INTRODUCTION
Diabetes mellitus type 2 is the most common and chronic metabolic disease in the world, and the most significant secondary complication associated with diabetes is diabetic foot ulcer. It is a serious clinical condition caused mostly by diabetic neuropathy and/or peripheral vascular dysfunction [1–3]. In a diabetic individual’s lifetime, the chance of acquiring a chronic foot wound is estimated to be 15%–25% cases [4]. Despite new therapies and research, foot ulcers continue to be the main reason for lower limb amputation, a reduction in quality of life, and an elevated risk of death [5]. According to numerous studies, the increased inflammation that results in the accumulation of pro-inflammatory cytokines and the failure to activate anti-inflammatory pathways is the primary factor contributing to the development of chronic diabetic foot ulcers [6].
One of the key contributors to inflammatory homeostasis is Annexin A1 (ANXA1), a highly abundant 37 kDa glucocorticoid-regulated protein member of the annexin superfamily [7,8]. ANXA1 has been known to exert an anti-inflammatory effect by inhibiting the activity of phospholipase A2 (PLA2), a key enzyme responsible for inflammation and cytotoxicity [9]. ANXA1 decreases neutrophil recruitment and the synthesis of pro-inflammatory mediators by encouraging neutrophil apoptosis, controlling monocyte recruitment, and enhancing macrophage clearance of apoptotic cells [10]. Recent research reveals that ANXA1 is involved in the reprogramming of macrophages to reduce inflammation and restore tissue homeostasis. ANXA1 is also associated with inflammation that is pro-resolving, which is linked to a number of cellular and molecular processes in the inflammatory response and is connected to the endogenous mechanisms that are triggered to bring about inflammation resolution [9,11]. In the peripheral blood cells, ANXA1 is highly expressed in subcellular granules of neutrophils, eosinophils, and monocytes, with small amounts expressed in subsets of T lymphocytes, mast, and B cells [12]. The low levels of anti-inflammatory ANXA1 in various chronic inflammatory disorders, including rheumatoid arthritis, diabetes mellitus, coronary heart disease, Crohn’s disease, and others indicate the role in inflammation [13–16]. Even though there is a sufficient amount of circulatory ANXA1, several chronic inflammatory conditions fail to activate inflammation homeostasis. We speculate that it may be because there are insufficient amounts of ANXA1 in the wound microenvironment, which is preventing the body from producing its own anti-inflammatory activity and delaying wound healing. However, as far as we are aware, no research has linked serum and tissue ANXA1 levels to diabetic foot ulcers. Therefore, this study aimed to determine the levels of the anti-inflammatory ANXA1 and a few other inflammatory regulators, including IL1β, IL6, IL10, and TNFα in the serum and wound tissue of diabetic and non-diabetic subjects. In conclusion, this study’s calculation of endogenous tissue-protective anti-inflammatory molecules, such as ANXA1, in diabetic wounds may be useful in evaluating the effectiveness of treatment for diabetic foot ulcers.
MATERIALS AND METHODS
Study subjects
After receiving approval from the institutional ethics committee (No.: NU/CEC/2020/0335 dated 09-09-2020), this cross-sectional study enrolled 40 participants and divided into 20 diabetic foot ulcer subjects and 20 non-diabetic subjects of both sexes who visited the department of general surgery at Justice K S Hegde Charitable Hospital, Mangaluru, for wound care. The sample size for this single-center pilot study was calculated using the formula, where n is the sample size, Z1-α meaning confidence interval at 5% type 1 error is 1.96, SD of variable is 16, and the precision/absolute error (d) is 5% [17]. After obtaining the written informed consent, the diabetic subjects except those who were pregnant, lactating, having radiotherapy, chemotherapy, immunotherapy, taking any form of steroid treatment, or suffering from any other immunodeficiency or inflammatory illnesses such as rheumatoid arthritis, inflammatory bowel disease, etc., with foot wounds were recruited for the study. The non-diabetic subjects with wounds from trauma or burns were recruited; however, wounds with infection were eliminated for this study.
Clinico-investigative characteristics
A detailed history (family and personal) and physical examination details including age, sex, body mass index (BMI), duration of diabetes, blood pressure, and biochemical parameters such as glycated hemoglobin (HbA1c), and blood glucose findings data were collected from the study subject case sheet. All the biochemical parameters data were expressed as mean ± SD. For diabetic subjects, the wound size was determined by multiplying the longest and widest diameter of a wound and expressed in centimeter squares. Wagner’s classification system was used to grade the ulcers as grade I (superficial ulcer or subcutaneous tissue), grade II (ulcers that extended into tendon, bone, or capsule), grade III (deep ulcer with osteomyelitis or abscess), grade IV (gangrene of toes), and grade V (gangrene of entire foot).
Blood and debrided tissue collection
To determine the levels of ANXA1 and few other inflammatory regulators in the serum, 2 ml of venous blood was collected in a plain vacutainer from the recruited individuals. Then, the vacutainers containing blood were left at room temperature for 30 minutes to clot, centrifuged at 3,000 rpm for 10 minutes to separate serum, and stored in aliquots at −80°C until further investigation. Furthermore, debrided tissue was collected, washed, and prepared the tissue homogenate in phosphate-buffered saline, centrifuged, and the final supernatant was stored at −80°C until analysis.
Estimation of ANXA1 and other inflammatory molecules in serum and tissue
The levels of ANXA1 (#EH0855, Wuhan fine Biotech Co. Ltd, China) and other inflammatory regulators IL1β (#850.006.096, Diaclone, France), IL6 (#950.030.096, Diaclone, France), TNFα (#950.090.096, Diaclone, France), and IL10 (#950.060.096, Diaclone, France) in both serum and tissue were determined by using human quantitative sandwich enzyme-linked immune-sorbent assay (ELISA) kits according to manufacturer’s protocols. The color change was detected at 450 nm using a multimode microplate reader (Tecan spark®, Bioscreen, Switzerland). The levels of all inflammatory molecules in each sample were calculated according to the standard curve method, and the results were reported as mean ± SD.
Statistical analysis
All data were analyzed using the Statistical Package for the Social Sciences, version 22.0, (IBM Corp. Armonk, NY). Continuous variables normally distributed in the groups were compared using Independent Student’s t-test. Using normally distributed variables, Pearson’s correlation coefficient analysis was used to identify the correlation of ANXA1 with the clinico-investigative profile and other inflammatory regulators in serum and tissue samples of study subjects. The area under the curve (AUC) for ANXA1 was calculated using receiver operating characteristic (ROC) curve analysis. Statistical significance was defined as a p-value < 0.001, <0.01, and <0.05.
RESULTS
Clinico-investigative profile of study subjects
The anthropometric and biochemical characteristics of study subjects diabetic and non-diabetics (n = 40) with foot ulcers are summarized in Table 1. There were no significant variations among the study subjects in terms of age, gender, height, weight, BMI, systolic and diastolic blood pressure. However, the blood glucose and glycated HbA1c levels were significantly different (p < 0.05) between diabetic and non-diabetic subjects with wounds (Table 1). Further, the wound grades among the diabetic subjects according to Wagner’s grading method found that 14 subjects had grade 0–III wounds, while 6 had grade IV–V wounds. The duration of diabetes in study subjects was divided into three categories, namely 5–10 years (n = 10), 10–20 years (n = 6), and >20 years (n = 4) (Table 1).
Levels of ANXA1 and other inflammatory molecules in diabetics and non-diabetics
The levels of ANXA1 were observed significantly different between serum (353.50 ± 39.17 pg/ml) and tissue homogenates (414.10 ± 70.39 pg/ml) of diabetic subjects, while similar results were observed in non-diabetic subjects (serum, 436.20 ± 140.76 pg/ml; tissue, 509.5 ± 33.93 pg/ml) (Table 2 and Fig. 1). However, the levels were considerably higher with little variations in tissue homogenate samples compared serum of study subjects. In addition, we found similar results for the other inflammatory molecules IL1β, IL6, and IL10, where tissue levels were considerably higher than the serum levels in both diabetic and non-diabetic study subjects. Furthermore, when compared to non-diabetics, the levels of ANXA1 were significantly lower in both serum and tissue homogenate samples from diabetic individuals. Similarly, other inflammatory molecules IL10, IL6, and TNFα levels were significantly different in both serum and tissue samples, except ILβ levels between diabetics and non-diabetic subjects (Table 2 and Fig. 1). Overall, the anti-inflammatory ANXA1 was found to be very much significantly different between diabetic and non-diabetic serum and tissue homogenate samples, indicating that monitoring its levels in the wound microenvironment would provide a better clinical estimation of foot ulcer healing during the course of treatment. Furthermore, the determination of changes in the ANXA1 levels might be useful to the treating physician for understanding the therapeutic effect of undergoing treatment in healing foot ulcers.
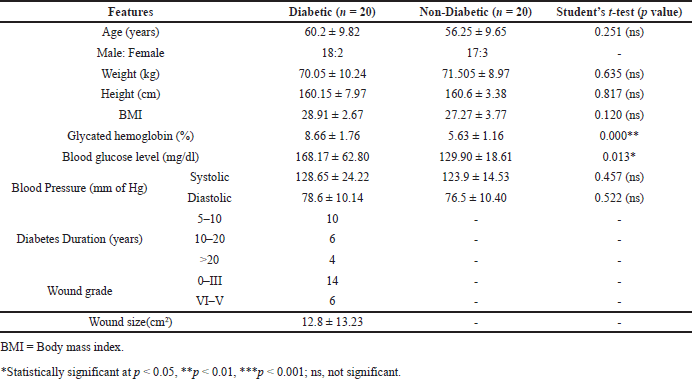 | Table 1. Clinical and biochemical characteristics of study subjects. [Click here to view] |
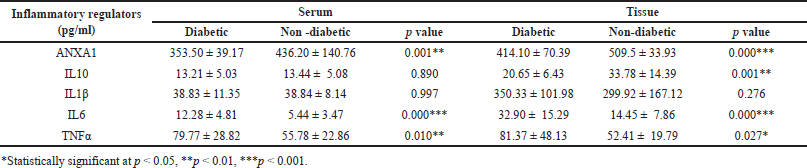 | Table 2. Comparison between the levels (mean ± SD) of pro- and anti-inflammatory regulators observed in diabetic and non-diabetic subjects. [Click here to view] |
Association of ANXA1 levels with other inflammatory molecules and clinico-investigative profile of study subjects
Pearson’s correlation analysis revealed that the PLA2 inhibitor ANXA1 levels in serum (r = 0.685, p = 0.000) and tissue (r = 0.331, p = 0.037) had a positive correlation with glycated hemoglobin of diabetic subjects with foot ulcers. In addition, a positive correlation was also observed for the serum ANXA1 levels with the blood glucose (r = 0.434, p = 0.05) and wound grade (r = 0.454, p = 0.044) of diabetic subjects (Table 3).
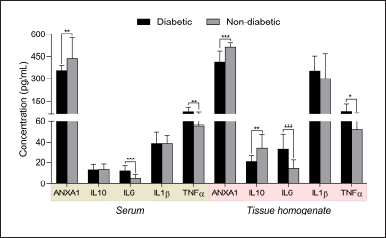 | Figure 1. The levels of ANXA1 and other inflammatory molecules IL6, IL1β, TNFα, and IL10 in serum and tissue homogenates of diabetic and non-diabetic subjects. [Click here to view] |
The relationship analysis with other inflammatory molecules found that a significant negative correlation was observed between anti-inflammatory ANXA1 levels in serum with the pro-inflammatory molecules like IL6 (r = −0.669, p = 0.002) and TNFα (r = −0.515, p = 0.020) in diabetic subjects. Meanwhile, ANXA1 levels in serum (r =−−0.559, p = 0.016) had a significant negative correlation with the other anti-inflammatory IL10 in non-diabetic subjects. (Table 4 and Fig. 2).
Efficacy of ANXA1 in the diagnosis of diabetic foot ulcer healing
The ROC curve analysis found that the area under the curve values of ANXA1 in serum (AUC = 0.973, p = 0.000) and tissue (AUC = 0.816, p = 0.001) showed a statistically significant (Fig. 3). Overall, these results indicate that determining anti-inflammatory ANXA1 in serum and tissue homogenates would be useful in understanding the wound healing process in diabetic subjects.
DISCUSSION
Diabetes mellitus is frequently associated with decreased wound healing because of diminished angiogenesis and the inability to effectively resolve inflammation. Because there is a dearth of clinical evidence demonstrating the connection between pro- and anti-inflammatory regulators for healing, diabetes patients with wounds are at an increased risk of postoperative problems. In this work, we looked at the levels of anti-inflammatory ANXA1 and other inflammatory molecules in debrided tissue and serum from diabetes and non-diabetic wound patients and compared the results to their clinico-investigative profiles. To the best of our knowledge, this is the first study to explore ANXA1 levels in debrided tissue samples from diabetic foot ulcer patients and compare them to serum concentrations using an ELISA technique. In this pilot study, there was a significant difference in blood glucose and HbA1C levels observed between diabetic and non-diabetic subjects, suggesting that the study subjects were classified appropriately. Our results indicate that both diabetic and non-diabetic subjects had higher levels of anti-inflammatory ANXA1 levels in tissue compared to the serum samples. The first class of endogenous anti-inflammatory mediators to be successfully used in ulcer therapy was glucocorticoids. Further, glucocorticoids are known to regulate ANXA1 production and activity by inhibiting the pro-inflammatory cytokines IL6 and TNFα [18]. Glucocorticoids can generate a PLA2 inhibitory protein ANXA1 in inflammatory cells and exert many anti-inflammatory properties in addition to arachidonic acid metabolism. Recent research suggests that ANXA1 may selectively target cytosolic PLA2 by inhibiting the enzyme directly and suppressing the enzyme’s cytokine-induced activation.
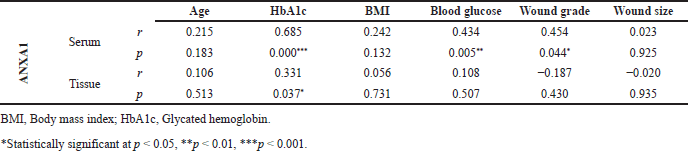 | Table 3. Association of ANXA1 levels in serum and tissue homogenate samples with clinico-investigative characteristics of diabetic subjects with foot ulcers. [Click here to view] |
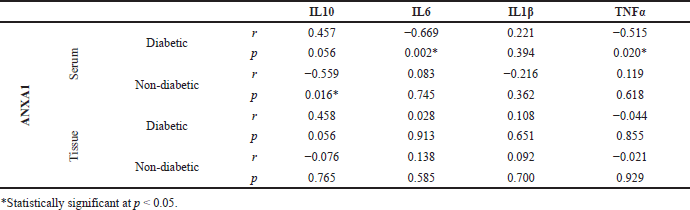 | Table 4. Association of ANXA1 levels in serum and tissue homogenate samples with other inflammatory regulators of both diabetic and non-diabetic subjects. [Click here to view] |
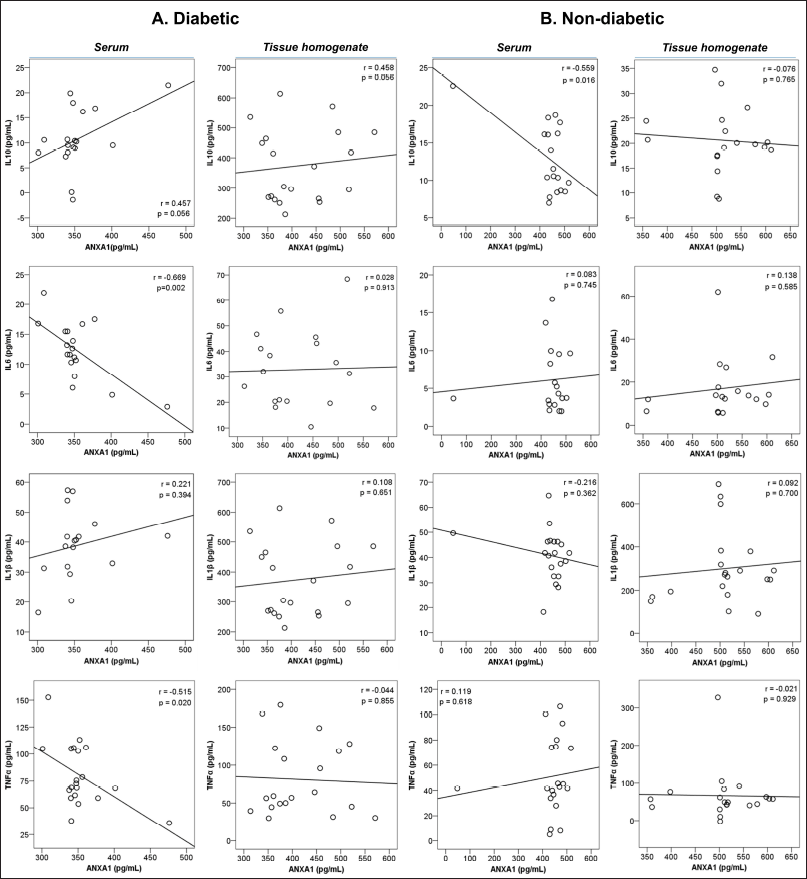 | Figure 2. Pearson’s correlation analysis of ANXA1 serum and tissue homogenate levels with inflammatory regulators of diabetic subjects. [Click here to view] |
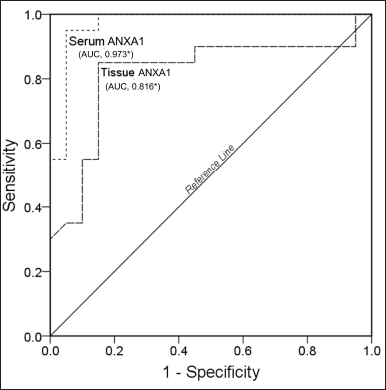 | Figure 3. ROC graph of ANXA1 in serum and tissue homogenate samples of study subject [Click here to view] |
Further, in our study, we have found that the levels of pro-inflammatory regulators IL6, TNFα, and IL1β in tissue were comparatively higher in diabetic subjects compared to the non-diabetic subjects. Similarly, several studies have observed that IL6 levels in diabetics are much higher than in healthy subjects [19,20]. In addition, TNFα is known to operate as a local intensification signaling molecule in the pathogenic processes associated with chronic inflammation, where TNFα levels were considerably greater in serum and tissue homogenates of study subjects [21,22]. In another study, researchers discovered that IL1β levels were higher in diabetic foot ulcers, but that they reduced as the ulcers healed. The anti-inflammatory IL10 is secreted by T helper cells, regulatory T cells, macrophages, and dendritic cells where it will inhibit the release of pro-inflammatory mediators, suppresses the antigen presentation and enhances the phagocytosis [23]. Human studies have observed that the diabetic foot ulcers have decreased expression of IL10, particularly in keratinocytes and endothelial cells at the wound margin [24]. The elevation of IL10 release generated by ANXA1 in macrophages could be causing the suppression of iNOS expression. In addition, the glucocorticoids like ANXA1 have a strong inhibitory effect on neutrophil and monocyte migration during inflammation. The N-terminal Ac2-26 peptide of ANXA1 stimulates macrophage phagocytosis of apoptotic neutrophils under hyperglycemic conditions through the fibroblast migration [25]. Inflammation, angiogenesis, and mast cell infiltration are all linked to high ANXA1 expression in a variety of cells from the breast tumor microenvironment, including fibroblasts, as well as angiogenesis, or the formation of new blood vessels [26]. ANXA1 is known to stimulate angiogenesis, which is affected in diabetic patients, leading to impaired wound healing. There are several intrinsic (neuropathy, vascular issues) and extrinsic factors (wound infection, calculus formation, and excessive pressure to the region) involved in the delayed healing of wounds in diabetics. Our study discovered that anti-inflammatory ANXA1 levels were extremely low in diabetic foot ulcer patients, suggesting that there may have been a breakdown in the body’s defense against diabetes complications. As a result, elevated levels of ANXA1 may aid in wound healing. The small sample size resulting from the rigorous inclusion criteria is one of the study’s drawbacks, and adding more participants would have reduced the statistical power of our conclusions. The numerous inflammatory regulator levels found in the wound microenvironment have, as of this point, produced good significant changes when compared to the circulatory serum levels in diabetic foot ulcers, but similar research studies are necessary to corroborate this concept. We believe that anti-inflammatory ANXA1 exhibited substantial outcomes among all the examined inflammatory molecules and could be used as a foretelling biomarker in the healing of diabetic foot ulcers.
CONCLUSION
Overall, the findings of this study suggest that measuring the levels of PLA2 inhibitor ANXA1 could be beneficial as a predictive biomarker in predicting diabetic foot ulcer healing and also helps in determining the current state of foot ulcer pathophysiology as well as the effectiveness of treatment. Furthermore, the ANXA1 would be established as an early predictor based on its serum or tissue levels, and also a prognosticator as well as a new therapeutic target for diabetic foot ulcer management. Additionally, the molecular mechanisms-oriented studies are warranted to understand the role of ANXA1 in resolving the inflammation underlying the defective healing of diabetic foot ulcers.
ACKNOWLEDGMENTS
The authors are thankful to the Staff and Faculty of Central Research Laboratory, K S Hegde Medical Academy, and the Registrar, Nitte (Deemed to be University), Mangalore, India, for providing all the support and facilities to complete this work. The authors express heartfelt thanks to Dr Suchetha Kumari N and Dr. Sachidananda Adiga for extending their guidance and support to complete this work.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICT OF INTERESTS
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The peripheral blood and wound tissue were collected from diabetic and non-diabetic subjects. For which, the Institutional Ethics Committee (No.: NU/CEC/2020/0335 dated 09-09-2020) approval was obtained.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation
REFERENCES
1. Guarnotta V, Radellini S, Vigneri E, Cernigliaro A, Pantò F, Scondotto S, et al. Diabetic foot ulcers: retrospective comparative analysis from Sicily between two eras. PLoS One. 2021;16:e0259405. doi: https://doi.org/10.1371/JOURNAL.PONE.0259405
2. Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Kaabi J Al. Epidemiology of type 2 diabetes—global burden of disease and forecasted trends. J Epidemiol Glob Health. 2020;10:107. doi: https://doi.org/10.2991/JEGH.K.191028.001
3. Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. doi: https://doi.org/10.1016/j.diabres.2019.107843
4. Andrews KL, Houdek MT, Kiemele LJ. Wound management of chronic diabetic foot ulcers: from the basics to regenerative medicine.Prosthet Orthot Int. 2015;39:29–39. doi: https://doi.org/10.1177/0309364614534296
5. Nazarko L. Preventing foot ulcers. Nurs Resid Care. 2013;15:187. doi: https://doi.org/10.12968/nrec.2013.15.4.187
6. Doupis J, Lyons TE, Wu S, Gnardellis C, Dinh T, Veves A. Microvascular reactivity and inflammatory cytokines in painful and painless peripheral diabetic neuropathy. J Clin Endocrinol Metab. 2009;94:2157–63. doi: https://doi.org/10.1210/jc.2008-2385
7. Ferraro B, Leoni G, Hinkel R, Ormanns S, Paulin N, Ortega-Gomez A, et al. Pro-angiogenic macrophage phenotype to promote myocardial repair. J Am Coll Cardiol. 2019;73:2990–3002. doi: https://doi.org/10.1016/j.jacc.2019.03.503
8. Lim LHK, Pervaiz S. Annexin 1: the new face of an old molecule. FASEB J. 2007;21:968–75. doi: https://doi.org/10.1096/FJ.06-7464REV
9. Sugimoto MA, Vago JP, Teixeira MM, Sousa LP. Annexin A1 and the resolution of inflammation: modulation of neutrophil recruitment, apoptosis, and clearance. J Immunol Res. 2016;2016. doi: https://doi.org/10.1155/2016/8239258
10. D’Acquisto F, Perretti M, Flower RJ. Annexin-A1: a pivotal regulator of the innate andadaptive immune systems. Br J Pharmacol. 2008;155:152. doi: https://doi.org/10.1038/BJP.2008.252
11. Perretti M, D’Acquisto F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat Rev Immunol. 2009;91:62–70. doi: https://doi.org/10.1038/nri2470
12. Gastardelo TS, Damazo AS, Dalli J, Flower RJ, Perretti M, Oliani SM. Functional and ultrastructural analysis of annexin A1 and its receptor in extravasating neutrophils during acute inflammation. Am J Pathol. 2009;174:177–83. doi: https://doi.org/10.2353/AJPATH.2009.080342
13. Haridas V, Shetty P, Sarathkumar E, Bargale A, Vishwanatha JK, Patil V, et al. Reciprocal regulation of pro-inflammatory annexin A2 and anti-inflammatory annexin A1 in the pathogenesis of rheumatoid arthritis. Mol Biol Rep. 2019;46:83–95. doi: https://doi.org/10.1007/s11033-018-4448-5
14. Pietrani NT, Ferreira CN, Rodrigues KF, Perucci LO, Carneiro FS, Bosco AA, et al. Proresolving protein annexin A1: the role in type 2 diabetes mellitus and obesity. Biomed Pharmacother. 2018;103:482–9. doi: https://doi.org/10.1016/j.biopha.2018.04.024
15. Särndahl E, Bergström I, Nijm J, Forslund T, Perretti M, Jonasson L. Enhanced neutrophil expression of annexin-1 in coronary artery disease. Metabolism. 2010;59:433–0. doi: https://doi.org/10.1016/J.METABOL.2009.07.044
16. Sena A, Grishina I, Thai A, Goulart Larissa, Macal M, Fenton A, et al. Dysregulation of anti-inflammatory annexin A1 expression in progressive crohns disease. PLoS One. 2013;8:e76969. doi: https://doi.org/10.1371/journal.pone.0076969
17. Charan J, Biswas T. How to calculate sample size for different study designs in medical research? Indian J Psychol Med. 2013;35:121–6. doi: https://doi.org/10.4103/0253-7176.116232
18. Yang YH, Aeberli D, Dacumos A, Xue JR, Morand EF. Annexin-1 regulates macrophage IL-6 and TNF via glucocorticoid-induced leucine zipper. J Immunol. 2009;183:1435–45. doi: https://doi.org/10.4049/jimmunol.0804000
19. Tuttolomondo A, La Placa S, Di Raimondo D, Bellia C, Caruso A, Lo Sasso B, et al. Adiponectin, resistin and IL-6 plasma levels in subjects with diabetic foot and possible correlations with clinical variables and cardiovascular co-morbidity. Cardiovasc Diabetol. 2010;9:50. doi: https://doi.org/10.1186/1475-2840-9-50
20. Kartika RW, Alwi I, Suyatna FD, Yunir E, Waspadji S, Immanuel S, et al. The role of vegf, pdgf And Il-6 On diabetic foot ulcer after platelet rich fibrin Þ hyaluronic therapy. Heliyon. 2021;7:e07934. doi: https://doi.org/10.1016/j.heliyon.2021.e07934
21. Ahmad J, Zubair M, Malik A. Plasma adiponectin, IL-6, hsCRP, and TNF-α levels in subject with diabetic foot and their correlation with clinical variables in a North Indian tertiary care hospital. Indian J Endocrinol Metab. 2012;16:769. doi: https://doi.org/10.4103/2230-8210.100672
22. Dhamodharan U, Viswanathan V, Krishnamoorthy E, Rajaram R, Aravindhan V. Genetic association of IL-6, TNF-α and SDF-1 polymorphisms with serum cytokine levels in diabetic foot ulcer. Gene. 2015;565:62–7. doi: https://doi.org/10.1016/j.gene.2015.03.063
23. Nanda R, Patel S, Ghosh A, Asha KS, Mohapatra E. A study of Apolipoprotein A1(ApoA1) and interleukin-10(IL-10) in diabetes with foot ulcers. BioMedicine. 2022;12:30–8. doi: https://doi.org/10.37796/2211-8039.1279
24. Van Exel E, Gussekloo J, De Craen AJM, Frölich M, Van Der Wiel AB, Westendorp RGJ. Low production capacity of interleukin-10 associates with the metabolic syndrome and type 2 diabetes: the Leiden 85-plus study. Diabetes. 2002;51:1088–92. doi: https://doi.org/10.2337/diabetes.51.4.1088
25. Bizzarro V, Fontanella B, Carratù A, Belvedere R, Marfella R, Parente L, et al. Annexin A1 N-terminal derived peptide Ac2-26 stimulates fibroblast migration in high glucose conditions. PLoS One. 2012;7:e45639. doi: https://doi.org/10.1371/JOURNAL.PONE.0045639
26. Yi M, Schnitzer JE. Impaired tumor growth, metastasis, angiogenesis and wound healing in annexin A1-null mice. Proc Natl Acad Sci USA. 2009;106:17886. doi: https://doi.org/10.1073/PNAS.0901324106