INTRODUCTION
Traditional medicine has been widely used by people who use traditional plants, including plants from the Myristicaceae family. Myristicaceae grows considerably in tropical rainforests (Tallei and Kolondam, 2015), such as in Indonesia, China, Taiwan, Malaysia, India, Grenada, South America, and Sri Lanka (Naeem et al., 2016; Quigley et al., 2020). The use of nutmeg is widely used in parts of the world as a spice (Sharma and Armstrong, 2013) and as herbal medicine to overcome various diseases (Anaduaka et al., 2022). In addition, nutmeg is also commercially utilized in care products, active packaging of food (Pilevar et al., 2019), beverages, and antimicrobial agents in food preservation derived from trimyristin and its derivatives in nutmeg butter content, namely, myristic acid, myristyl alcohol, and glycerol (Bahrami et al., 2020; Singh, 2003).
The pharmaceutical importance of nutmeg lies in its capacity to produce a wide variety of secondary metabolites. Gas chromatography–mass spectrometry (GC-MS) analysis showed that nutmeg contains 37 metabolites, such as saccharides (monosaccharides and disaccharides), fats, amino acids, organic acids, alkaloids, and nonvolatile metabolites that explain nutmeg flavor as a spice (Farag et al., 2018). In addition, several carbazole alkaloids define nutmeg fragrance at maximum levels in the arillus (24%), followed by the seeds (7%) (Farag et al., 2018), which has analgesic, antinociceptive, and antidepressant activities (Hayfaa et al., 2013; Muchtaridi et al., 2010).
The chemical components of nutmeg include fats, proteins, starch, fixed oils, and essential oils (EOs) (Cao et al., 2020). Nutmeg EO (NEO) has antimicrobial, antiseptic, antiparasitic, anti-inflammatory, and antioxidant properties (Matulyte et al., 2020; Muchtaridi et al., 2010). The main components of this oil are sabinene (21.38%), 4-terpineol (13.92%), and myristicin (13.57%). At the same time, the dominant compounds in nutmeg seeds are alkylbenzene and propylbenzene derivatives (pelican, safrol, eugenol, and its derivatives) (Muchtaridi et al., 2010).
In controlled laboratory studies, nutmeg was shown to have antioxidant and antimicrobial activities (Gupta et al., 2013; Nikolic et al., 2021). However, long-term use of nutmeg may cause degenerative changes in the kidneys, spleen, liver, heart, medial geniculate body, and superior colliculus trialed in albino rats (Olaleye et al., 2006). Nutmeg toxicity experiments in rats have also been analyzed (Anaduaka et al., 2022). The results of the research above prove that information needs to be debated between the benefits and the impact. Some previous nutmeg reviews discussed chemical compounds, biological potentials, and toxic effects of nutmeg, which focused on NEO content and compiled from the literature of 2000–2020 (Warsito, 2021).
Secondary metabolite content, pure compound extraction methods, and recent approaches to the total synthesis of several major components have also been reported, such as NEO rich in terpenes and phenylpropanoids and nutmeg containing nonvolatile lignan/neolignan type (Abourashed and El-Alfy, 2016). Other studies have reported chemical and pharmacological compounds, focusing on pure compounds (Ha et al., 2020). Previous reviews have not discussed nutmeg as the main topic but briefly discussed chemical and pharmacological compounds such as ginger, turmeric, cumin, garlic, cinnamon, and vanilla (Johnson-Arbor and Smolinske, 2021; Mehmood et al., 2019). Although some nutmeg reviews have been widely reported, as far as we are concerned, it is still rare to provide a comprehensive review that focuses on the pharmacology, phytochemicals, and toxicity of nutmeg seeds. The increasingly developing chemical compound synthesis and analysis technologies allow identifying new compounds not discovered in previous research. As a varied source of metabolism with substantial as a prototyping agent in drug discovery, nutmeg requires greater attention within several limits, including the provision of sustainable bioactive through the development of analytical methods (Cz?onka et al., 2020).
In line with this, there are possible scientific gaps in the phytochemical literature on nutmeg. Therefore, this study aims to assess the landscape, map the published nutmeg phytochemical studies, and toxicological and pharmacological properties, and identify research gaps in this area. Scoping reviews are a practical methodology for understanding the breadth of research and knowledge gaps in a particular field (Peters et al., 2020). This review presents comprehensive data on nutmeg research using eight large databases for the last 10 years. The resulting findings are expected to inform the scientific community and facilitate decision-making about the future research direction in this area.
METHODS
Research design
This study employed a scoping review design. This review provides a preliminary assessment of the potential size and scope of the available research literature. It aims to identify the nature and extent of research evidence on a topic (Arksey and O’Malley, 2005; Grant and Booth, 2009). It is also a transparent method for mapping literature and answering broad research questions (Sarrami-Foroushani et al., 2015). Scoping reviews provide a comprehensive study to answer more general questions than a more specific systematic review of effectiveness or qualitative evidence (Peters et al., 2020). This methodology was chosen because it facilitates an efficient and detailed review of the scope, properties, and extent of nutmeg’s phytochemicals, pharmacology, and toxicity. The Preferred Reporting Item guided this scoping review for Systematic Review and the Meta-Analysis extension for Scope Review (PRISMA-ScR) (Tricco et al., 2018).
Research questions
This review is based on the main research question: “How does research on phytochemicals, pharmacology and nutmeg toxicity advance?” These key questions are further extended to secondary research questions, including the following.
1. What are the phytochemicals contained in nutmeg?
2. What are the pharmacological potentials of nutmeg?
3. What is the toxicity of the nutmeg content it has?
The following Population, Intervention, Comparison, and Outcomes (PICO) framework was used to answer the research questions (Table 1).
Search strategy
The search was limited to full-text articles published in English for 10 years (2011–2020). This study was conducted to find in-depth information on research topics tested using modern methods and technologies. During the search, articles were collected according to the research questions and filtered to select those that discuss relevant and promising results (Fig. 1).
The literature search was conducted using the databases of Springer, Scopus, Taylor and Francis, ScienceDirect, ProQuest, SAGE, Wiley, and PubMed. The investigation used keywords and titles of the study subjects with the following search terms: “secondary metabolite Nutmeg” or “metabolite Nutmeg,” “pharmacology Nutmeg” or “pharmacology Myristica fragrans,” and “potentiality Nutmeg” or “potentiality Myristica fragrans,” “phytochemical Nutmeg” or “phytochemical Myristica fragrans,” and “toxicity Nutmeg” or “toxicity Myristica fragrans.”
Exclusion and inclusion criteria
Studies were selected based on inclusion and exclusion criteria regarding research questions and PICO elements (Table 1). Studies were included when they (1) focused on the phytochemical, pharmacological, and toxicity activities of nutmeg; (2) had clear methodology; (3) were written in English; (4) were open access. Moreover, studies that did not contain clear or sufficient detailed methods or results were excluded. Specific inclusion and exclusion criteria were applied to each question to create an overall safety profile of nutmeg consumption (Table 2). This also allowed the most relevant data to be included in the study.
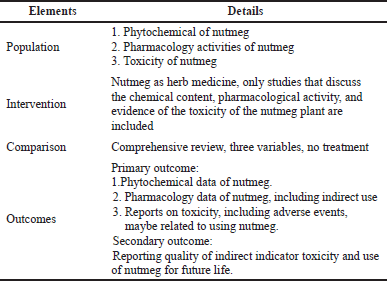 | Table 1. PICO framework. [Click here to view] |
RESULTS AND DISCUSSION
Demographics of the study area
This work presents data on the pharmacological, phytochemical, and toxicity of nutmeg from 2011 to 2020. A literature search found 2,110 articles, of which 1,315 have been deleted based on open-access journals, journals of the last 10 years, and journals of a research nature, leaving 792 articles. In total, 792 of those full-text articles were reviewed for notability based on inclusion criteria; 728 articles were removed because they were not eligible, and 67 remained. At least 39 articles were deleted because they were not related to the main topic. Thus, 28 articles were included for analysis and discussion (Table 4).
The studies included (Table 3) by categorizing them into pharmacology (n = 15), phytochemicals (n = 5), toxicity (n = 4), and combinations that included more than one variable (n = 4). All articles presented are characteristic of research variables. Most articles are randomized controlled trials on nutmeg pharmacology, with the USA as the leading country (21.42%). Three articles included a combination study type by investigating two to three variables and related nutmeg (10.71%). Studies of general and specific toxicity in rodents were published, with most reported by the UAE.
The scoping review results revealed a significant lack of studies to expand and deepen the knowledge of nutmeg. These included metabolites, phytochemicals, and pharmacology, which will be discussed further in detail.
Phytochemistry
Nutmeg is one of the most commonly used spices due to its EOs’ unique taste and aroma (Rizwana et al., 2021; Singh, 2003). Six active compounds were isolated by bioassay-guided fractionation, identified as eugenol, methyl eugenol, methyl isoeugenol, elemicin, myristicin, and safrole (Du et al., 2014). Myristicin and the active metabolite of nutmeg have psychoactive activity, which is mainly responsible for its toxicity (Seneme et al., 2021). However, this activity can also be explored as a potential therapeutic agent for treating central nervous system (CNS) disorders (Sivathanu et al., 2014). In addition, other studies agreed that myristicin is able to suppress the inflammatory response stimulated by low-density lipoprotein oxidation (ox-LDL) by regulating the signaling pathway PI3K/Akt/NF-κB in the development of atherosclerosis so that it can provide potential therapeutic targets that are useful for atherosclerosis (Luo et al., 2022).
Nutmeg hexane extracts showed the highest amounts of steroids, tannins, and terpenoids evaluated based on color production in phytochemical tests. In contrast, other extracts inhibited the formation of melanin at higher concentrations (Hoda et al., 2020). In addition, the phytochemical tests revealed the presence of steroids, carbohydrates, tannins, alkaloids, and terpenoids in nutmeg extract (Hoda et al., 2020). However, other studies reported the absence of terpenoids in its extract (Iyer et al., 2017). This variation was possible due to the plant source, climatic conditions, geographical origin, cultivation conditions, harvest season, and extraction methodology (Nikolic et al., 2021; Suhr and Nielsen, 2003). Another study revealed that nutmeg phytochemicals consist of various compounds that have been identified (Table 5).
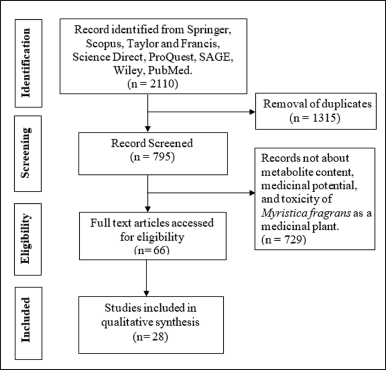 | Figure 1. PRISMA flowchart illustrating the process of selecting articles for scoping review search. [Click here to view] |
 | Table 2. Inclusion and exclusion criteria. [Click here to view] |
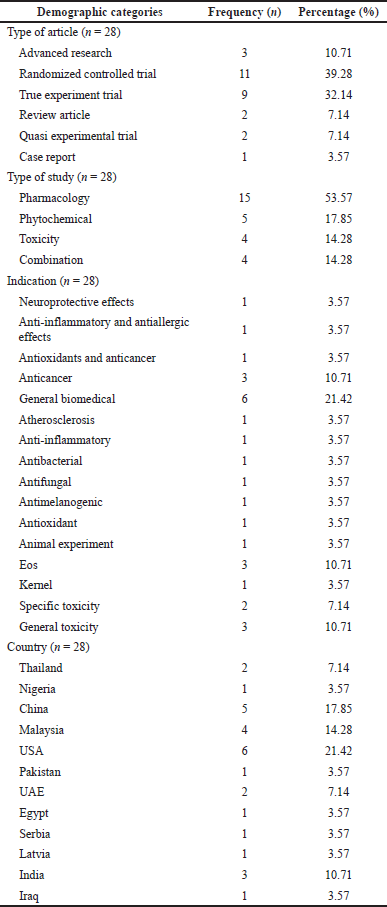 | Table 3. Demographics of included articles. [Click here to view] |
Phenolic acids
The total phenolic content of 50% acetone and 80% methanol extract from each plant was determined using a Folin–Ciocalteu reagent (Yu et al., 2002). This result underlined testing the phenolic content in nutmeg. Nutmeg’s main antioxidant is less polar than other botanical materials under experimental conditions (Chatterjee et al., 2007). Nutmeg still shows a very significant and positive correlation between the content of total phenolics and antioxidant activity, especially in fresh fruits (Chatterjee et al., 2007). The tendency for phenolic content reported in nutmeg is acetone > ethanol > methanol > aqueous > butanol (Gupta et al., 2013).
Myristicin
Myristicin is the main component of NEO. Various reports of nutmeg consumers showed toxicological side effects from myristicin compounds (Ehrenpreis et al., 2014). Side effects include phenytoin toxicity, which can affect the CNS and gastrointestinal tract, vomiting, hypotension, and, very rarely, visual dysfunction (Ehrenpreis et al., 2014; Sivathanu et al., 2014). However, systematic follow-up research is still needed for further application of nutmeg.
Lignans
Lignans are a group of compounds derived from plants with various biological activities such as antitumor, antimitotic, antiviral, and antiatherosclerotic activities (Akinboro et al., 2011). It was identified in nutmeg seeds and flowers that lignans and neolignans were the most abundant secondary metabolites. They were proven on the mass spectral fragmentation pattern of individual peaks completed with GC-MS consisting of elemicin, erythro-neolignane, and their derivatives (Zálešák et al., 2019).
Flavonoids
Resistance to ultraviolet (UV) radiation (and high temperatures) is associated with the chemical structure of nutmeg seeds containing extractive organics, such as polyphenols, quinones, or flavonoids (Cz?onka et al., 2020). There are three flavonoids in nutmeg, including quercetin 3-O-α-L-rhamnopyranosyl-(1→6)-O-[α-L-rhamnopyranosyl-(1 → 2)]-O-β-D-galactopyranoside (203), which was found in aril 5,7-diacetyl chrysin (204), catechins (205) (Morikawa et al., 2018).
Pharmacological evaluation of nutmeg compounds
Antioxidant activities
Ethanol extract in mace nutmeg has more significant antioxidant activity than aqueous extract (Suthisamphat et al., 2020). However, other studies evaluated nutmeg extract using the Allium cepa test, which was shown to have cytotoxic potential and antimutagenic effects on A. cepa chromosome division and cell partition (Akinboro et al., 2011). A temporary conjecture that nutmeg extract is quite promising in developing cancer therapeutic agents. In addition to nutmeg extract, nutmeg oil can potentially be used for chronic inflammation and as a painkiller (Zhang et al., 2016). EOs have been identified as having a more robust content of the chemical compound elemicin, but terpinen-4-ol has been shown to contribute the most to antioxidant activity (Nikolic et al., 2021). Furthermore, limonene in NEO was found to have an antioxidant effect when testing Chinese maye frying in sunflower oil (Wang et al., 2019). This fact showed that limonene in NEO was a safe and effective vegetable with oxidative stability and a unique taste.
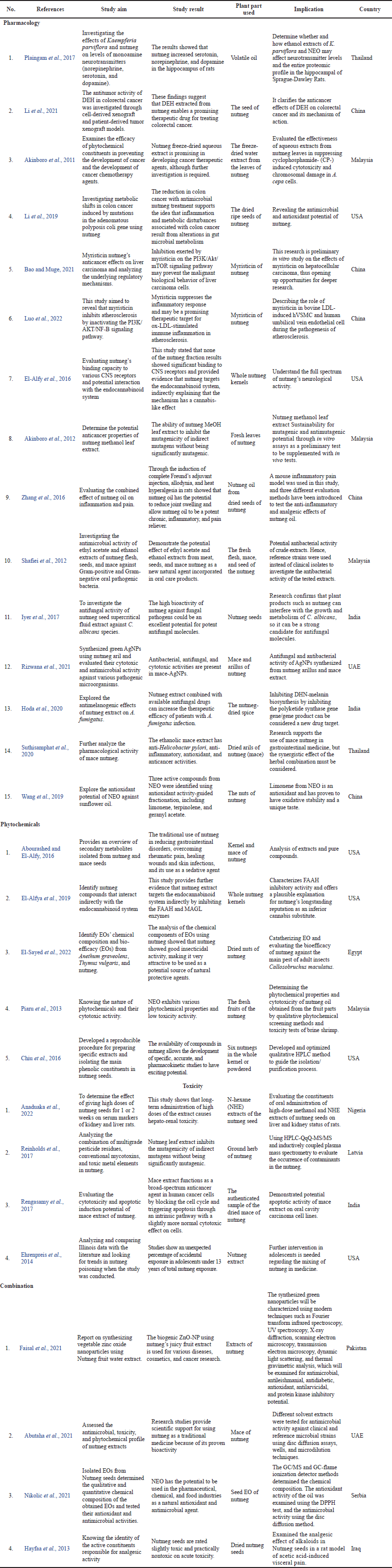 | Table 4. General study characteristics. [Click here to view] |
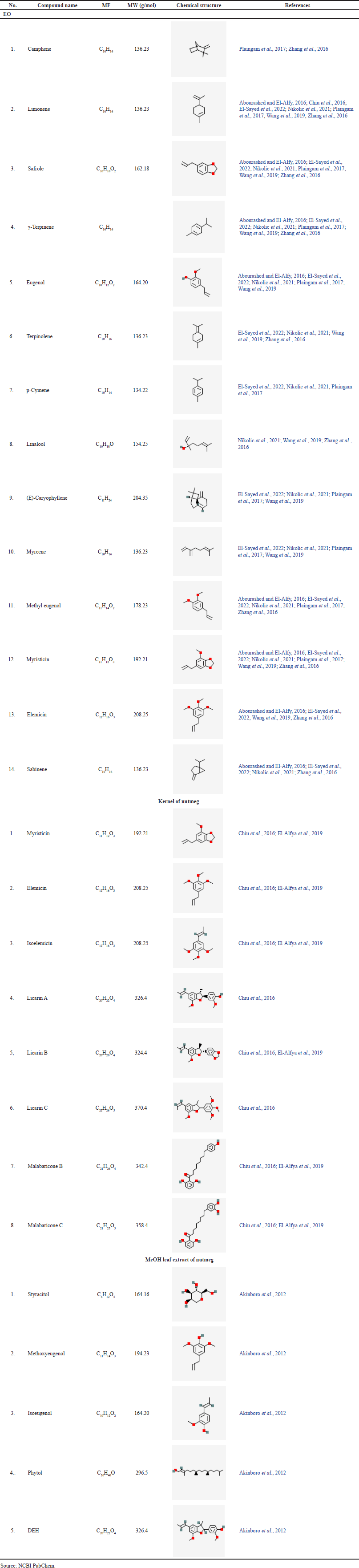 | Table 5. The compound contents of nutmeg. [Click here to view] |
Antibacterial activities
The presence of antibacterial activity in ethyl acetate and ethanol extracts in all parts of the dry sample (mesocarp, arillus, and seeds) indicated low antibacterial activity (Shafiei et al., 2012). On the other hand, a significant effect of NEO activity in inhibiting fungi is comparable to conventional nystatin antifungal drugs. The potential is beneficial in dentistry as an oral care product such as toothpaste and mouthwash (Sokmen et al., 2004). Another analysis performed against the antibacterial activity of arillus extracts and mace-mediated silver nanoparticles (AgNPs) from nutmeg showed that arillus-AgNPs are very effective in inhibiting bacterial test isolates and making them have good benefits for the agrochemical sector, industry, pharmaceuticals, and some biomedical applications (Rizwana et al., 2021).
Antimelanogenic activities
Aging can be caused by an increase in the production of melanin pigmentation in response to damage caused by UV radiation (Oh et al., 2020). One of the studies concluded that nutmeg hexane extract has antimelanogenic potential, as evidenced by inhibition of melanin synthesis, loss of cell surface protrusion, formation of fine cell walls, and decreased ergosterol concentration and hydrophobicity of cell surfaces (Hoda et al., 2020). Furthermore, the study stated that combining with antifungal drugs will help many patients suffering from Aspergillus fumigatus infection.
Antifungal activities
One of the reasons for researching nutmeg as a plant is that it can reduce Candida albicans infection and antimicrobial resistance that humans are concerned about (Iyer et al., 2017). The study results show the high bioactivity of nutmeg extract to fungal pathogens and may be a potential candidate for a potent antifungal molecule. Nutmeg seed extract showed antimicrobial activity at a significant difference of 5% (p ≤ 0.05) during trials on nystatin. So nutmeg extract can meet the needs of developing effective and safe antifungal and antibacterial agents with few side effects (Abutaha et al., 2021).
Anticancer activities
Ehydrodiisoeugenol [Dehydrodiisoeugenol (DEH), CAS: 83377-50-8] is a benzofurane-type neolignan extracted from nutmeg that has long been prescribed in Chinese medicine (Lv et al., 2017). One of the studies has shown that DEH has a role in treating colorectal cancer that can represent a new treatment strategy with exceptional anticancer activity and low toxicity (Li et al., 2021). The part of DEH was obtained based on the research process by inhibiting cell growth and proliferation and inducing endoplasmic reticulum stress and autophagy to exert an apparent anticancer effect on colorectal cancer cells (Li et al., 2021).
Myristicin can potentially be a therapeutic agent for liver carcinoma that can prevent the biological behavior of malignant liver carcinoma cells by inhibiting the signaling pathway PI3K/Akt/mTOR (Bao and Muge, 2021). Li et al. (2019) stated that nutmeg could treat pathogenic bacteria associated with gastrointestinal diseases, reduce colon cancer, and improve metabolic disorders by regulating microbial metabolism in the gut, which can be a potential method to treat colon cancer. Other studies have shown that nutmeg leaf methanol extract induces cytotoxicity and mutagenesis at higher concentrations and indirectly inhibits the induction of mutagenic agents without significant mutagenesis; thus, it could be a promising candidate for cancer treatment therapy (Akinboro et al., 2012).
Neuroprotective effects
Nutmeg increased levels of serotonin (5-HT), norepinephrine, and dopamine in the hippocampus of rats. The data show that nutmeg can target and regulate multiple pathways involved in the underlying molecular therapy mechanisms, proving therapeutic effects in preventing and treating neurodegenerative diseases (Plaingam et al., 2017). A study reported the effects of nutmeg on the endocannabinoid system on the tissues of complex neuromodulators involved in various physiological functions such as appetite, pain, reward, motoric control, memory, and cognition (El-Alfy et al., 2016).
Environmental applications
The antibacterial, antidiabetic, antioxidant, antiparasitic, and larvicidal properties of nutmeg have been evaluated before. The outcomes of the analysis revealed that ZnO nanoparticles synthesized from nutmeg could be used as potential candidates for biomedical and environmental applications that are environmentally friendly, nontoxicity, and biocompatibility (Faisal et al., 2021). Another study explored the use of nutmeg as vegetable oil. Vegetable oils have a fat composition that can cause the production of free radicals and eventually damage their oxidative (Aladedunye and Matthäus, 2014). More profound research was conducted on NEO in maintaining oxidative stability in sunflower oil, and this proves that NEO has antioxidant effects (Wang et al., 2019).
Toxicity activities
Extracts of arillus and ethanolic nutmeg show the presence of cytotoxic activity. Arillus extract has a selective cytotoxic effect in inducing apoptosis between cancer and normal cells, so arillus is a potential candidate as a potent chemotherapy agent (Rengasamy et al., 2017). Meanwhile, ethanolic extract in nutmeg showed significant cytotoxic activity against Kato III gastric cancer cells (IC50 = 26.06 g/ml) with the sulforhodamine B assay test (Suthisamphat et al., 2020). However, the study admitted that ethanolic extract in nutmeg could support the potential of arillus, which was used as a preparation component for treating gastrointestinal symptoms. The treatment experiment was carried out on male and female rats given alkaloids on raw nutmeg and concluded that the administration of 4 g/kg or more exhibited abnormal behavior, including hypoactivity, unstable gait, or dizziness that lasted for several hours. The administration of 3 g/kg or less did not give rise to abnormal behavior (Hayfaa et al., 2013). However, the study agreed that the treatment did not lead to death and only excess alkaloids (5.1 g/kg), which caused slight toxicity and was nontoxic. Using nutmeg in high doses and for long periods is not recommended (Anaduaka et al., 2022). Similarly, other articles demonstrated that the lowest concentrations of toxic metals were found in nutmeg samples compared to plants of peppers, thyme, basil, oregano, and black paper (Reinholds et al., 2017). Such effects are associated with myristicin (Carstairs and Cantrell, 2011).
According to data from the Illinois Poison Center (IPC), from January 2001 to December 2011, there were 32 cases in children and adolescents of intentional or unintentional consumption of nutmeg (Ehrenpreis et al., 2014). Accidental cases are caused by a mixture of drugs containing nutmeg and consumed in excess, while in intentional cases, one is by consuming duloxetine, clonazepam, K2 (synthetic cannabinoids), and acetaminophen. Nutmeg was used in a suicide attempt, and another case involved a 16-year-old girl who reported consuming 25 g of nutmeg after reading that nutmeg was a “bowel cleanser” in a popular teen magazine. Overdose of nutmeg use provides serious effects, namely, urinary retention, tremors, and seizures. The literature shows tremors in animals receiving toxic nutmeg doses associated with the anticholinergic effects of nutmeg attributed to myristicin and elemicin (Barceloux, 2009). Further research provides evidence that nutmeg extract works indirectly on the endocannabinoid system by inhibiting the enzymes FAAH and MAGL, which explains the cannabis-like effects of nutmeg (El-Alfya et al., 2019).
Finally, we must acknowledge the limitations of our study in using such an extensive research database. There are many phytochemical and biological studies on nutmeg outside our libraries, and this famous spice plant has been proven to have many benefits that can be studied continuously. Seeing its wide distribution, we suggest further investigating this plant, for example, the phytochemical constituents of nutmeg and its pharmacological properties from different geographical areas or cultivation strategies to increase productivity which are still rare.
CONCLUSION
Nutmeg’s use as an alternative for various ailments has resulted in a massively popular effort to compare nutmeg to different extracts. The available nutmeg literature suggests that this medicinal plant is essential for use in biomedical and environmental applications, such as the treatment of neurodegenerative diseases, disorders of the CNS, atherosclerosis, cancer, chronic inflammation, and pain relief, A. fumigatus infections, gastrointestinal, oral care such as toothpaste including mouthwashes, and environmentally friendly vegetable oils. Alkaloids, tannins, carbohydrates, lignans, neolignans, diphenyl alkanes, phenylpropanoids, terpenoids, alkanes, fatty acids, fatty acid esters, and some minor constituents such as steroids, saponins, triterpenoids, and flavonoids are the main chemical constituents that have been proven in nutmeg. Various subsubjects of nutmeg chemical compounds were found to have significant potential as drug discovery agents. Studies have shown that multiple nutmeg extracts have a wide range of pharmacological activities, such as antioxidant, antibacterial, antimelanogenic, antifungal, anticancer, and cytotoxicity activities. Nutmeg still has its toxic effects, although it tends to be low compared to the side effects of chemical drugs. Therefore, given its versatile usefulness, more in-depth research studies on this plant are warranted.
ACKNOWLEDGMENTS
The author would like to thank the Universitas Muhammadiyah Prof. DR. Hamka for supporting the research.
AUTHOR CONTRIBUTIONS
HYE and SS designed the study. HYE collected the data. SS analyzed the samples. All authors contributed to the drafting of the final manuscript. All authors read and approved the final manuscript.
FINANCIAL SUPPORT
No funding was received for this study.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
REFERENCES
Abourashed EA, El-Alfy AT. Chemical diversity and pharmacological significance of the secondary metabolites of nutmeg (Myristica fragrans Houtt.). Phytochem Rev, 2016; 15:1035–56; doi:10.1007/s11101-016-9469-x
Abutaha N, Al-Keridis LA, Mohamed RAEH, AL-Mekhlaf FA. Potency and selectivity indices of Myristica fragrans Houtt. mace chloroform extract against non-clinical and clinical human pathogens. Open Chem, 2021; 1096–107; doi: 10.1515/chem-2021-0097
Akinboro A, Mohamed K Bin, Asmawi MZ. Antioxidants in aqueous extract of Myristica fragrans (Houtt.) suppress mitosis and cyclophosphamide-induced chromosomal aberrations in Allium cepa L. cells. J Zhejiang Univ B (Biomedicine Biotechnol), 2011; 12:915–22; doi:10.1631/jzus.B1000315
Akinboro A, Mohamed K Bin, Asmawi MZ, Othman S, Ying TH, Maidin SM. Mutagenic and antimutagenic assessment of methanol leaf extract of Myristica fragrans (Houtt.) using in vitro and in vivo genetic assays. Drug Chem Toxicol, 2012; 35; doi:10.3109/01480545.2011.638300
Aladedunye F, Matthäus B. Phenolic extracts from Sorbus aucuparia (L.) and Malus baccata (L.) berries: antioxidant activity and performance in rapeseed oil during frying and storage. Food Chem, 2014; 159:273–81; doi:10.1016/j.foodchem.2014.02.139
Anaduaka EG, Okagu IU, Uchendu NO, Ezeanyika LUS, Nwanguma BC. Hepato-renal toxicity of Myristica fragrans Houtt. (Myristicaceae) seed extracts in rats. J King Saud Univ Sci, 2022; 34:101694; doi:10.1016/j.jksus.2021.101694
Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol Theory Pract, 2005; 8:19–32; doi:10.1080/1364557032000119616
Bahrami A, Moaddabdoost Baboli Z, Schimmel K, Jafari SM, Williams L. Efficiency of novel processing technologies for the control of Listeria monocytogenes in food products. Trends Food Sci Technol, 2020; 96:61–78; doi:10.1016/j.tifs.2019.12.009
Bao H, Muge Q. Anticancer effect of myristicin on hepatic carcinoma and related molecular mechanism. Pharm Biol, 2021; 59:1126–32; doi:10.1080/13880209.2021.1961825
Barceloux DG. Nutmeg (Myristica fragrans Houtt.). Dis Mon, 2009; 55:373–9; doi:10.1016/j.disamonth.2009.03.007
Cao Z, Xia W, Zhang X, Yuan H, Guan D, Gao L. Biomedicine & pharmacotherapy hepatotoxicity of nutmeg?: a pilot study based on metabolomics. Biomed Pharmacother, 2020; 131:110780; doi:10.1016/j.biopha.2020.110780
Carstairs SD, Cantrell FL. The spice of life?: an analysis of nutmeg exposures in California. Clin Toxicol, 2011:177–80; doi:10.3109/15563650.2011.561210
Chatterjee S, Niaz Z, Gautam S, Adhikari S, Variyar PS, Sharma A. Antioxidant activity of some phenolic constituents from green pepper (Piper nigrum L.) and fresh nutmeg mace (Myristica fragrans). Food Chem, 2007; 101:515–23; doi:10.1016/j.foodchem.2006.02.008
Chiu S, Wang T, Belski M, Abourashed EA. HPLC-guided isolation, purification and characterization of phenylpropanoid and phenolic constituents of nutmeg kernel (Myristica fragrans). Nat Prod Commun, 2016; doi:10.1177/1934578X1601100416
Cz?onka S, Str?kowska A, Kairyt? A, Kremensas A. Nutmeg filler as a natural compound for the production of polyurethane composite foams with antibacterial and anti-aging properties. Polym Test, 2020; 86; doi:10.1016/j.polymertesting.2020.106479
Du S, Yang K, Wang C, You C, Geng Z. Chemical constituents and activities of the essential oil from Myristica fragrans against cigarette beetle lasioderma serricorne. Chem Biodivers, 2014; 11:1449–56.
Ehrenpreis JE, Deslauriers C, Lank P, Armstrong PK, Leikin JB. Nutmeg poisonings?: a retrospective review of 10 years experience from the Illinois Poison Center, 2001–2011. Toxicol Investig, 2014: 148–51; doi:10.1007/s13181-013-0379-7
El-Alfy AT, Joseph S, Brahmbhatt A, Akati S, Abourashed EA. Indirect modulation of the endocannabinoid system by specific fractions of nutmeg total extract nutmeg total extract. Pharm Biol, 2016; 54; doi:10.1080/13880209.2016.1194864.
El-Alfya AT, Abourasheda EA, Patel C, Mazharib N, An H, Jeon A. Phenolic compounds from nutmeg (Myristica fragrans Houtt.) inhibit the endocannabinoid-modulating enzyme fatty acid amide hydrolase. J Pharm Pharmacol, 2019; 71:1879–89; doi:10.1111/jphp.13174
El-Sayed KK, Sherif RM, Kamal A. Chemical composition and bio-efficacy of essential oils isolated from seeds of Anethum graveolens L., leaves of Thymus vulgaris L., and nuts of Myristica fragrans Houtt. Against Callosobruchus maculatus (Fab.) (Coleoptera: Bruchidae). J Essent Oil Bear Plants, 2022; doi:10.1080/0972060X.2021.2016498
Faisal S, Jan H, Shah SA, Shah S, Khan A, Akbar MT. Green synthesis of zinc oxide (ZnO) nanoparticles using aqueous fruit extracts of Myristica fragrans?: their characterizations and biological and environmental applications. ACS Publ, 2021; doi:10.1021/acsomega.1c00310
Farag MA, Mohsen E, El-Gendy AENG. Sensory metabolites profiling in Myristica fragrans (Nutmeg) organs and in response to roasting as analyzed via chemometric tools. LWT, 2018; 97:684–92; doi:10.1016/j.lwt.2018.08.002
Grant MJ, Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies. Health Info Libr J, 2009; 26:91–108; doi:10.1111/j.1471-1842.2009.00848.x
Gupta AD, Bansal VK, Babu V, Maithil N. Chemistry, antioxidant and antimicrobial potential of nutmeg (Myristica fragrans Houtt). J Genet Eng Biotechnol, 2013; 11:25–31; doi:10.1016/j.jgeb.2012.12.001
Ha MT, Vu NK, Tran TH, Kim JA, Woo MH, Min BS. Phytochemical and pharmacological properties of Myristica fragrans Houtt: an updated review. Arch Pharm Res, 2020; 43:1067–92; doi:10.1007/s12272-020-01285-4
Hayfaa AAS, Sahar AAMAS, Awatif MAS. Evaluation of analgesic activity and toxicity of alkaloids in Myristica fragrans seeds in mice. J Pain Res, 2013; 6:611–5; doi:10.2147/JPR.S45591
Hoda S, Vermani M, Joshi RK, Shankar J, Vijayaraghavan P. Anti-melanogenic activity of Myristica fragrans extract against Aspergillus fumigatus using phenotypic based screening. BMC Complement Med Ther, 2020; 20:67; doi:10.1186/s12906-020-2859-z
Iyer M, Gujjari AK, Gowda V, Angadi S. Antifungal response of oral?associated candidal reference strains (American Type Culture Collection) by supercritical fluid extract of nutmeg seeds for geriatric denture wearers?: an in vitro screening study. J Indian Prosthodont Soc, 2017; 17:267–72; doi:10.4103/jips.jips
Johnson-Arbor K, Smolinske S. Stoned on spices: a mini-review of three commonly abused household spices. Clin Toxicol, 2021; 59:101–5; doi:10.1080/15563650.2020.1840579
Li Changhong, Zhang K, Pan G, Ji H, Li Chongyang, Wang X, Hu X, Liu R, Deng L, Wang Y, Yang L, Cui H. Dehydrodiisoeugenol inhibits colorectal cancer growth by endoplasmic reticulum stress-induced autophagic pathways. J Exp Clin Cancer Res, 2021; 9:1–15; doi:10.1186/s13046-021-01915-9
Li F, Yang X, Krausz KW, Nichols RG, Xu W, Patterson AD, Gonzalez FJ. Modulation of colon cancer by nutmeg. J Proteome Res, 2019; 14:1937–46; doi:10.1021/pr5013152.Modulation
Luo L, Liang H, Liu L. Myristicin regulates proliferation and apoptosis in oxidized low-density lipoprotein-stimulated human vascular smooth muscle cells and human umbilical vein endothelial cells by regulating the PI3K / Akt / NF-κ B signalling pathway. Pharm Biol, 2022; 60:56–64; doi:10.1080/13880209.2021.2010775
Lv Q-Q, Yang X-N, Yan D-M, Liang W-Q, Liu H-N, Yang X-W, Li F. Metabolic profiling of dehydrodiisoeugenol using xenobiotic metabolomics. J Pharm Biomed Anal, 2017; 145:725–33; doi:10.1016/j.jpba.2017.07.045
Matulyte I, Jekabsone A, Jankauskaite L, Zavistanaviciute P, Sakiene V, Bartkiene E, Ruzauskas M, Kopustinskiene DM, Santini A, Bernatoniene J. The essential oil and hydrolats from myristica fragrans seeds with magnesium aluminometasilicate as excipient: antioxidant, antibacterial, and anti-inflammatory activity. Foods, 2020; 9; doi:10.3390/foods9010037
Mehmood A, Zhao L, Wang C, Nadeem M, Raza A, Ali N, Shah AA. Management of hyperuricemia through dietary polyphenols as a natural medicament: a comprehensive review. Crit Rev Food Sci Nutr, 2019; 59:1433–55; doi:10.1080/10408398.2017.1412939
Morikawa T, Hachiman I, Ninomiya K, Hata H, Sugawara K, Muraoka O, Matsuda H. Degranulation inhibitors from the arils of Myristica fragrans in antigen-stimulated rat basophilic leukemia cells. J Nat Med, 2018; 72:464–73; doi:10.1007/s11418-017-1170-x
Muchtaridi, Subarnas A, Apriyantono A, Mustarichie R. Identification of compounds in the essential oil of nutmeg seeds (Myristica fragrans Houtt.) that inhibit locomotor activity in mice. Int J Mol Sci, 2010; 11:4771–81; doi:10.3390/ijms11114771
Naeem N, Rehman R, Mushtaq A, Ghania B. Nutmeg: a review on uses and biological properties. Int J Chem Biochem Sci, 2016; 9:107–0.
Nikolic V, Nikolic L, Dinic A, Gajic I, Urosevic M, Stanojevic L, Danilovic B. Chemical composition, antioxidant and antimicrobial activity of nutmeg (Myristica fragrans Houtt.) seed essential oil. J Essent Oil Bear Plants, 2021; 24:218–7; doi:10.1080/0972060X.2021.1907230
Oh YS, Shin SY, Kim S, Lee KH, Shin JC, Park KM. Comparison of antiaging, anti-melanogenesis effects, and active components of Raspberry (Rubus occidentalis L.) extracts according to maturity. J Food Biochem, 2020; 44:1–0; doi:10.1111/jfbc.13464
Olaleye MT, Akinmoladun AC, Akindahunsi AA. Antioxidant properties of Myristica fragrans (Houtt) and its effect on selected organs of albino rats. Afr J Biotechnol, 2006; 5:1274–8; doi:10.4314/ajb.v5i15.43113
Peters MDJ, Marnie C, Tricco AC, Pollock D, Munn Z, Alexander L, McInerney P, Godfrey CM, Khalil H. Updated methodological guidance for the conduct of scoping reviews. JBI Evid Synth, 2020; 18:2119–26; doi:10.11124/JBIES-20-00167
Piaru SP, Mahmud R, Ismail S. Studies on the phytochemical properties and brine shrimp toxicity of essential oil extracted from Myristica fragrans Houtt. (Nutmeg). J Essent Oil Bear Plants, 2013; 5026; doi:10.1080/0972060X.2012.10644019
Pilevar Z, Bahrami A, Beikzadeh S, Hosseini H, Jafari SM. Migration of styrene monomer from polystyrene packaging materials into foods: characterization and safety evaluation. Trends Food Sci Technol, 2019; 91:248–61; doi:10.1016/j.tifs.2019.07.020
Plaingam W, Sangsuthum S, Angkhasirisap W. Kaempferia parviflora rhizome extract and Myristica fragrans volatile oil increase the levels of monoamine neurotransmitters and impact the proteomic profiles in the rat hippocampus?: mechanistic insights into their neuroprotective effects. J Tradit Complement Med, 2017; 7:538–2; doi:10.1016/j.jtcme.2017.01.002
Quigley DTG, MacNamara L, Gainey PA. First records of stranded nutmegs Myristica fragrans Houttuyn, 1774 (Magnoliales: Myristicaceae) on the Irish Coast and a review of North Atlantic records. Bull Irish Biogeogr Soc, 2020; 44:178–88.
Reinholds I, Pugajeva I, Bavrins K, Kuckovska G. Mycotoxins, pesticides and toxic metals in commercial spices and herbs. Food Addit Contam Part B, 2017; 10:5–14; doi:10.1080/19393210.2016.1210244
Rengasamy G, Venkataraman A, Veeraraghavan VP, Jainu M. Cytotoxic and apoptotic potential of Myristica fragrans Houtt. (mace) extract on human oral epidermal carcinoma KB cell lines. Brazilian J Pharm Sci, 2017; 1–8; doi:10.1590/s2175-97902018000318028 Article
Rizwana H, Bokahri NA, Alkhattaf FS, Albasher G, Aldehaish HA. Antifungal, antibacterial, and cytotoxic activities of silver nanoparticles synthesized from aqueous extracts of mace-arils of Myristica fragrans. J Mol, 2021; doi:10.3390/ molecules26247709
Sarrami-Foroushani P, Travaglia J, Debono D, Clay-Williams R, Braithwaite J. Scoping meta-review: introducing a new methodology. Clin Transl Sci, 2015; 8:77–81; doi:10.1111/cts.12188
Seneme EF, Carla D, Marcela E, Silva R, Edwirges Y, Franco M, Longato GB. Pharmacological and therapeutic potential of myristicin: a literature review. J Mol, 2021:1–15; doi:10.3390/molecules26195914
Shafiei Z, Shuhairi NN, Md Fazly Shah Yap N, Harry Sibungkil CA, Latip J. Antibacterial activity of Myristica fragrans against oral pathogens. Evid Based Complement Altern Med, 2012:825362; doi:10.1155/2012/825362
Sharma MV, Armstrong JE. Pollination of Myristica and other nutmegs in natural populations. Trop Conserv Sci, 2013; 6:595–607; doi:10.1177/194008291300600502
Singh RH. The nutmeg and spice industry in Grenada?: innovations and competitiveness. Sci Technol, 2003. Available via https://www.researchgate.net/publication/275771715
Sivathanu S, Sampath S, David HS. Myristicin and phenytoin toxicity in an infant. Case Rep, 2014:2013–5; doi:10.1136/bcr-2013-203000
Sokmen A, Gulluce M, Akpulat HA, Daferera D, Tepe B, Polissiou M, Sökmen A. The in vitro antimicrobial and antioxidant activities of the essential oils and methanol extracts of endemic Thymus spathulifolius. Food Control, 2004; 15:627–34; doi:10.1016/j.foodcont.2003.10.005
Suhr KI, Nielsen PV. Antifungal activity of essential oils evaluated by two different application techniques against rye bread spoilage fungi. J Appl Microbiol, 2003; 94:665–74.
Suthisamphat N, Dechayont B, Phuaklee P, Prajuabjinda O, Vilaichone R, Itharat A, Mokmued K, Prommee N. Anti-Helicobacter pylori, anti-inflammatory, cytotoxic, and antioxidant activities of mace extracts from Myristica fragrans. Evid Based Complement Altern Med, 2020; doi:10.1155/2020/7576818
Tallei TE, Kolondam BJ. DNA barcoding of sangihe nutmeg (Myristica fragrans) using matk gene. Hayati J Biosci, 2015; 22:41–7; doi:10.4308/hjb.22.1.41
Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, Moher D, Peters MDJ, Horsley T, Weeks L, Hempel S, Akl EA, Chang C, McGowan J, Stewart L, Hartling L, Aldcroft A, Wilson MG, Garritty C, Lewin S, Godfrey CM, Macdonald MT, Langlois EV, Soares-Weiser K, Moriarty J, Clifford T, Tunçalp Ö, Straus SE. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med, 2018; 169:467–73; doi:10.7326/M18-0850
Wang D, Dong Y, Wang Q, Wang X, Fan W. Limonene, the compound in essential oil of nutmeg displayed antioxidant effect in sunflower oil during the deep-frying of Chinese Maye. Food Sci Nutr, 2019; 8:511–20; doi:10.1002/fsn3.1333
Warsito MF. A review on chemical composition, bioactivity, and toxicity of Myristica fragrans Houtt. Essent Oil Indones J Pharm, 2021; 32:304–13.
Yu L, Perret J, Davy B, Wilson J, Melby CL. Antioxidant properties of cereal products. J Food Sci, 2002; 67:2600–3; doi:10.1111/j.1365-2621.2002.tb08784.x
Zálešák F, Bon DJYD, Pospíšil J. Lignans and neolignans: plant secondary metabolites as a reservoir of biologically active substances. Pharmacol Res, 2019; 146:104284; doi:10.1016/j.phrs.2019.104284
Zhang WK, Tao S, Li T, Li Y, Li X, Cong R, Ma FL, Wan CJ. Nutmeg oil alleviates chronic inflammatory pain through inhibition of COX-2 expression and substance P release in vivo. Food Nutr Res, 2016:6628; doi:10.3402/fnr.v60.30849