INTRODUCTION
Coronavirus disease (COVID-19) is a virus-borne infection arising from the severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2 virus) [1]. In December 2019, an unidentified pneumonia epidemic was recorded to have emerged from Wuhan in China. Inoculation of bronchoalveolar lavage material from patients with unexplained pneumonia into human airway epithelial cells resulted in the discovery of a new coronavirus, SARS-CoV-2, heretofore, acknowledged as 2019-nCov [2]. As of April 10, 2022, over 496 million confirmed cases and over 6 million deaths have been reported globally [3], with India reporting 43,036 132 cases and 521,691 deaths [4]. Commencing April 19, 2022, Karnataka disclosed that there were 3,946,422 cases and 40,057 deaths [5] alongside Udupi, Karnataka, reporting 95,606,cases and 546 deaths.
Although children have accounted for a small proportion of COVID-19 infections [6], families with higher infection rates due to Delta [7], along with increased vaccination rates in older adults, are causing the infection frequency in younger age groups [8,9]. However, most of the children’s conditions remain mild. This can result from children’s nasal epithelial tissue exhibiting lesser amounts of the Angiotensin-converting enzyme 2 supermolecule (where SARSCoV2 connects to the cells), with expression rising as they get older [10,11]. Due to lower vaccination rates in adolescents, current epidemiology suggests that young adults, rather than children, are more likely to constitute a core of increased transmission [12]. Immunization, according to the Centers for Disease Control and Prevention (CDC), is predicted to diminish the complications of the virus, such as long-term COVID-19 and multisystem-inflammatory syndrome (MISC) syndrome [13]. Reports of MISC among fully vaccinated children are infrequent, and they can assist in averting significant COVID-19 ailments, such as hospitalizations, critical care admissions, and morbidity. This reinforces the view that COVID-19 immunization prevents the occurrence of complications.
Hypoalbuminemia is a sign of poor outcomes in chronically unwell children [14] with COVID-19. Previous analysis has linked hypoalbuminemia to vascular illness and mortality [15,16]. In distinction to a previous study [17], we tend to fail to determine hypoalbuminemia in kids with milder infection; however, we tend to realize hypoalbuminemia in kids was observed in critical illness, like those with MISC [18], where it is predicted to be higher. Albumin of less than 3.0 g/dl is considered a highly inflammatory state and is observed in pediatrics with severe COVID-19 who require critical care [19]. Several studies have shown that even though COVID-19 is regarded as a mild infection in the pediatric population, it has caused a considerable number of complications, including respiratory distress syndrome (RDS) [20,21] and MISC [22,23], being reported to be the most common complication. In contrast, others included COVID-19 pneumonia [24] and neurological complications such as Guillain–Barre syndrome (GBS) [25,26] and COVID-19 encephalitis [27]. Several costs are involved in the treatment of COVID-19 in children. Some of these include admission, consultation, super-specialization for children, laboratory, nursing, and medication charges. With respect to a Korean study [28], individual treatment expenditures for youngsters with COVID-19 under the Korean National Insurance Service System are calculable to be around USD 2,192. COVID-19 treatment techniques tailored to every age bracket could save expenses and improve the effectiveness of resource allocation. COVID-19-related monetary difficulties could push additional families into difficult financial situations, pushing them to ration food and choose cheaper, unhealthy food options to pay for other essentials such as rent and prescriptions.
MATERIALS AND METHODS
Study design
This was a retrospective observational study. The study was carried out at Kasturba Hospital, Manipal, and Dr. TMA Pai Hospital, Udupi, Karnataka. Before conducting the study, the necessary ethical approval was obtained from the Institutional Ethical Committee of Kasturba Hospital, Manipal (IEC 623/2021). In addition, the study was registered with the Clinical Trial Registry of India (CTRI) under the registration number CTRI/2022/01/039676. The total sample consisted of 176 pediatric patients admitted to these hospitals between March 2020 and August 2021 and included pediatric patients of both genders, less than 18 years of age with a confirmed diagnosis of COVID-19 by reverse transcription-polymerase chain reaction test (RT-PCR). Patients on other alternative medicine systems such as Ayurveda, homeopathy, and Unani systems and incomplete medical records were excluded. The data were collected from patient case records and finance records. The financial data were collected on how much was spent during the patient’s hospitalization on medicines, nursing, bed, physician charges, and personal protective equipment (PPE) kit. The costs spent on the various laboratory tests and Intensive Care Unit (ICU) admission were collected.
Statistical analysis
Statistical analysis was done using SPSS version 20.0 (IBM Corp., Armonk, NY). The chi-square test was used, and statistical significance was determined at p < 0.05. Cost analysis was performed using all continuous variables. The mean SD and median interquartile range were analyzed.
RESULTS
Frequency of lab tests and the relation between gender, complications, and risk factors
The present study comprised 176 COVID-19-positive patients from two hospitals. 43.75% (n, 77) of females were confirmed either by RT-PCR or by Rapid Antigen Test (RAT) test. The children were found to be 5 (1–12) years old and were hospitalized for 6 (3–9) days. The most common diagnostic test performed was aspartate transaminase (AST) [36.9% (n, 65)] while the least was d-dimer [8.5% (n, 15)]. As shown in Table 1, of the 176 children, 60.23% (n, 106) had a history of association with a COVID-19 case while 50% (n, 88) had a travel history. Out of our study population, 87.5% (n, 154) were symptomatic patients, 71.60% (n, 126) were admitted to the pediatric ward, and 28.4% (n, 50) were on ICU admissions.
COVID-19 complications and outcomes
Among 176 children, 33.53% (n, 59) children had COVID-19 complications as mentioned in Table 2. The majority of the children, i.e., 89.20% (n, 157) had improved from COVID-19 infection with only mild symptoms. Nonetheless, 2.84% (n, 5) children had expired as a result of COVID-19 and its complications, such as multiple organ failure, viral encephalitis, and RDS, whereas 5.11% (n, 9) children remained unchanged unaffected by COVID-19 symptoms during their hospital stay, and 2.84% (n, 5) children were discharged against medical advice.
Treatment pattern for COVID-19 in pediatric patients
Paracetamol [36.0% (n, 64)] was the most commonly used drug followed by Oral/iv corticosteroids [20.5% (n, 36)] (methylprednisolone, hydrocortisone, and dexamethasone) and anticoagulants [9.0% (n, 16)] (Table 3).
Direct cost burden of COVID-19 on pediatric patients
The medications were found to be one of the common reasons for the financial burden on the pediatric population and on their parents/guardians with the total cost of COVID-19 drugs and non-COVID-19 drugs amounting to Indian rupees (INR) 2,183.06 (1,099.23–13,032.64) [USD 26.95 (13.57–160.87)] and INR 1,631.48 (192.0–6,533.84) [USD (20.14 (2.37–80.65)], respectively, and the direct cost burden in our study for the COVID-19 therapy in Kasturba Hospital, Manipal, and TMA Pai Hospital, Udupi, was calculated to be with a mean of INR 25,813 (17,358–42,320) [USD 318.64 (214.27–522.4)]. The cost for asymptomatic patients amounting to INR 19,008.50 (14,303.50–22,210.00) [USD 234.64 (176.56–274.16)] while symptomatic patients amounting to INR 31,435.50 (21,184.00–61,721.25) [USD 388.04 (261.5–761.89)]. On the other hand, the cost of therapy for patients who were admitted to the ICU amounted to INR 70490 (45,595–105,309) [USD 870.13 (562.82–1,299.93)] in comparison to INR 21,507 (15,700–29,511) [USD 265.48 (193.8–364.28)] for patients who were admitted to the pediatric wards. Table 4 shows a detailed economic breakdown of all the components involved.
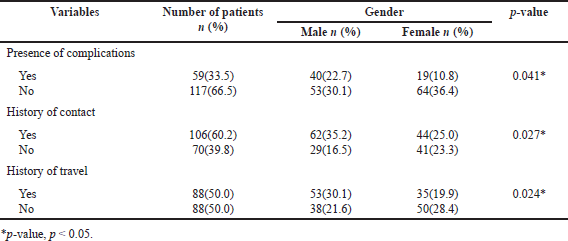 | Table 1. Relation between gender, complications, and risk factors. [Click here to view] |
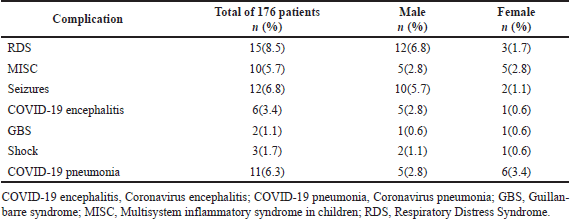 | Table 2. Frequency of complications. [Click here to view] |
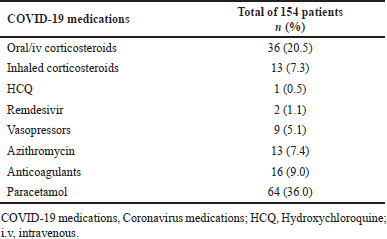 | Table 3. Frequency of COVID-19 medications used. [Click here to view] |
DISCUSSION
There is a fair amount of information available for adult COVID-19 patients regarding the complications, comorbidities, and treatment; however, the data for pediatric COVID-19 is limited. As a result, our study aimed to assess the comorbidities, complications, treatment patterns, and the direct cost of illness in pediatric patients hospitalized with COVID-19. Our study’s findings were seen to be consistent with many other research findings. For instance, a study conducted by Chua et al. [29] found that 55.4% of the infected patients were male, similar to our study’s findings where 51.7% of the patients were male. Simultaneously, according to a multicentre study in Italy, the age was reported to be similar to the median age as reported in our study [30].
The patients’ COVID-19 test results were all positive for the virus, similar to results for adults, even though 12.5% of them were found to be asymptomatic Other most commonly used diagnostic tests for pediatrics include AST (36.9%), alanine transaminase (ALT) (23.86%), C-reactive protein (CRP) (25.57%), total bilirubin (15.9%), direct bilirubin (15.9%), albumin (14.47%), and d-dimer (8.5%), which were consistent to the studies described by Samprathi et al. [31] and Cui et al. [32]. The diagnostic tests confirmed that in mild cases, creatine kinase-MB was mostly seen to be elevated while CRP, procalcitonin, and lactate dehydrogenase levels were frequently elevated in severe disease. Leukocyte indices in children were not consistent, unlike the reports in adults that note specific leukocyte trends, as per earlier COVID-19 studies [33]. Fisler et al. [34] and Guo et al. [35] conducted retrospective studies in two New York city pediatric hospitals and found that 39% of the total patients were admitted to the pediatric intensive care unit (PICU). These results were similar to our finding of 28.4% PICU admissions.
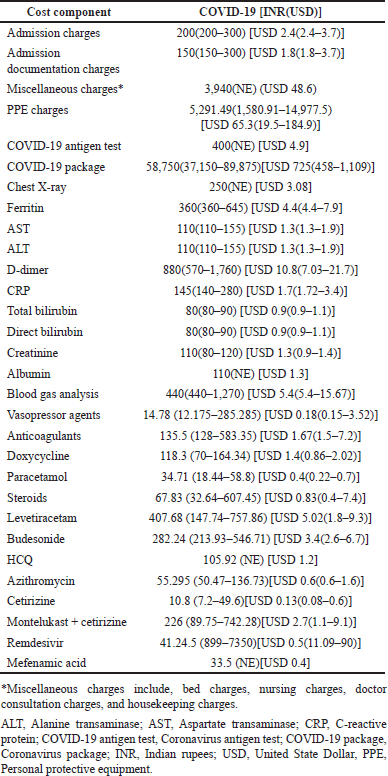 | Table 4. Direct cost of illness components. [Click here to view] |
A study by Guo et al. [35] found that 66% of pediatrics were infected with COVID-19 via a family member, which is on par with the results we concluded, showing a 60.22% history of contact. On the other hand, thyroid, cardiovascular, liver, respiratory, renal disorders, dehydration, immunocompromised, iron deficiency anemia, and malnutrition were observed in 26.15% of our patients, while a previous study conducted by Prata-Barbosa et al. [36] concluded that 41% of the patients had comorbidities which included predominantly neuromuscular diseases (30%), chronic respiratory disease (20%), once-hematological disease (20%), congenital heart defect (13%), diabetes (7%), chronic liver disease (3%), undernutrition (13%), and obesity (3%). Subsequently, we reported that 43.18% of patients suffered from complications caused by COVID-19, which included RDS, MISC, seizures, COVID-19 encephalitis, GBS, shock, and pneumonia. Whereas a study by Garazzino et al. [30] reported 19.6% complications, including pneumonia, severe acute respiratory illness, gastroenterological complications, and neurological complications. These differences are likely due to the small sample size at our hospital during the study period.
As per the guidelines for the management of COVID-19 in children, Ministry of Health and Family Welfare Government of India, corticosteroids (methylprednisolone, hydrocortisone, and dexamethasone) were administered to 20.45% of the patients in our study, compared to 7.6% in a study by Garazzino et al. [30] in Italy. Remdesivir was used in 1.1% of the patients in our study, which was consistent with another study in Europe that reported 3% use [37]. hydroxychloroquine (HCQ) was used in 0.5% of the patients in our study, which is significantly lower than the 7%–47% reported in the other studies [30,36,37]. De Jacobis et al. [38], reported that 44% of the patients received paracetamol, whereas we concluded that 36.3% of the patients received paracetamol. An observational cohort study performed by Korkmaz et al. [39] in Turkey, found that 7.3% of the patients received inhaled corticosteroids; however, just 2% of our pediatric patients received inhaled corticosteroids. Kainth et al. [40], reported the use of vasopressors to be 12% in US Children’s hospitals, and according to a previously performed study in Italy [32], the use of anticoagulants was reported to be 9%, both of which were significantly lower in our study by 5.1% and 6.5%, respectively. Furthermore, Shekerdemian et al. [41] reported that azithromycin was used in 17% of the patients. However, only a handful (7.38%) of the pediatric patients received azithromycin in our study. Not enough studies support the use of vitamin C, vitamin D, and zinc in children with COVID-19. Moreover, Lee et al. [28] reported the mean direct cost of treatment to be 2,192 USD in Korea, while on the other hand, we observed that the mean direct cost of treatment in our study was INR 25,813 (17,358–42,320) [USD 312.81 (210.35–512.84)].
CONCLUSION
COVID-19 has had a significant impact on both the adult and pediatric populations. The main cost drivers are PPEs, advanced and basic disease management programs, and d-dimer. While most children were fortunate to have only a minor infection and recover without any major concerns, some have had a significant illness and have died as a result of COVID-19 complications. The most frequently reported complication of COVID-19 was respiratory distress followed by seizures. Early identification of the specific features of severe pediatric patients and timely treatment is crucial. The current study recommends conducting future research to determine the total cost of illness, including both direct and indirect costs.
LIMITATIONS OF THE STUDY
The study’s small sample size but diverse age group and heterogeneity characteristics may jeopardize the research’s findings. Another limitation was that the COVID-19 diagnosis in the hospital was not classified (mild, moderate, or severe), so we were unable to identify the class of infection suffered by the children. Furthermore, the study was conducted in India; therefore, it is impossible to extrapolate the costs of medical care and the available treatments to other nations.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There was no external funding for this study.
CONFLICTS OF INTEREST
The author(s) declare(s) that there is no conflict of interest regarding the publication of this article. All the authors have collectively approved the manuscript.
INFORMED CONSENT, CONSENT TO PARTICIPATE/CONSENT TO PUBLISH
Not relevant as the study was done retrospectively.
ETHICS APPROVAL
The ethical approval was obtained from the Institutional Ethical Committee of Kasturba Hospital, Manipal (IEC 623/2021). In addition, the study was registered with the Clinical Trial Registry of India (CTRI) under the registration number CTRI/2022/01/039676.
DATA AVAILABILITY
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request. All data is confidential and de-identified individual participant data will not be made available.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Coronavirus. Geneva, Switzerland; 2022 [cited 2022 Apr 19]. Available from https://www.who.int/health-topics/coronavirus#tab=tab_1
2. Wu A, Peng Y, Huang B, Ding X, Wang X, Niu P, et al. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Amp Microbe. 2020;27(3):325–8. CrossRef
3. Weekly epidemiological update on COVID-19—12 April 2022. 2022 [cited 2022 May 10]. Available from: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-COVID-19---12-april-2022
4. 2022 [cited 2022 Apr 19]. Available from: https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases
5. Home—COVID-19 Information Portal. Covid19.karnataka.gov.in; 2022 [cited 2022 Apr 19]. Available from: https://covid19.karnataka.gov.in/english
6. Williams P, Howard-Jones A, Hsu P, Palasanthiran P, Gray P, McMullan B, et al. SARS-CoV-2 in children: spectrum of disease, transmission and immunopathological underpinnings. Pathology. 2020;52(7):801–8. CrossRef
7. Lam-Hine T, McCurdy SA, Santora L, Duncan L, Corbett-Detig R, Kapusinszky B, et al. Outbreak associated with SARS-CoV-2 B.1.617.2 (Delta) variant in an Elementary School—Marin County, California, May–June 2021. MMWR Morb Mortal Wkly Rep. 2022 [cited 2022 Apr 19];70:1214–19. Available from: https://www.cdc.gov/mmwr/volumes/70/wr/mm7035e2.htm
8. Sheikh A, McMenamin J, Taylor B, Robertson C. SARS-CoV-2 Delta VOC in Scotland: demographics, risk of hospital admission, and vaccine effectiveness. Lancet. 2021;397:2461–62. CrossRef
9. Herlihy R, Bamberg W, Burakoff A, Alden N, Severson R, Bush E, et al. Rapid increase in circulation of the SARS-CoV-2 B.1.617.2 (Delta) Variant—Mesa County, Colorado, April–June 2021. MMWR Morb Mortal Wkly Rep. 2022 [cited 2022 Apr 19];70:1084–87. Available from: https://www.cdc.gov/mmwr/volumes/70/wr/mm7032e2.htm
10. Yonker L, Neilan A, Bartsch Y, Patel A, Regan J, Arya P, et al. Pediatric severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): clinical presentation, infectivity, and immune responses. J Pediatr. 2020;227:45–52.e5. CrossRef
11. Bunyavanich S, Do A, Vicencio A. Nasal gene expression of angiotensin-converting enzyme 2 in children and adults. JAMA. 2020;323(23):2427. CrossRef
12. Howard-Jones A, Bowen A, Danchin M, Koirala A, Sharma K, Yeoh D, et al. COVID-19 in children: I. Epidemiology, prevention and indirect impacts. J Paediatr Child Health. 2021;58(1):39–45. CrossRef
13. 2022 [cited 2022 Apr 19]. Available from: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-06/05-COVID-Wallace-508.pdf
14. Horowitz I, Tai K. Hypoalbuminemia in critically Ill children. Arch Pediatr Amp Adolesc Med. 2007;161(11):1048. CrossRef
15. Violi F, Cangemi R, Romiti G, Ceccarelli G, Oliva A, Alessandri F, et al. Is albumin predictor of mortality in COVID-19? Antioxid Amp Redox Signal. 2021;35(2):139–42. CrossRef
16. Violi F, Ceccarelli G, Cangemi R, Alessandri F, D’Ettorre G, Oliva A, et al. Hypoalbuminemia, coagulopathy, and vascular disease in COVID-19. Circ Res. 2020;127(3):400–1. CrossRef
17. Wu H, Zhu H, Yuan C. Clinical and immune features of hospitalized pediatric patients with coronavirus disease 2019 (COVID-19) in Wuhan, China. JAMA Netw Open. 2020;3(6):e2010895. CrossRef
18. Feldstein L, Rose E, Horwitz S, Collins J, Newhams MM, Son MB, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383(4):334–46. CrossRef
19. Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395(10237):1607–8. CrossRef
20. Das S, Sarkar M, Roychowdhoury S, Das B, Mahapatra M, Konar M. A retrospective analysis of clinical manifestations, management and outcome of acute respiratory distress syndrome associated with coronavirus disease-2019 infection in children. Indian J Crit Care Med Peer-reviewed Off Publ Indian Soc Crit Care Med. 2022;26(3):331. CrossRef
21. Seth S, Rashid F, Khera K. An overview of the COVID-19 complications in paediatric population: a pandemic dilemma. Int J Clin Pract. 2021 Sep;75(9):e14494.
22. Waseem M, Shariff M, Tay E, Mortel D, Savadkar S, Lee Horton, et al. Multisystem inflammatory syndrome in children. J Emerg Med. 2022;62(1):28–37. CrossRef
23. Ahmed M, Advani S, Moreira A, Zoretic S, Martinez J, Chorath K, et al. Multisystem inflammatory syndrome in children: a systematic review. EClinicalMedicine. 2020;26:100527. CrossRef
24. Li F, Zhang Y, Shi P, Cao L, Su L, Zhang Y, et al. Epidemiology of viruses causing pediatric community acquired pneumonia in Shanghai during 2010–2020: what happened before and after the COVID-19 outbreak? Infect Dis Ther. 2021;11(1):165–74. CrossRef
25. Abu-Rumeileh S, Abdelhak A, Foschi M, Tumani H, Otto M. Guillain–Barré syndrome spectrum associated with COVID-19: an up-to-date systematic review of 73 cases. J Neurol. 2020;268(4):1133–70. CrossRef
26. Khera D, Didel S, Panda S, Tiwari S, Singh K. Concurrent longitudinally extensive transverse myelitis and Guillain-Barré syndrome in a child secondary to COVID-19 infection. Pediatr Infect Dis J. 2021;40(6):e236–9. CrossRef
27. McAbee G, Brosgol Y, Pavlakis S, Agha R, Gaffoor M. Encephalitis associated with COVID-19 infection in an 11-year-old child. Pediatr Neurol. 2020;109:94. CrossRef
28. Lee J, Kwak B, Choi J, Choi E, Kim J, Kim D. Financial burden of hospitalization of children with coronavirus disease 2019 under the National Health Insurance Service in Korea. J Korean Med Sci. 2020;35(24):e224. CrossRef
29. Chua G, Wong J, Lam I, Ho P, Chan W, Yau F, et al. Clinical characteristics and transmission of COVID-19 in children and youths during 3 waves of outbreaks in Hong Kong. JAMA Netw Open. 2021;4(5):e218824. CrossRef
30. Garazzino S, Lo Vecchio A, Pierantoni L, Carducci F, Marchetti L, Meini A, et al. Epidemiology, clinical features and prognostic factors of pediatric SARS-CoV-2 infection: results from an Italian multicenter study. Front Pediatr. 2021;9:649358. CrossRef
31. Samprathi M, Jayashree M. Biomarkers in COVID-19: an up-to-date review. Front Pediatr. 2021;8:607647. CrossRef
32. Cui Y, Tian M, Huang D, Wang X, Huang Y, Fan L, et al. A 55-day-old female infant infected with 2019 novel coronavirus disease: presenting with pneumonia, liver injury, and heart damage. J Infect Dis. 2020;221(11):1775–81. CrossRef
33. Henry BM, Benoit SW, de Oliveira MHS, Hsieh WC, Benoit J, Ballout RA, et al. Laboratory abnormalities in children with mild and severe coronavirus disease 2019 (COVID-19): a pooled analysis and review. Clin Biochem. 2020;81:1–8. CrossRef
34. Fisler G, Izard S, Shah S, Lewis D, Kainth M, Hagmann S, et al. Characteristics and risk factors associated with critical illness in pediatric COVID-19. Ann Intensive Care. 2020;10(1):1–8. CrossRef
35. Guo C, He L, Yin J, Meng X, Tan W, Yang G, et al. Epidemiological and clinical features of pediatric COVID-19. BMC Med. 2020;18(1):1–7. CrossRef
36. Prata-Barbosa A, Lima-Setta F, Santos G, Lanziotti V, Castro R, Carla de Souza D, et al. Pediatric patients with COVID-19 admitted to intensive care units in Brazil: a prospective multicenter study. J Pediatr (Rio J). 2020;96(5):582–92. CrossRef
37. Götzinger F, Santiago-García B, Noguera-Julián A, Lanaspa M, Lancella L, Carducci F, et al. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Amp Adolesc Health. 2020;4(9):653–61. CrossRef
38. De Jacobis I, Vona R, Cittadini C, Marchesi A, Cursi L, Gambardella L, et al. Clinical characteristics of children infected with SARS-CoV-2 in Italy. Ital J Pediatr. 2021;47(1):1–5. CrossRef
39. Korkmaz M, Türe E, Dorum B, K?l?ç Z. The epidemiological and clinical characteristics of 81 children with COVID-19 in a pandemic hospital in Turkey: an observational cohort study. J Korean Med Sci. 2020;35(25):e236. CrossRef
40. Kainth M, Goenka P, Williamson K, Fishbein J, Subramony A, Barone S, et al. Early experience of COVID-19 in a US Children’s Hospital. Pediatrics. 2020;146(4):e2020003186. CrossRef
41. Shekerdemian L, Mahmood N, Wolfe K, Riggs B, Ross C, McKiernan C, et al. Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian Pediatric Intensive Care Units. JAMA Pediatr. 2020;174(9):868. CrossRef