect. 2021;11(6):629–41. doi: https://doi.org/10.1007/s13659-021-00320-1
25. Kan WL, Yin C, Xu HX, Xu G, To KK, Cho CH, et al. Antitumor effects of novel compound, guttiferone K, on colon cancer by p21Waf1/Cip1-mediated G(0)/G(1) cell cycle arrest and apoptosis. Int J Cancer. 2013;132(3):707–16. doi: https://doi.org/ 10.1002/ijc.27694
26. Li Y, Xi Z, Chen X, Cai S, Liang C, Wang Z, et al. Natural compound oblongifolin C confers gemcitabine resistance in pancreatic cancer by downregulating Src/MAPK/ERK pathways. Cell Death Dis. 2018;9:538. doi: https://doi.org/10.1038/s41419-018-0574-1
27. Boonyong C, Pattamadilok C, Suttisri R, Jianmongkol S. Benzophenones and xanthone derivatives from Garcinia schomburgkiana induced P-glycoprotein overexpression in human colorectal Caco-2 cells via oxidative stress-mediated mechanisms. Phytomedicine. 2017;27:8–14. doi: https://doi.org/10.1016/j.phymed.2017.01.011
28. Lertnitikul N, Suttisri R, Sitthigool S, Pattamadilok C, Piewpong K, Khanboon A, et al. Antioxidant, antimicrobial and cytotoxic activities of amides and aristolactams from Piper wallichii (Miq.) Hand.-Mazz. Stems. J Biol Act Prod Nat. 2023;13:1–11. doi: https://doi.org/10.1080/22311866.2023.2211043
29. Daina A, Michielin O, Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep. 2017;7:1–13. doi: https://doi.org/10.1038/srep42717
30. Yang H, Lou C, Sun L, Li J, Cai Y, Wang Z, et al. admetSAR 2.0: web-service for prediction and optimization of chemical ADMET properties. Bioinformatics. 2019;35(6):1067–9. doi: https://doi.org/10.1093/bioinformatics/bty707
31. Jarc T, Novak M, Hevir N, Rižner TL, Kreft ME, Kristan K. Demonstrating suitability of the Caco-2 cell model for BCS-based biowaiver according to the recent FDA and ICH harmonised guidelines. J Pharm Pharmacol. 2019;71(8):1231–42. doi: https://doi.org/10.1111/jphp.13111
32. Szewczyk P, Tao H, McGrath AP, Villaluz M, Rees SD, Lee SC, et al. Snapshots of ligand entry, malleable binding and induced helical movement in P-glycoprotein. Acta Crystallogr D Biol Crystallogr. 2015;71(Pt 3):732–41. doi: https://doi.org/10.1107/S1399004715000978
33. Yang H, Figueroa M, To S, Baggett S, Jiang B, Basile MJ, et al. Benzophenones and biflavonoids from Garcinia livingstonei fruits. J Agric Food Chem. 2010;58(8):4749–55. doi: https://doi.org/10.1021/jf9046094
34. Zheng Y, Zhang W, Xu L, Zhou H, Yuan M, Xu H. Recent progress in understanding the action of natural compounds at novel therapeutic drug targets for the treatment of liver cancer. Front Oncol. 2022;11:795548. doi: https://doi.org/10.3389/fonc.2021.795548
35. Comte G, Daskiewicz JB, Bayet C, Conseil G, Viornery-Vanier A, Dumontet C, et al. C-Isoprenylation of flavonoids enhances binding affinity toward P-glycoprotein and modulation of cancer cell chemoresistance. J Med Chem. 2001;44(5):763–8. doi: https://doi.org/10.1021/jm991128y
36. Jabeen I, Pleban K, Rinner U, Chiba P, Ecker GF. Structure-activity relationships, ligand efficiency, and lipophilic efficiency profiles of benzophenone-type inhibitors of the multidrug transporter P-glycoprotein. J Med Chem. 2012;55(7):3261–73. doi: https://doi.org/10.1021/jm201705f
37. Chen C, Lee MH, Weng CF, Leong MK. Theoretical prediction of the complex P-glycoprotein substrate efflux based on the novel hierarchical support vector regression scheme. Molecules. 2018;23(7):1–25. doi: https://doi.org/10.3390/molecules23071820
38. Ohashi R, Watanabe R, Esaki T, Taniguchi T, Torimoto-Katori N, Watanabe T, et al. Development of simplified in vitro P-glycoprotein substrate assay and in silico prediction models to evaluate transport potential of P-glycoprotein. Mol Pharm. 2019;16(5):1851–63. doi: https://doi.org/10.1021/acs.molpharmaceut.8b01143
39. Zhu T, Howieson C, Wojtkowski T, Garg JP, Han D, Fisniku O, et al. The effect of verapamil, a P-glycoprotein inhibitor, on the pharmacokinetics of peficitinib, an orally administered, once-daily JAK inhibitor. Clin Pharmacol Drug Dev. 2017;6(6):548–55. doi: https://doi.org/ 10.1002/cpdd.344
40. Chieli E, Romiti N, Rodeiro I, Garrido G. In vitro effects of Mangifera indica and polyphenols derived on ABCB1/P-glycoprotein activity. Food Chem Toxicol. 2009;47(11):2703–10. doi: https://doi.org/10.1016/j.fct.2009.07.017
41. Farman S, Javed A, Arshia Khan KM, Nasir A, Khan AU, Lodhi MA, et al. Benzophenone sulfonamide derivatives as interacting partners and inhibitors of human P-glycoprotein. Anticancer Agents Med Chem. 2020;20(14):1739–51. doi: https://doi.org/10.2174/1871520620666200516144403
42. Lai JI, Tseng YJ, Chen MH, Huang CF, Chang PM. Clinical perspective of FDA approved drugs with P-glycoprotein inhibition activities for potential cancer therapeutics. Front Oncol. 2020;10:561936. doi: https://doi.org/10.3389/fonc.2020.561936
43. Leopoldo M, Nardulli P, Contino M, Leonetti F, Luurtsema G, Colabufo NA. An updated patent review on P-glycoprotein inhibitors (2011-2018). Expert Opin Ther Pat. 2019;29(6):455–61. doi: https://doi.org/10.1080/13543776.2019.1618273