INTRODUCTION
Indonesia has a lot of medicinal plants and could be a source of natural drugs. Melinjo (Gnetum gnemon L.) is a plant used to treat many diseases. Several studies have reported extensions from the melinjo seed shell extract (MSE) to have various therapeutic effects, including an anti-obesity [1], anti-tumor [2], antihypertensive [3], antioxidants [4], and melanin biosynthesis [36]. Resveratrol (RES), the primary bioactive component of melinjo, has multiple purposes [6].
Previous research demonstrated that MSE has an anti-hyperuricemic effect compared to a placebo. Other studies also described a similar trend, i.e., ethanol extracts of melinjo (G. gnemon L.) leave and seed shells decrease uric acid (UA) blood levels in a model of hyperuricemic rats [7]. MSE decreased serum UA by increasing UA excretion in the ileum via increased expression of the ABCG2 protein. ABCG2, a urate transporter in the ileal crypt of the intestine, is essential for UA excretion outside of the kidneys [8].
Currently, the global prevalence of hyperuricemia (HU) cases remains high. Research by McAdams-DeMarco et al. [9] showed the cumulative incidence was 4% (5% in men and 3% in women) from 8,342 people studied for 9 years. In Indonesia, there was no actual prevalence of HU. In North Kayong Regency, West Kalimantan, Indonesia, it was shown that 36.36% of the individuals, or 44 out of 121, had HU [10]. HU is a disease in which serum UA exceeds the normal range due to abnormal purine metabolism for various reasons. HU drugs are currently available, such as allopurinol. Although allopurinol is a well-tolerated medication, it has many side effects, such as mild gastric upset hepatitis, nephropathy, allergies, and agranulocytosis [11]. Apart from medication, HU can also be managed by adjusting the diet. It has been reported that side-chain amino acids, including leucine, play a role in reducing oxidative stress, a potential risk factor for HU, by improving mitochondrial function. Increased leucine consumption will reduce the risk of HU by activating antioxidant mechanisms and reducing reactive oxygen species production in the liver. The reduced risk of HU due to dietary branched-chain amino acids is mediated by total cholesterol, HbA1c, fasting blood glucose, and triglyceride levels [12].
Considering those problems, exploration for better and safer antihyperuricemia drugs is encouraged. According to our previous report, MSE promises to be an alternative natural source in the future. RES is the primary active substance present in MSE [13,14]. RES is a bioactive compound in many foods. RES has antioxidant, anti-inflammatory, antihyperuricemic, immunomodulatory, glucose and lipid regulatory, neuroprotective, and cardiovascular protective effects. Therefore, RES has been regarded as a potent candidate for developing nutraceuticals and pharmaceuticals to prevent and treat certain chronic diseases [15].
Suitable formulations are also an important issue in increasing efficacy and acceptability. Numerous studies have shown that RES is efficiently ingested but rapidly eliminated, primarily as sulfate and glucuronides in the small intestinal lumen and liver [16]. RES has a half-life of 1–3 hours after single doses and 2–5 hours after repeated doses [17]. In another investigation, the plasma half-life of RES in rats was determined to be 4.4 ± 0.12 hours.
Many novel drug delivery systems have been found to boost resveratrol’s duration of therapeutic action, plasma half-life, bioavailability, and stability. The inclusion complexes were made by combining RES with -cyclodextrin and hydroxyl—cyclodextrin. In vitro testing improved the solubility and antioxidant activity [18]. Incorporating RES into crosslinked chitosan microspheres by vanillin resulted in controlled release and stabilization of RES. RES encapsulation efficiency within microspheres reached up to 93.68% [19]. A modified heat homogenization process was used to create solid lipid nanoparticles of RES. The in vitro studies given in this research reveal that this weakly soluble chemical has increased oral bioavailability [20]. Saturated phosphatidyl-choline and cholesterol liposome compositions improve solubility and bioavailability [21]. However, they have benefits and drawbacks, such as instability, quick clearance, immunotoxicity, toxicity, high cost, and cytotoxicity [22].
RES is a Biopharmaceutical Classification System (BCS) class II substance with poor solubility (3 mg/100 ml) and high permeability (log P 3.1) [23]. Its in vivo bioavailability is limited by its poor aqueous solubility. As a result, it is an excellent candidate for the controlled-release dosage form. The physicochemical properties of RES, such as pKa 8.99 and the molecular relative 228.24, meet the requirements for formulation into controlled-release oral preparations, apart from the short half-life. Therefore, this study reports the formulation approach used to prolong the biological half-life of RES through a controlled release particulate system using hydrophilic and hydrophobic polymers. hydrophilic matrix (HPMC) is the first choice for formulating HPMC systems because it has a robust mechanism and a wide range of viscosity grades. It is nonionic, has consistently reproducible release profiles, is cost-effective, and can be used with conventional equipment and procedures [24]. Eudragit RSPO was selected because of its more hydrophobic nature, which can be attributed to its structure, which has fewer quaternary ammonium groups [25].
This study intended to improve the bioavailability of the natural antihyperuricemia MSE in the blood to boost its efficacy. This investigation also aimed to develop a pilot and production-scale technology for an unexplored new MSE technology.
MATERIALS AND METHODS
Materials
Melinjo shell extract from P.T. Phytochemindo Bogor Indonesia, HPMC K100M (Bratachem Bandung Indonesia), Eudragit RSPO (Evonik Rohm Pharma. Polymers), Avicel PH 101 (Bratachem Bandung Indonesia), PVP K30 (Bratachem Bandung Indonesia), RES (Sigma- Aldrich, USA), and oxonic acid potassium salt (Sigma-Aldrich, USA). The HPLC reagents and other chemical components were acquired from Merck (Germany).
Methods
Preparation of controlled release particulate system of MSE
Controlled release particulate systems of MSE were prepared by wet granulation techniques using various percentages of HPMC K100M (hydrophilic polymer) and Eudragit RSPO (hydrophobic polymer) as release-controlling polymers. The composition of the formulations is listed in Table 1. All ingredients were weighed as per the quantities defined in Table 1. The required amount of the extract, various polymers, and other components were mixed thoroughly, and a sufficient granulating agent (1% of PVP Solution in ethanol 70%) was added gradually. After sufficient cohesiveness mass was obtained, the wet mass was sieved through sieve 14 mesh. The granules were dried at 60°C for 2 hours, and then the dried granules were passed through a 16 mesh sieve.
Physical evaluation of controlled release particulate system of MSE
Flow rate and angle of repose
The angle of repose is calculated by the time it takes for a certain amount of particulate matter to fall freely through the funnel.
The cone’s height (h) and radius (r) are measured. The angle of repose equation is as follows:
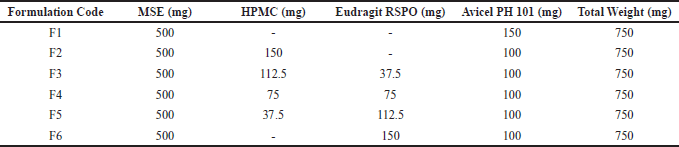 | Table 1. Formulation of melinjo seed shell extract granule containing HPMC:Eudragit RSPO. [Click here to view] |
Bulk density
The particulate system was put into a graduated cylinder, and the volume (V) and weight (W) were measured to obtain the apparent bulk density (ρb) of the powder mixture (M).
Tapped density
Tapped density was estimated by putting the correctly weighed grains into the graduated cylinder and measuring (volume) V. Then bulk density device tapped the graduated cylinder to maintain a constant capacity. The following equation determined tapped density:
Carr’s index
Carr’s index can be calculated using a material’s poured density and tapped density values. Theoretically, the less compressible a substance is, the greater its flowability. It can be calculated by substituting the values for poured density (ρb) and tapped density (ρt) into the following equation:
Hausner’s ratio
Hausner’s ratio is an indicator of particulate system flow and was determined by comparing the density of the tapped sample to the aggregate density.
In vitro study
Drugs release profile studies
The in vitro release profile studies were done with a USP Apparatus 3 (revolving cylinder) dissolution tester at 10 dpm in 250 ml of 0.1 N HCl at 37 0.5°C for 24 hours. The samples were taken at certain times (0.25; 1; 2; 3; 5; 8; 24 hours). At certain times, 5 ml of the dissolution medium was removed and replaced with 5 ml of fresh 0.1N HCl solution in each glass vessel to compensate for the volume loss.
A preliminary test release of the active substance
Preliminary tests were performed to select the most appropriate formula. This initial test was carried out on a particulate system formula that had been made previously by determining the total dissolved phenol content using the Folin-Ciocalteu method [26]. The reaction mixture in this method consisted of 0.5 ml of sample, 5.0 ml of distilled water, and 0.5 ml of the Folin-Ciocalteu reagent. 1.0 ml of a saturated sodium carbonate solution was added after 3 minutes. These combinations were shaken and set aside for an hour. PG Instruments Ltd Type T92+ Uv-Vis Spectrophotometer was used to measure total dissolved phenol at 760 nm.
HPLC analysis of RES
The selected formula was tested for re-dissolution. The release of the active substance from the selected formula was tested using RES as a substance marker. The RES content in the controlled release particulate system was calculated by HPLC analysis of the sample media. HPLC analysis was conducted using an HPLC Knauer German. A reserve phase column C-18 (4.6 mm by 250 mm by 5 m, Reliant: Ireland). Here are the conditions for the analysis: The flow rate was 1 ml/minute, the detection wavelength was set to 280 nm, and the injection volume was 100 μl. The mobile phase was a 45:55 mix of acetonitrile and water.
In vivo study
Animal model
The animal care committee of the Department of Pharmacy at Institut Teknologi Bandung (ITB) ensured that all protocols for in-vivo studies and taking care of lab animals were followed to the letter. The Universitas Airlangga Faculty of Dental Medicine Health Research Ethical Clearance Commission gave the in vivo experiment the green light: Certificate of Ethical Clearance No. 493/HRECC/FODM/VIII/2021. Male Rattus norvegicus (Wistar strain) rats 8–9 weeks old and weighed between 200 and 300 g were chosen for the study, which looked at pharmacokinetics and antihyperuricemic activity. Animals were taken from the animal house at Sekolah Ilmu dan Teknologi Hayati ITB, Indonesia. All of the animals lived in the animal house of the Pharmacology Research Laboratory, Sekolah Farmasi, ITB, Bandung. Before the experiment began, the animals had a week to get used to their new surroundings. There were six of these in each cage. The room temperature was 24°C, and the humidity was 60%. Before the study began, the animals were fed standard food and given clean water as much as they wanted.
HPLC method validation
Peak area ratios of each analyte versus plasma concentration were used to make calibration curves. Five different samples were examined to determine the accuracy and precision for the same day. Five different days were used to test the accuracy and precision between days. Precision is measured by the relative standard deviation (RSD) of the concentrations that have been found. The following equation was used to determine accuracy: Accuracy = (mean measured concentration minus nominal concentration)/nominal concentration 100. Metabolite recovery was examined using five different plasma samples with low, medium, and high concentrations.
Hyperuricemic induction
The rat was induced by potassium oxonate in 0.9% saline solution with a dose of 250 mg/kg rat body weight/day and administered intraperitoneally to each rat on days 1, 3, and 7 of the experiment.
Pharmacokinetic study of controlled release particulate system of MSE
Pharmacokinetic studies of the optimized formulations were carried out using a double crossover design. After overnight fasting, the rats were divided into three groups (n = 3 for each group). The study was performed as a single-dose crossover design with seven days washout period. Group 1 received the pure MSE, Group 2 received the F1 formulation, and Group 3 received the F3 formulation. All groups that received a dose equivalent to 125 mg MSE per kg of body weight were dispersed in distilled water. Following oral drug administration, the rats kept in the cages were allowed access to food and water ad libitum. During experiments, blood samples (1 ml) were taken from the tail and transferred to tubes at 0, 0.25, 0.5, 1, 2, 3, 5, 8, 12, and 24 hours after drug administration. Immediately, blood samples were centrifuged at 10,000 rpm for 10 minutes and stored at 20°C until analyzed. Analysis for RES using HPLC methods is based on validation that has been done previously. Instrument using an HPLC Knauer: German. A reserve phase column C–18 (4.6 mm × 250 mm × 5μm, Reliant: Ireland) column was used. The analytical conditions were as follows: the mobile phase was an acetonitrile-water mixture (45:55, v/v), the flowing rate was 1 ml/minute, detection wavelengths were set at 280 nm, and injection volumes 100 μl. The pharmacokinetic parameters [Ka, Kel, t1/2, tmax, Cmax, and area under curve (AUC)0-24] were calculated from the plasma concentrations of the RES.
Antihyperuricemic activity study of controlled release particulate system of MSE
Five potassium oxonate-induced hyperuricemic groups were used in this study: the group received MSE, F1, F3, standard drug allopurinol, and untreated as a positive control (PC). The healthy group was only given the media (distilled water) as a negative control. MSE, F1, F3, and allopurinol samples were dispersed in distilled water. The volume or amount of suspension given to animals was based on body weight weighing immediately before administering each dose. All groups received the sample orally through gavage. Approximately 500 μl of whole blood was taken from each rat 2 hours after administering test compounds through the tail vein. The sampling times were set to 1, 2, 3, 5, 8, 12, and 24 hours. The blood sample was centrifuged at 10,000 rpm for 10 minutes to obtain the serum. The detail groups used in this experiment are shown as follows:
NC= Healthy rat
PC= Hyperuricemic rat
A= Hyperuricemic rat + allopurinol (10 mg/kg body weight).
MSE= Hyperuricemic rat + MSE (125 mg/kg body weight)
F1 = Hyperuricemic rat + F1 Formula (equivalent MSE 125 mg/kg body weight)
F3= Hyperuricemic rat + F3 Formula (equivalent MSE 125 mg/kg body weight)
Determination of UA
UA levels were measured by enzymatic colorimetric method using a UA reagent kit (Greiner Diagnostic GmbH Bahlingen Germany) and determined by Microlab 300. About 20 μl of serum was added to a test tube containing 1 ml of reagent mixture, was kept aside for 5 minutes at 37°C, and the absorbance was taken against the reagent blank at 546 nm.
Statistical analysis
Pharmacokinetic parameter and UA level data were expressed as mean ± standard deviation, n = 6. Data were analyzed using IBM SPSS statistical computer program, version 25. The Shapiro-Wilk test is used to check for normality in the data. If p > 0.05, then the data are regularly distributed. Next, we perform a data homogeneity test, and the p value > 0.05 indicates that the data is distributed homogeneously. The different effects of different intervention groups were analyzed using a one-way analysis of variance parametric test if the data were normally and homogeneously distributed, followed by a post-hoc test using the Tukey test. The difference was significant if p-value < 0.05.
RESULTS AND DISCUSSION
Physical properties of controlled release particulate system
The evaluation of the Controlled Release Particulate system is shown in Table 2. The moisture content results of each formula’s granules meet the requirements of a suitable granule, ranging from 2% to 4% (2.08 ± 0.04–2.81 ± 0.02). This result can ensure the storage stability of the particulate. The stability of particulate is affected by its relative humidity. The stability decreases as the potential for microbial life increases with increasing humidity. If the water content is too high, it can cause the granules to become sticky and rigid, whereas a low water content will generate dry, easily broken granules. The angle of repose was found between 24.96 ± 1.32 and 27.63 ± 2.42. All formulas show excellent flow, i.e., in the range of excellent flow between 25° and 30°. The Car index was in the field of 7.33 ± 0.58 and 12.33 ± 1.53. A compressibility index below 15% indicates good flow. In this study, the Hausner ratio was in the range of 1.08 ± 0.006–1.14 ± 0.019. The results are obtained within limits so that the granules will not cause problems for further process.
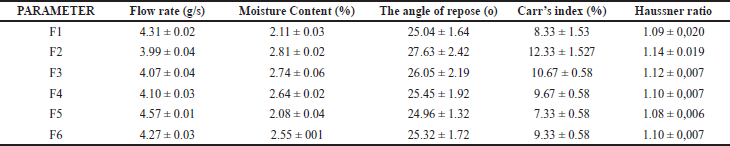 | Table 2. Physical properties of control release granule. Data represented as mean ± standard deviation. n = 3. [Click here to view] |
Our main approach is to extend the half-life of RES in MSE by formulating a controlled-release dosage form. Controlled-release solid dosage forms have several advantages: reduced dosing frequency, constant drug levels, blood plasma concentrations, reduced toxicity due to overdose, reduced fluctuations in peak-valley concentrations, reduced or eliminated systemic adverse effects, decreased or eliminated local side effects, and reduced or eliminated drug buildup with chronic dosages, economic, and easy to manufacture. Maintaining plasma concentrations of active substances over long periods aims to maintain minimum effective concentrations, thereby providing efficacy for long periods, and is a desirable treatment option for controlling HU.
In vitro study
Controlled release oral formulation was chosen by matrix method using release control polymer HPMC K100M (Hydrophilic polymer) and Eudragit RSPO (Hydrophobic polymer). The interaction between swelling and insoluble polymers created a physical barrier to drug release and increased matrix diffusion resistance. The polymer used in this study’s matrix was 20% w/w formula weight. Previous studies reported that the higher the polymer content, the slower the release. The optimal concentration in this study was at a concentration of 20%–30% [27].
The effect of HPMC K100M and Eudragit RSPO concentrations on the release of active substances is shown in Figure 1. Based on the preliminary dissolution test data, the release of total phenolic as an active substance in the nonpolymer particulate system (F1) showed the fastest release, nearly 79.89% in 0.25 hours. This might be no polymer function as an extended-release component. The cumulative percentage of phenol release of F1 at 8 hours reached 96.16%. Previous research reported on the conventional dosage form of G. gnemon fruit dry extract capsules showing a dissolution profile of 0.4 g per capsule in the 35-minute drug release of 93.17% [28]. Preliminary tests were carried out to select a suitable polymer composition. Active substance release with the HPMC matrix was higher than with using Eudragit. At 8 hours of observation, the release of the F2 formula (HPMC matrix) and F6 formula (Eudragit matrix) was 82.56% and 93.28%, respectively. These phenomena can be explained based on the evidence that HPMC chemistry promotes the formation of a robust and firm gel compared to other celluloses. As a result, drug release rates are maintained longer with HPMC than with equivalent levels of methylcellulose, hydroxyethylcellulose, or carboxymethylcellulose.
HPMC is often the polymer of choice over other celluloses. HPMC shows a better release profile and higher tolerability for most formulation variations. HPMC handles soluble and insoluble drugs with high and low dosages. In addition, HPMC exhibits good material for high variations manufacturing procedures, yielding consistent product quality. HPMC has been proven to modulate medication release for poorly soluble drugs [29]. The HPMC effect of hydrophilic polymer on drugs BCS II classification release by hydrating polymer chains, predominantly through H- bonding of oxygen atoms in the many ether links, causes them to stretch and form relatively open random coils, causing the viscosity of HPMC solutions. A given hydrated random coil is H linked to more water molecules, entrapping water molecules within, and may be entangled with other random coils. The medication release rate dropped in the HPMC K4M > K15M > K100M rank order. HPMC limited the release rate of glipizide in matrix tablets for 16 hours [30]. Another study reported HPMC K100M (20 mg) shows rosuvastatin release up to 99.4% in 20 hours [31].
Applying hydrophilic HPMC K100M and hydrophobic Eudragit RSPO (0.75:0.25) polymers in this study revealed the most effective strategy in controlling the release of active substances, i.e., 76.45% at 8 hours (F3). Hence, F3 formula is the selected formula for further studies. Previous studies reported that using a combination of hydrophilic polymers (HPMC) and hydrophobic polymers (ethylcellulose) showed longer release of the active ingredients (up to 12 hours) due to higher viscosity resulting from the polymer [32]. The selected formula (F3) was tested for re-dissolution using RES as a marker. Figure 2 displays the RES release profile. The formulation of MSE using an HPMC and Eudragit matrix (F3) showed extended drug release for 12 hours, according to the results of the in-vitro dissolution test. Figure 2 shows that MSE and F1 released quickly and completely within 3 hours. HPMC is a hydrophilic polymer that produces a gel layer when it comes into contact with water. The dissolution solvent flows through the gel layer, dissolving the drug’s polymers and diffusing it in a controlled manner. The dissolving rate is determined by the thickness of the gel layer, diffusion through the gel layer, and erosion of the gel layer. HPMC facilitates the release of insoluble drugs (RES) due to its solubilizing effect [33]. Depending on the drug’s solubility, hydrophilic matrices release drugs by diffusion through the gelatinous layer, erosion (polymer dissolution), or a combination of the two. HPMC has successfully prepared sustained-release curcumin, a hydrophobic polyphenolic compound. Experiments showed that the release of curcumin from the HPMC mixture was significantly slower due to the sustained-release property of HPMC as a typical excipient [34].
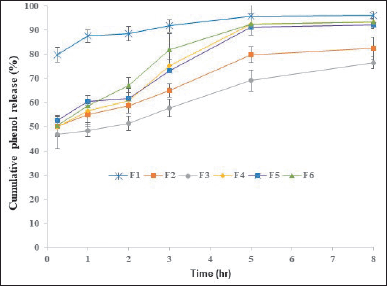 | Figure 1. The release profile of the proposed formulation is coded as F1 = Melinjo shell seed extract + Avicel PH101; F2 = (HPMC K100M; Eudragit RSPO = 1;0); F3 = (HPMC; Eudragit RSPO = 0.75: 0.25); F4 = (HPMC; Eudragit RSPO = 0.5: 0.5); F5 = (HPMC; Eudragit RSPO = 0.25; 0.75); F6 = (HPMC; Eudragit RSPO = 0:1); Date represented as mean ± standard deviation, n = 5. [Click here to view] |
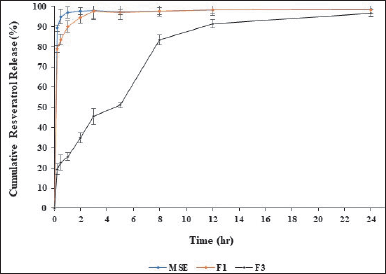 | Figure 2. Cumulative resveratrol release profiles of MSE = Melinjo; seed shell extract; F1 = Melinjo shell seed extract + Avicel; and F3 = (HPMC K100M: Eudragit RSPO = 0.75: 0.25). Data represented as mean ± standard deviation. n = 5. [Click here to view] |
Eudragit RSPO, as a hydrophobic polymer, is combined with HPMC to obtain better and easier drug release. Eudragit serves as a matrix in which the active ingredients are embedded. The matrix structure is obtained by granulation [35]. Hydrophobic polymers exhibit a slower release rate in poorly soluble substances due to the matrix’s lower water affinity and reduced water infiltration. As the relative concentration of Eudragit RSPO rises, it provides a more hydrophobic environment, preventing the dissolution medium from penetrating the matrix and delaying drug release [30]. The combination of insoluble and swellable polymers creates a physical barrier to drug release and increases the resistance of the matrix to diffusion. As a result, even though the overall number of polymers is reduced compared to a single polymer system, the interaction of both polymers can maintain the ability to control drug release [36].
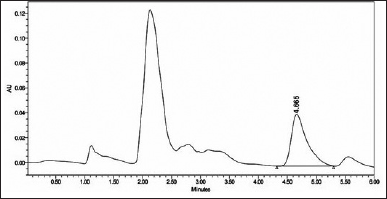 | Figure 3. HPLC chromatogram at 306 nm of trans-resveratrol in plasma. [Click here to view] |
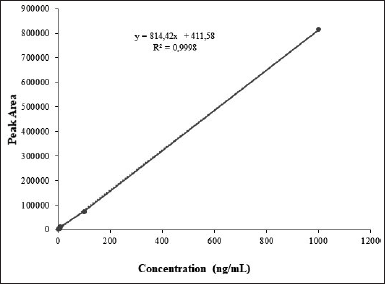 | Figure 4. The linearity curve of trans-resvertrol in plasma was linera over the 1-1000 ng/ml range with a correlation coefficient; r2 = 0.9998. [Click here to view] |
The combination of HPMC and Eudragit in other BCS II Classification drugs, namely domperidone, can extend drug release for up to 24 hours [37].
In vivo study
HPLC validation method
The selectivity, sensitivity [lower limit of detection (LOD) and limit of quantitation (LOQ)], linearity, precision (intra- and inter-day), accuracy (analytical recovery), and complete recovery of this HPLC technique were evaluated. The proposed method was found to be simple, precise, and linear. The results of linearity studies have provided a linear relationship in the 1–1,000 ng/ml. Previous studies showed that the obtained linearity values were 10–1,000 ng/ml [38]. The concentration range for RES in the regression equation y = 814.42 × + 411,11 (r2 > 0.9998). The regression analysis yields a linear equation with a goodness-of-fit (r2) of 0.9998, showing a linear relationship between analyte concentration and area under the peak. The graph’s LOD and LOQ were 6.81 ng/ ml and 22.70 ng/ ml, respectively.
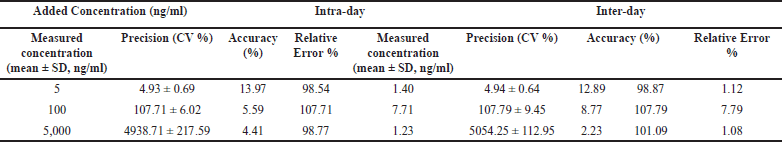 | Table 3. Accuracy and precision of Res in plasma. Data represented as mean ± standard deviation. n = 6. [Click here to view] |
Figure 3 illustrates a typical chromatogram. The proposed method was simple, precise, and linear, with a regression coefficient of 0.9998 (Fig. 4). It analyzed samples in the nanogram range with a mean percent recovery within the acceptable range of 92.84%–101.42%. Table 3 displays the precision and accuracy of data for analytical procedures. The methods’ intraday and interday precision (%RSD) were below 10%. They were within 15% of the permissible range for compliance with the United States Pharmacopeial Norms method validation guidelines (RSD). The deviation between the nominal concentration and calculated concentration for RES was well below the limits of 15%, indicating that the methods were accurate. Data on precision and accuracy indicate that the extraction methodologies for RES are highly robust and reproducible. The precision and accuracy values were adequate by the international recommendation that they did not exceed 15%.
Pharmacokinetic study of controlled release particulate system of MSE
The in vivo study of the MSE matrix polymer particulate system (F3) was done to determine the pharmacokinetic parameters. It was compared with a pharmacokinetic parameter of the pure MSE and MSE non-polymer particulate system (F1). The mean RES serum concentration-time profile is shown in Figure 5. Our results demonstrated a significant improvement in the pharmacokinetic profiles of MSE formulated as a controlled release particulate system (F3). Compared to pure MSE and nonpolymer particulate systems, the AUC and t1/2 of the MSE controlled release particulate system (F3) were increased significantly. As shown in Table 4, the Cmax (ng/ml), Tmax (hour), Kel (/hour), Ka (/hour), t1/2 (hour), and AUC0- (ng. hour/ml) values represented the mean pharmacokinetic parameters of RES from different formulations (MSE, F1, and F3). The pharmacokinetic parameters of RES in healthy rats align with previous studies [38].
The Cmax value parameter in healthy and hyperuricemic rats has increased significantly. In a healthy group, the Cmax of the group receiving the F3 formula was 22.41 ± 0.96 ng/ml, while in the group receiving the MSE sample, it was 20.10 ± 1.13 ng/ml, and in the group receiving the F1 formula, it was 17.53 ± 1.25 ng/ml. Whereas in the HU group, the Cmax of the group receiving the F3 formula was 11.16 ± 2.26 ng/ml, the group receiving the MSE pure was 5.64 ± 0.88 ng/ml, and the group receiving F1 formula was 2.82 ± 0.71 ng/ml. Earlier studies report of Cmax RES in humans with oral administration of 500 mg dose yielded results ranging from 261 to 967 ng/ml [39].
The t1/2 values ??obtained for healthy rats were F3 (1.57 ± 0.14 hour), F1 (1.78 ± 0.27 hour), and pure MSE (1.78 ± 0.27 hour), whereas in HU rats, F3 (1.24 ± 0.78 hour), pure MSE (0.80 ± 0.28 hour) and F1 (1.20 ± 0.31 hour). The half-life parameter values ??obtained for healthy rats were F3 (16.20 ± 2.48 hour), F1 (4.34 ± 0.81 hour), and pure MSE (4.48 ± 0.45 hour), whereas in HU rats, F3 (22.56 ± 14.14 hour), pure MSE (6.37 ± 1.86 hour) and F1 (6.37 ± 1.86 hour). The t1/2 F3 was longer than that of MSE and F2, which the sustained absorption may cause.
The mean AUC0-24 parameter values ??obtained for healthy rats were F3 (552.75 ± 91.25 ng.hour/ml), F1 (126.98 ± 17.77 ng.hourr/ml), and pure MSE (146.95 ± 13.13 ng.hour/ml), whereas in HU rats, F3 (116.42 ± 14.53 ng.hour/ml), pure MSE (55.51 ± 8.28 ng.hour/ml) and F1 (91.8 ± 59.5). In healthy and hyperuricemic rats, AUC0-∞ F3 increased compared to MSE and F1. MSE formulation with hydrophilic and hydrophobic matrices that regulate the release of active substances improves bioavailability by increasing the t1/2 and AUC0-∞ F3 formula in healthy and hyperuricemic rat groups. One of the main reasons for such a big difference between controlled-release RES is that the absolute bioavailability of RES was extremely low because of the poor solubility of the drug [40]. The administration of RES oral doses of 500 mg in healthy men and women gave a value of AUC0-inf 179.1 ng/ml [41].
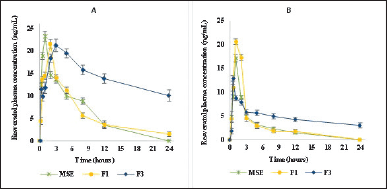 | Figure 5. Plasma concentration- time of the (a) resveratrol in the healthy rat. (b) in hyperuricemic rat oral administration MSE = Melinjo seed shell extract (125 mg/kg boby wight); F1 = F1 Formula (equivalent melinjo seed shell ectract 125 mg/kd boby weight) F3 = F3 Formula (equivalent melinjo seed shall extract 155mg/kg boby weight). Date represented as mean ± standard deviation, n = 5. [Click here to view] |
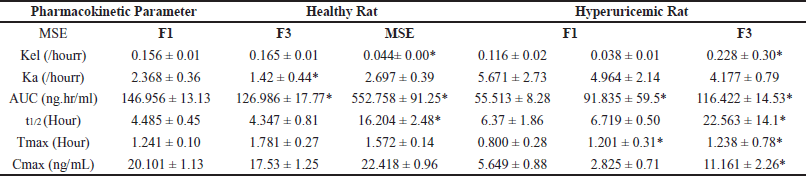 | Table 4. Summary of pharmacokinetic parameters particulate system MSE, F1, and F3 (n = 6). [Click here to view] |
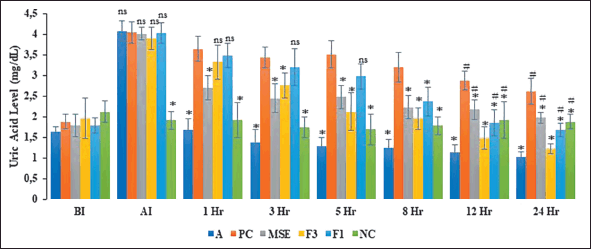 | Figure 6. The mean serum uric acid level in hyperuricemic group BI = Before Induction AI = Induction. Date represented as mean ± standard deviation, n = 6. ns = not significant, *indicate significant p < 0.05 when compared to the serum uric acid level of hyperuricemic control group (PC); #indicates significant p < 0.05 when compared to the serum uric acid level of Allopurinol treatment group (A); NC = healty rate; A = Hyperuricemic rat + allopurinol (10 mh/kg boby weight);MSE = Hyperuricemic rat + Melinjo seed shall extract (125 mg/kg boby weight ); F1 = Hyperuricemic rat + F1 Formula (equivalent melinjo seed shall extract 125mg/kg; boby weight); F3 = Hyperuricemic rat + F3 Formula (equivalent melinjo seed shall extract 125mg/kg; boby weight). [Click here to view] |
Antihyperuricemic activity study of controlled release particulate system of MSE
The effectivity data of a controlled release particulate system as anti-hyperuricemia activity of MSE is shown in Figure 6. The UA level was significantly higher before (BI) and after (AI) induction with 250 mg/kg body weight of potassium oxonate. This finding demonstrated that the rats were successfully hyperuricemic. Potassium oxonate is a hepatic uricase competitive inhibitor. It inhibits the conversion of UA to allantoin in part [42,43]. Urinary acid levels in the A and MSE groups declined significantly faster than in the other groups within the first hour. The F3 and F1 groups’ UA levels significantly differed from those of the PC group, indicating that the F3 and F1 groups did not experience a decline in UA within the first hour after treatment.
After 24 hours of treatment, the UA levels of groups receiving MSE, F1, and F3 decreased significantly compared to the PC group (hyperuricemic rats). RES, the primary active substance in MSE, decreases UA levels by decreasing mGLUT9 expression to inhibit urate reabsorption and upregulating mOAT1 expression to increase urate secretion in HU rat kidneys [44]. Another report described that Melinjo (G. gnemon L) was promising as an anti-hyperuricemic drug candidate because of the presence of polyphenolic compounds [45]. Tamura et al. [8] discovered that Melinjo seed extract therapy upregulated the expression of a G member of the ATP-binding cassette (ABCG2) subfamily in the ileum but not in the kidney. Melinjo increased ABCG2 expression in a human epithelial colorectal cell line. These data suggest that Melinjo seed extract decreases serum UA by increasing UA excretion via feces in the ileum by boosting ABCG2 protein expression [8].
The UA level of the F3 group was not significantly different from that of group A. These phenomena showed that the ability to reduce UA levels of the F3 formula was equivalent to allopurinol. These results indicated that the MSE formulated with a combination of hydrophilic and hydrophobic polymers enhanced its efficacy as an anti-hyperuricemia. RES RES, the active compound in MSE, ameliorates HU and kidney inflammation. RES may improve kidney inflammation through TLR4 and NLRP3 signaling pathways and reduce the expression of UA trans?porter proteins in the kidney, thereby reducing blood UA levels. Urate transport-associated proteins regulate serum UA levels; urate transporter 1 (URAT1) and glucose transporter 9 (GLUT9) are essential in renal UA reabsorption. RES decreases GLUT9 and URAT1 protein expression mediated through anti-inflammatory effects [46].
The combination of a HPMC and a hydrophobic matrix (Eudragit) enhanced the dissolution rate, pharmacokinetic parameters, and antihyperuricemic effect of MSE. Allopurinol was used as a PC with a dose of 10 mg/kg and showed significantly decreased UA levels compared to normal control rodents [47]. Primarily, HU is treated with Xantin Oxidase Inhibitors, such as allopurinol, which inhibit the final step in UA synthesis from purines. However, allopurinol has been observed to cause adverse effects such as skin allergy, Stevens–Johnson syndrome, and kidney injury [48]. Therefore, medicinal plants containing phytochemical constituents promise a better alternative to allopurinol.
CONCLUSION
Development of a particulate system of MSE using a combination of hydrophilic (HPMC K100M) and hydrophobic (Eudragit RSPO) polymers seems to be effective in achieving therapeutic outcomes by improving the RES pharmacokinetic profile and bioavailability. This also suggests a promising strategy for decreasing the frequency of drug administration and increasing oral bioavailability, subsequently improving the effectivity of MSE to decrease UA levels.
ACKNOWLEDGMENT
This project was partly financed by The Ministry of Research and Technology and Higher Education Republic of Indonesia (2021) in the scheme of the doctoral research grant. the authors also thank the Bandung Institute of Technology for supporting all research facilities.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This project was partly financed by the Ministry of Research and Technology, Republic of Indonesia (contract number: 2/E1/KP.PTNBH/2021).
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The study protocol was approved by the Ethical Clearance Commission of Universitas Airlangga, Faculty of Dental Medicine Health Research (Approval number: 493/HRECC/FODM/VIII/2021).
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Yanagihara M, Yoshimatsu M, Inoue A, Kanno T, Tatefuji T, Hashimoto K. Inhibitory effect of gnetin C, a resveratrol dimer from melinjo (Gnetum gnemon), on tyrosinase activity and melanin biosynthesis. Biol Pharm Bull. 2012;35(6):993–6. doi: https://doi.org/10.1248/bpb.35.993
2. Kunimasa K, Ohta T, Tani H, Kato E, Eguchi R, Kaji K, et al. Resveratrol derivative-rich melinjo (Gnetum gnemon L.) seed extract suppresses multiple angiogenesis-related endothelial cell functions and tumor angiogenesis. Mol Nutr Food Res. 2011;55(11):1730–4
3. Uson-Lopez RA, Kataoka S, Mukai Y, Sato S, Kurasaki M. Melinjo ( Gnetum gnemon ) seed extract consumption during lactation improved vasodilation and attenuated the development of hypertension in female offspring of fructose-fed pregnant rats. Birth Defects Res. 2017;110(1):27–34. doi: https://doi.org/10.1002/bdr2.1109
4. Yoneshiro T, Kaede R, Nagaya K. Saito M, Aoyama J, Elfeky M, et al. Melinjo (Gnetum gnemon L.) seed extract induces uncoupling protein 1 expression in brown fat and protects mice against diet-induced obesity, inflammation, and insulin resistance. Nutr Res. 2018;58:17–25. doi: https://doi.org/10.1016/j.nutres.2018.06.012
5. Wazir D, Ahmad S, Muse R, Mahmood M, Shukor MY. Antioxidant activities of different parts of Gnetum gnemon L. J Plant Biochem Biotechnol. 2011;20(2):234–40. https://doi.org/10.1007/s13562-011-0051-8
6. Chan EW, Wong CW, Tan YH, Foo JP, Wong SK, Chan HT. Resveratrol and pterostilbene: a comparative overview of their chemistry, biosynthesis, plant sources and pharmacological properties. J Appl Pharm Sc. 2019;9(7):124–9. doi: https://doi.org/10.7324/JAPS.2019.90717
7. Sari NK, Soemardji AA, Fidrianny I. The effect of melinjo (Gnetum gnemon L.) leaves and melinjo peel extracts on induced-hyperuricemia male rats model. J Med Health. 2019;2(4):956–64. doi: https://doi.org/10.28932/jmh.v2i4.1840
8. Tamura Y, Morimoto C, Kuribayashi-Okuma E, Uchida S, Hosoyamada M, Nakagawa T, et al. Melinjo seed extract stimulates intestinal ABCG2 expression to reduce serum uric acid levels in hyperuricemic rats. J Funct Foods. 2021;87:104849. Available from: https://www.sciencedirect.com/science/article/pii/S1756464621004989
9. McAdams-DeMarco MA, Law A, Maynard JW, Coresh J, Baer AN. Risk factors for incident hyperuricemia during mid-adulthood in African American and white men and women enrolled in the ARIC cohort study. BMC Musculoskeletal Disord.2013;14(1):1–8. doi: https://doi.org/10.1186/1471-2474-14-347
10. Usman SY, Darmawan G, Hamijoyo L, Wachjudi RG. Hyperuricemia prevalence and metabolic syndrome profiles: a pilot cross sectional study in North Kayong regency, West Kalimantan, Indonesia. Indonesian J Rheumatol. 2019;11(2):175–80. doi: https://journalrheumatology.or.id/index.php/ijr/article/download/118/113
11. Mari E, Ricci F, Imberti D, Gallerani M. Agranulocytosis: an adverse effect of allopurinol treatment. Italian J Med. 2011;5(2):120–23. doi: https://doi.org/10.1016/j.itjm.2011.02.006
12. Ren X, Wu S, Xie W, Liu Y, Yang S. Association between the risk of hyperuricemia and changes in branched-chain amino acids intake over twelve years: a latent class trajectory analysis from the china health and nutrition survey, 1997–2009. Front Nutr. 2022;9(August):1–8. doi: https://doi.org/10.3389/fnut.2022.916446
13. Iliya I, Ali Z, Tanaka T, Iinuma M, Furusawa M, Nakaya KI, et al. Stilbene derivatives from Gnetum gnemon Linn. Phytochemistry. 2003;62(4):601–6. doi: https://doi.org/10.1016/S0031-9422(02)00670-2
14. Tani H, Koshino H, Taniguchi T, Yoshimatsu M, Hikami S, Takahashi S. Structural studies on Stilbene oligomers isolated from the Seeds of Melinjo (Gnetum gnemon L.). ACS Omega. 2020;5(21):12245–50. doi: https://doi.org/10.1021/acsomega.0c00910
15. Ren S, Meng F, Liu Y, Meng Y, Tao N, Liu R, et al. Effects of external application of compound Qingbi granules on acute gouty arthritis with dampness-heat syndrome: a randomized controlled trial. Chin Med (United Kingdom). 2020;15(1):1–13. doi: https://doi.org/10.1186/s13020-020-00398-8
16. Tani H, Hikami S, Iizuna S, Yoshimatsu M, Asama T, Ota H, et al. Pharmacokinetics and safety of resveratrol derivatives in humans after oral administration of melinjo (Gnetum gnemon L.) seed extract powder. J Agric Food Chem. 2014;62(8):1999–2007. doi: https://doi.org/10.1021/jf4048435
17. Almeida L, Vaz-da-Silva M, Falcão A, Soares E, Costa R, Loureiro AI, et al. Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Mol Nutr Food Res. 2009;53(SUPPL. 1):7–15. doi: https://doi.org/10.1002/mnfr.200800177
18. Lu Z, Cheng B, Hu Y, Zhang Y, Zou G. Complexation of resveratrol with cyclodextrins: Solubility and antioxidant activity. Food Chem. 2009;113(1):17–20. doi: https://doi.org/10.1016/j.foodchem.2008.04.042
19. Peng H, Xiong H, Li J, Xie M, Liu Y, Bai C, et al. Vanillin cross-linked chitosan microspheres for controlled release of resveratrol. Food Chem. 2010;121(1):23–8. doi: https://doi.org/10.1016/j.foodchem.2009.11.085
20. Teska? K, Kristl J. The evidence for solid lipid nanoparticles mediated cell uptake of resveratrol. Int J Pharm. 2010;390(1):61–9. doi: https://doi.org/10.1016/j.ijpharm.2009.10.011
21. Bonechi C, Martini S, Ciani L, Lamponi S, Rebmann H, Rossi C, et al. Using liposomes as carriers for polyphenolic compounds: the case of trans-resveratrol. PLoS One. 2012;7(8). doi: https://doi.org/10.1371/journal.pone.0041438
22. Milan A, Mioc A, Prodea A, Mioc M, Buzatu R, Ghiulai R, et al. Theoptimized delivery of triterpenes by liposomal nanoformulations: overcoming the challenges. Int J Mol Sci. 2022;23(3):1140. doi: https://doi.org/10.3390/ijms23031140
23. Amri A, Chaumeil JC, Sfar S, Charrueau C. Administration of resveratrol?: what formulation solutions to bioavailability limitations?? J Control Release. 2012;158(2):182–93. doi: https://doi.org/10.1016/j.jconrel.2011.09.083
24. Ghimire M, Hodges LA, Band J, O’Mahony B, McInnes FJ, Mullen AB, et al. In-vitro and in-vivo erosion profiles of hydroxypropylmethylcellulose (HPMC) matrix tablets. J Controlled Release. 2010;147(1):70–5. doi: https://doi.org/10.1016/j.jconrel.2010.06.015
25. Akhgari A, Tavakol A. Prediction of optimum combination of eudragit RS/eudragit RL/ethyl cellulose polymeric free films based on experimental design for using as a coating system for sustained release theophylline pellets. Adv Pharm Bull. 2016;6(2):219–25. doi: https://doi.org/10.15171/apb.2016.030
26. Li L, Teng M, Liu Y, Qu Y, Zhang Y, Lin F, et al. Anti-gouty arthritis and antihyperuricemia effects of sunflower (Helianthus annuus) head extract in gouty and hyperuricemia animal models. Bio Med Res Int. 2017;2017:5852076. doi: https://doi.org/10.1155/2017/5852076
27. Kumpugdee-Vollrath M, Helmis M. Controlled release of resveratrol and lignan by matrix tableting. Adv Mat Res. 746;330–336. doi: https://doi.org/10.4028/www.scientific.net/AMR.746.330
28. Samuel, AJSJ, RSaid RB, Anandarajagopal K, Vimala AGKA, Khan A Muthumani M. Formulation and Evaluation of Herbal Capsules Containing Dried Ethanol Extract of Gnetum gnemon Fruits. Int J Pharm Pharm Res. 2018;12(1):273–80. www.ijppr.humanjournals.comwww.ijppr.humanjournals.com
29. Kandel R, Saha T, Masum ZU, Chowdhury JA. Formulation and In vitro release behavior of hydroxypropyl methylcellulose (HPMC) matrix tablet containing poorly soluble fenofibrate. Bangladesh Pharm J.2020;23(1):10–16. doi: https://doi.org/10.3329/bpj.v23i1.45314
30. Ige P, Swami B, Patil T, Pradhan J, Patil P, Nerkar P, et al. Design and development of sustained release swelling matrix tablets of glipizide for type ii diabetes mellitus. Farmacia. 201361(5):883–901.
31. Srinivasa Rao B, Ratnam BV. Formulation and optimization of sustained release tablets of rosuvastatin using HPMC K4M, HPMC K100M and carrageenan. Int J ChemTech Res. 2018;11(05):376–86. doi: https://doi.org/10.20902/ijctr.2018.110542
32. Hadi MA, Babu VL, Pal N. Formulation and evaluation of sustained release matrix tablets of glimepiride based on combination of hydrophilic and hydrophobic polymers. J Appl Pharm Sci. 2012;2(6):101–7. doi: https://doi.org/10.7324/JAPS.2012.2613
33. Khan NA, Khan A, Ullah R, Ullah M, Alotaibi A, Ullah R, et al Preparation and characterization of hydrophilic polymer based sustained-release matrix tablets of a high dose hydrophobic drug. Polymers. 2022;14(10). doi: https://doi.org/10.3390/polym14101985
34. Zheng J, Wang B, Xiang J, Yu Z. Controlled release of curcumin from HPMC (hydroxypropyl methyl cellulose) co-Spray-dried materials. Bioinorg Chem Appl. 2021;2021. doi: https://doi.org/10.1155/2021/7625585
35. Joshi, M. Role of Eudragit in targeted drug delivery. Int J Curr Pharm Res. 2013;5(2):58–62.
36. Ganesan K, Rajaram SK, Chinnathambi A, Murugesan V, Muruganantham K, Amanullah TR. A sustained release of tablet granules associated with ZnS nanocrystals using tamarind seed polysaccharide. J Appl Pharm Sci. 2013;3(4SUPPL.1):44–7. doi: https://doi.org/10.7324/JAPS.2013.34.S7
37. Biswas R, Basak SC, Shaikh SA. Formulation development and polymer optimization for once-daily sustained release matrix tablets of domperidone. J Pharma Sci Tech. 2011;1(1):28–34.
38. Gadag S, Narayan R, Nayak Y, Nayak UY. Bioanalytical RP-HPLC method validation for resveratrol and its application to pharmacokinetic and drug distribution studies. Jf Appl Pharm Sci. 2022;12(2):158–164. doi: https://doi.org/10.7324/JAPS.2021.120216
39. Kemper C, Benham D, Brothers S, Wahlestedt C, Volmar CH, Bennett D. Safety and pharmacokinetics of a highly bioavailable resveratrol preparation (JOTROL TM). AAPS Open. 2022;8(1):1–14. doi: https://doi.org/10.1186/s41120-022-00058-1
40. Briskey D, Rao A. Trans-resveratrol oral bioavailability in humans using lipisperseTM dispersion technology. Pharmaceutics. 2020;12(12):1–11. doi: https://doi.org/10.3390/pharmaceutics12121190
41. Sergides C, Chiril? M, Silvestro L, Pitta D, Pittas A. Bioavailability and safety study of resveratrol 500 mg tablets in healthy male and female volunteers. Exp Ther Med. 2016;11(1):164–70. doi: https://doi.org/10.3892/etm.2015.2895
42. Osada Y, Tsuchimoto M, Fukushima H, Takahashi K, Kondo S, Hasegawa M, et al. Hypouricemic effect of the novel xanthine oxidase inhibitor, TEI-6720, in rodents [Abstract]. Eur J Pharmacol. 1993;241:183–8.
43. Huang J, Wang S, Zhu M, Chen J, Zhu X. Effects of genistein, apigenin, quercetin, rutin, and astilbin on serum uric acid levels and xanthine oxidase activities in normal and hyperuricemic mice. Food Chem Toxicol. 2011;49:1943–7.
44. Shi YW, Wang CP, Liu L, Liu YL, Wang X, Hong Y, et al. Antihyperuricemic and nephroprotective effects of resveratrol and its analogues in hyperuricemic mice. Mol Nutr Food Res. 2012;56(9):1433–44. doi: https://doi.org/10.1002/mnfr.201100828
45. Tatefuji T, Yanagihara M, Fukushima S, Hashimoto K. Safety assessment of melinjo (Gnetum gnemon L.) seed extract: Acute and subchronic toxicity studies. Food Chem Toxicol. 2014;67:230–35. doi: https://doi.org/10.1016/j.fct.2014.02.030
46. Zhang X, Nie Q, Zhang Z, Zhao J, Zhang F, Wang C. Resveratrol affects the expression of uric acid transporter by improving inflammation. Mol Med Rep. 2021;24(2):1–9. doi: https://doi.org/10.3892/mmr.2021.12203