INTRODUCTION
The traditional Indian medical system is called Ayurveda. There is a long history of disease management in Ayurvedic practice dating back about 3000 years (Hankey, 2001). The Ayurvedic healing process relies heavily on plant-based preparations (Sastri, 2002). In Ayurvedic medicine, approximately 90% of the preparations are derived from plants (Kumar et al., 2017). There are many Asia’s subtropical regions, including China and India, where Curculigo orchioides grows as a perennial herb of the Amaryllidaceae family (Bafna and Mishra, 2005; Jiao et al., 2009; Wang et al., 2012). Curculigo orchioides Gaertn, of the Amaryllidaceae family has different names such as Golden Eye Grass, Talamuli, Kalimusli, Nilappani, and Nilapanaiin English, Sanskrit, Hindi, Malayalam, and in Tamil, respectively (Joy et al., 2004).
Originally native to India, C. orchioides occur everywhere, especially in rocky areas, especially at sea level and up to 2,300 m above the sea level (Mehta and Nama, 2014). Tonic medicine has been used for centuries with the rhizome of C. orchioides by the Chinese since the Tang Dynasty for the maintenance of health, energy, and nourishment of renal and hepatic systems. The root of C. orchioides was commonly used in the treatment of impotence, limb limping, lumbar and knee joint arthritis, and diarrheal water (Chauhan et al., 2010). Jaundice, asthma, urinary and skin diseases, and bladder and kidney infections were treated with C. orchioides in the Ayurvedic System of Medicines (Khare, 2007).
A variety of secondary metabolites are found in the genus Curculigo plants. Ten species: their chemistry and pharmacology have been studied to date. These species include Curculigo capitulata, Curculigo sinensis, Curculigo crassifolia, C. orchioides, Curculigo breviscapa, Curculigo gracilis, Curculigo recurvata, Curculigo glabrescens, Curculigo pilosa, and Curculigo latifolia. The main compounds found in Curculigo species include phenols, phenolic glucosides, terpenoids, and norlignans (Nie et al., 2013). Curculigo plants are increasingly studied phytochemically due to their traditional uses. This genus of plants contains more than 110 compounds, among which are as follows: phenolic glycosides and phenols (Chang and Lee, 1998; Xu and Xu, 1992; Zuo et al., 2010), lignan glycosides and lignans (Li, 1559; Li et al., 2005a, 2005b; Wang et al., 2008; Zhu et al., 2010), triterpenoid glycosides and triterpenes (Xu et al., 1992; Yokosuka et al., 2010; Zuo et al., 2012), eudesmanes, flavones (Tiwari and Mishra, 1976), alkaloids (Li et al., 2005a, 2005b), and other constituents. In China, C. orchioides is considered as a supplemental health product in the form of tea bags and alcoholic beverage (Liu et al., 2022). Curculigo orchioides is reported to have the effects of dissipating carbuncle, strengthening muscles and bones, dispelling cold and dampness, tonifying kidney yang, benefiting essence and blood, immunoregulation, hepatoprotective and neuroprotective activities (Fang et al., 2020). These plants are most likely characterized by norlignans, phenol glycosides, and triterpenoids as their major constituents. Bio-active compounds and extracts of Curculigo plants exhibited a variety of activities including antidiabetic, immunostimulatory, anti-oxidant, radical scavenging, mast cell stabilization, sweet-tasting and taste-modifying, estrogenic and sexual behaviour-modifying, anti-inflammatory, antihistaminic, antidepressant, antitumor, anti-asthmatic, anti-osteoporotic, neuroprotective, nephroprotective, antiarthritic, vasoconstrictor, anti-microbial, hepatoprotective, antistress activity, and adaptive activity (Table 1) (Wang et al., 2021). By using C. orchioides embryos for the green fabrication of gold nanoparticles, an environmentally friendly method for maintaining medicinal plants and preventing them from being overutilized was conducted (Thamilchelvan et al., 2023).
PHYTOCHEMISTRY
Curculigo plants have yielded about 111 secondary metabolites, 3 proteins, and 2 polysaccharides so far. A number of compounds are isolated from extracts of Curculigo species which includes phenolic glycosides, phenols, lignan glycosides, lignans, triterpenoid glycosides, triterpenes, polysaccharides such as COPb-1 and COPf-1, flavones, aliphatic compounds, alkaloids, eudesmanes, and bioactive proteins such as neoculin, curculin, and β-amylase (Goyal and Kabra, 2020; Nie et al., 2013). Phytochemicals such as alkaloids, phenols, flavonoids, and tannins were present in the extracts of methanol, ethanol, and chloroform but were found to be absent in the ethyl acetate extract of C. orchioides (Saxena, 2022). Curculigo orchioides rhizome methanolic extracts were found to contain alkaloids, carbohydrates, steroids, saponins, phenols, tannins, and flavonoids (Agrahari et al., 2010; Asif, 2012). Curculigo orchioides rhizome extracts contain an alkaloid lycorine, sterols including sitosterol, sapogenin, and flavone glycoside 5,7- dimethoxy glucopyranoside. Flavonoids include 5,7-dimethoxy glucopyranoside, and fatty acids such as linolenic, palmitic, behenic, arachidic, and oleic acids (Mehta et al., 1980). O-acetyl-glucomannan COP90-1, a compound soluble in water (Wang et al., 2017) and COP70-3, a homogeneous heteropolysaccharide compound (Wang et al., 2019) were found in the rhizomes of C. orchioides. In a preparative reversed-phase liquid chromatography, levoglucosan was obtained (Niu et al., 2020). The primary metabolites of Curculigo plants are phenolic compounds. Two phenolic glucosides named curculigoside H and orcinoside I, along with 10 known phenolic glucosides such as curculigoside A, B, C, and G, orcinol glucoside (OG) B, benzyl-O-β-D-glucopyranoside, 3-hydroxy-5-methylphenol-1-O-[β-glucopyranosyl-(1-6)-β-D-glucopyranoside], glucosyringic acid, 3-hydrox-5-methyl-phenol-1-O-[β-apiosyl-(1-6)-β-glucopyranoside], and OG were isolated from C. orchioides rhizomes (Wang et al., 2013, 2014) (Fig. 1). Four other known phenolic compounds, including orcinoside I and J, have been proclaimed to be new heterocyclic phenolic derivatives, including 3-(4-hydroxy-3-methoxyphenyl) propane-1,2-diol, 3-(4-hydroxy-3,5-dimeth-oxyphenyl) propane-1,2-diol, piperoside, 4-ally-2, and 6-dimeoxy phenol glucoside (Chen et al., 2017).
Curculigo rhizomes powdered and dried were used to isolate 3,5-Dihydroxy-4-methoxybenzoic acid and p-Hydroxycinnamic acid (Niu et al., 2020). Curculigo orchioides contains natural and rare chlorinated compounds called cuculligines. It was discovered that the rhizome of C. orchioides contains three chlorophenolic glucosides, B, C, and curculigine D (Cao et al., 2009; Xu et al., 1987; Xu and Xu, 1992). The C. orchioides rhizomes were collected for the purpose of obtaining 11 chlorophenolic glucosides, including curculigine E–G, I, and K–O (Wang et al., 2013, 2014, 2018). The C. orchioides rhizomes contain two new chlorophenolic glucosides named ascurculigine P and Q (Deng et al., 2021). Terpenoids are second metabolites produced by Curculigo species. There is evidence that C. orchioides produces cycloartane-type triterpenoid ketone in its rhizomes (Jiao et al., 2013) (3S,5R,6S,7E,9R)- megatigma-7-ene-3,5,6,9-tetrol, actinidioionoside, (6S,9R)-roseoside, (−)- angelicoidenol-2-O-β-D-glucopyranoside, (−)-angelicoidenol-2-O-β-apiofuranosyl-(1→6)-β-D-gluco-pyranosidetetillapyrone[(7R,9S,10R)-3-methyl-5-(4-hydroxyl-5-hydroxylmethyltetrahydrofuryl)]-6-hydroxypyran-2-one are six terpenoids content of C. orchioides (Zhang et al., 2019a, 2019b). Rhizomes of C. orchioides contain eight cyclodipeptides, such as cyclo-(L-Ala-L-Tyr), cyclo- (Gly-D-Val), cyclo-(LeuAla), cyclo-(Val-Ala), cyclo-(L-Ser-L-Phe), cyclo-(LeuThr), cyclo-(S-Pro-R-Leu), and cyclo- (Leu-Ser), which are previously known (Chen et al., 2017). The aqueous leaf extract of the plant was observed to contain different alkaloids and phenolic compound from the post preliminary phytochemical analysis using Fourier-transform infrared spectrophotometer (Umar et al., 2021). The presence of alkaloids, phenols, and saponins could be a plausible explanation for the observed toxic effects of C. orchioides’ AL extract in organismal-level toxicity in Drosophila (Kushalan et al., 2022a, 2022b).
PHARMACOLOGICAL ACTIVITY
Immunomodulatory activity
Adaptation to hypoxia and high temperatures is improved by C. orchioides ethanol extract. Extracts from C. orchioides have been found to be sedative, anticonvulsant, and androgen-like. In mice, immunological activity increased as well (Chen et al., 1989). Curculigo orchioides phenolic glucosides increased haemagglutination titer and delayed type hypersensitivity (DTH) response (Lakshmi et al., 2003). Although polysaccharides stimulate splenocyte proliferation, they do not affect thymocyte proliferation. Using ConA-induced splenocyte proliferation as a model, polysaccharides demonstrated an inhibitory effect on thymocyte and spleenocyte proliferation in vitro. Mice with immunosuppression had larger thymuses and spleens as a result of these effects (Zhou et al., 1996).
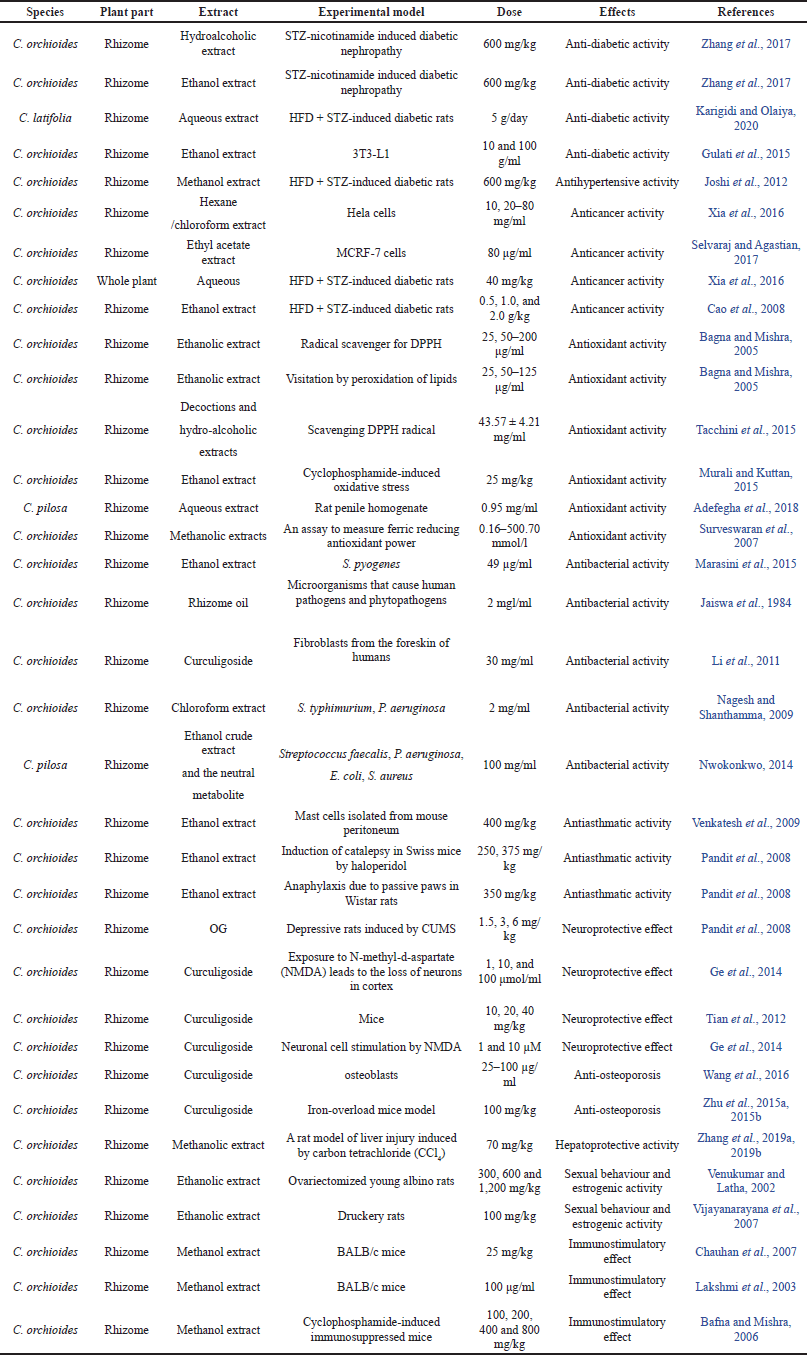 | Table 1. Effect of C. orchioides in many metabolic ailments. [Click here to view] |
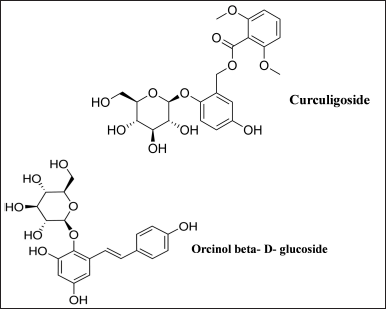 | Figure 1. Chief active constituents of C. orchioides. [Click here to view] |
It has been suggested that the methanolic extract of C. orchioides (MECO) Gaertn’s could be used to prevent the cytotoxic effects of drugs. When the extract was administered to normal mice or cyclophosphamide-induced immunosuppressed mice, humoral antibodies, DTH, and leukocytes increased depending upon the dose (Bafna and Mishra, 2006). One of the contents of C. orchioides rhizomes, Curculigo saponin, acycloartane-type triterpene saponin, enhanced the number of lymphocytes of the spleen remarkably in mice without affecting antibody production (Lacaille-Dubois and Wagner, 1996). Polysaccharides resulted in increased spleen and thymus indexes in normal mice, along with increased hemolytic index and thicker plantar tissue in serum; Mice immune function is enhanced by polysaccharides, according to these results (Ji, 2011).
Antioxidant activity
DPPH (2,2-diphenyl-1-picryl-hydrazyl-hydrate) and nitric oxide radicals are scavenged effectively by methanol extracts of C. orchioides rhizomes, but lipid peroxidation is moderately effective (Bafna and Mishra, 2005). In addition to DPPH testing, ferric reducing ability of plasma testing as well as 2,2’-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) testing has also been performed on C. orchioides to confirm its antioxidant activity (Surveswaran et al., 2007). DPPH, reducing power, and phosphomolybdenum assays were used to assess C. orchioides’ antioxidant potential ethanolic extract of C. orchioides root demonstrated significant anti-free radical activity, reducing power, and antioxidant properties compared with a reference standard, gallic acid (Table 1) (Ratnam et al., 2013). Methanolic extract of elevated rhizomes treated hepatotoxic rats showed increased levels of antioxidant enzymes such as glutathione transferase (Venukumar and Latha, 2002). Methanolic root extract showed DPPH radical scavenging activity (Kushalan et al., 2022a, 2022b). A major component of C. orchioides’ antioxidant activity is phenolic compounds (Wu et al., 2005).
Anti-inflammatory activity
Curculigoside A which is the vital component of C. orchioides, reduced paw swelling and arthritis index in mice significantly and decreased serum IL-1β, TNF-α, IL-6, and PGE2 levels, decreased malondialdehyde (MDA) and increased serum superoxide dismutase activity very effectively downregulated NF-κB/NLRP3 pathways in Freund’s complete adjuvant-induced rats with adjuvant arthritis (Ding et al., 2016). Type II collagen-induced arthritis rat showed arthritis scores and paw swelling inhibition, serum pro-inflammatory factor levels of IL-10, TNF-α, IL-17A, IL-6, IL-12, and IL-1β reduced, the expression of JAK3, STAT3, and JAK1 down-regulated and NF-κB p65 and IκBup-regulated because of the active compound curculigoside A (Tan et al., 2019). Carrageen-induced paw oedema in rats was significantly reduced by C. orchioides rhizome gel formulation (Dode et al., 2009).
Mice peritoneal mast cells were significantly inhibited from degranulating by an extract of C. orchioides rhizome in ethanol and mice exposed to 48/80-induced systemic anaphylaxis. Anti-inflammatory properties of C. orchioides are attributed to their inhibitory effects on mast cell degranulation and mast cell-derived immediate-type allergic reactions (Venkatesh et al., 2009).
Estrogenic activity
Total glucosides from C. orchioides were found to increase the thymus, uterus, and spleen indices, testosterone levels, and estrogen levels, and decrease luteinizing hormone levels in perimenopause model mice (Cao et al., 2016; Miao et al., 2017). Curculigo orchioides also alleviate streptozotocin-induced hyperglycaemia in male rats, improving sexual dysfunction as well. The effects of the treatment were observed in male sexual behaviour, penile erection index, seminal fructose content, and sperm counting the test samples (Thakur et al., 2012). It increases steroid synthesis and restores sexual function by enhancing the spermatogenesis process. It could also facilitate hormone absorption into the gonads if C. orchioides was administered (Chauhan et al., 2007).
Anti-osteoporosis activity
The rhizomes of C. orchioides are asserted to strengthen bones and tendons (Cao et al., 2008). The proliferation of bone marrow stromal cells was enhanced by 100 mM curculigoside, osteogenic genes were enhanced, and osteoprotegerin secretion was increased (Shen et al., 2013). A study indicates that cuculigoside A chemical compound inhibits the inflammatory cytokines TNF-a, IL-1a, IL-6, and COX-2 production by rat calvarial osteoblasts induced with dexamethasone and regulates osteoblast COX-2 expression, proliferation, and differentiation (Zhu et al., 2015a, 2015b).
The chlorophenolic glucosides (Curculigine M, Curculigine N, and Curculigine O) isolated from the dried rhizomes of C. orchioides Gaertn showed moderate effect on osteoblast proliferation against MC3T3-E1 cell line by using MTT assays (Wang et al., 2018). A novel homogeneous heteropolysaccharide, COP70-3, was isolated and purified from the crude polysaccharide (CO70) isolated from the rhizomes of C. orchioides (0.94 and 1.87 nM) significantly improved the osteogenic mineralization rate and has favorable anti-osteoporosis activity in vitro (Wang et al., 2018). Curculigoside was able to alleviate bone loss induced by oxidative stress resulting from iron overload, suggesting its potential use for the treatment of primary osteoporosis and bone loss in iron-overload–related diseases (Zhang et al., 2019a, 2019b). The major bioactive component of found in the plant’s rhizomes, curculigoside, a phenolic glycoside, exhibits neuroprotective and anti-osteoporotic properties (Zhu et al., 2021). COP50-4, a crude polysaccharide (CO50) from C. orchioides shows great potential for the treatment of osteoporosis (Yu et al., 2022).
Antidepressant activity
The immobility time of the forced swimming test, osmotic fragility test, and tail suspension test was significantly reduced by curculigoside treatment. In the hippocampus of chronic mild stress rats, serotonin, dopamine, and norepinephrine levels significantly increased along with brain-derived neurotrophic factor (BDNF) protein expression following the treatment. There is evidence that curculigoside can treat depression in this way (Wang et al., 2016).
Neuroprotective activity
Acetylcholinesterase was effectively inhibited by the extracts of C. orchioides rhizomes, suggesting their potential use in the treatment of Alzheimer’s disease (Pratap and Shantaram, 2019). Curculigoside remarkably reduced NMDA-induced loss of neuron cells, apoptosis, necrosis, excitotoxicity, and reactive oxygen species (ROS) production in cultured cortical neurons. The inhibitory properties of curculigoside in cultured cortical neurons may cause the production of intracellular ROS to be reduced and apoptosis may be inhibited. When neurons are exposed to NMDA-induced neuronal excitoxicity, curculigoside prevents them from dying and reduces their apoptosis and necrosis (Tian et al., 2012).
Spectrophotometry and autobiography were used to evaluate the anti-acetylcholinesterase activity of C. orchioides extracts in vitro. The anti-acetylcholinesterase role of rhizome extract methanol in the treatment of Alzheimer’s disease appears to be explained by its inhibition of the acetylcholinesterase enzyme (Pratap, 2020). Using curculigoside A, rats with Alzheimer’s can be effectively treated because it inhibits apoptosis in hippocampal neurons and reduces cellular damage (Li et al., 2019). In vitro, curculigoside A modulated VCAM-1/Egr-3/CREB/VEGF signalling in cerebroendothelial cells, providing stroke and brain injury therapies by neurovascular repair (Zhu et al., 2015).
It was demonstrated that OG decreased over activity of the hypothalamic-pituitary–adrenal axis and reduced depressive behavior in chronic unpredictable mild stress rats by up-regulating BDNF expression and phosphorylating ERK1/2 (Ge et al., 2014). Mice showed reduced anxiety-like behaviors after administration of OG, but no sedation was seen (Wang et al., 2016).
Hepatoprotective activity
Aspartate aminotransferase, alkaline phosphatase, gamma-glutamyl transpeptidase, and gamma-glutamyl transpeptidases were reduced in rats exposed to carbon tetrachloride. Food consumption and weight gain were increased by MECO rhizomes. In addition to lowering liver and serum protein levels, serum lipids, cholesterol, and phospholipids were normalized. These studies showed that C. orchioides rhizome has hepatoprotective properties (Venukumar and Latha, 2002).
Nephroprotective activity
Treatment of cyclophosphamide-induced hepatotoxicity and intestinal toxicities with C. orchioides does not compromise cyclophosphamide’s chemotherapeutic efficacy. When administered along with cyclophosphamide, the whole plant extract of C. orchioides significantly decreased serum creatinine levels and blood urea nitrogen levels. Induced urotoxicity and nephrotoxicity by cyclophosphamide are alleviated by C. orchioides (Murali and Kuttan, 2016)
Antidiabetic activity
Alloxan-induced diabetic rats and glucose-loaded diabetic rats were shown to be antihyperglycemic with aqueous and ethanol extracts (Chauhan et al., 2007). Using streptozotocin–nicotinamide-induced diabetic nephropathy rats, extracts of C. Orchiodies using ethanol and hydroalcoholreduced hyperglycemia-induced lipid changes, oxidative stress, and renal dysfunction (urea, creatinine, and andalbumin) (Singla and Singh, 2020). Curculigo orchioides was shown to inhibit adipogenesis and enhance glucose uptake in 3T3-L1 adipocytes in an ethanolic extract in a cell-based assay (Gulati et al., 2015).
Anti-microbial activity
As well as being antimicrobial against Bacillus anthracis and Bacillus subtilis, oil of C. orchioides rhizomes inhibited Fusarium solani, Salmonella newport, Staphylococcus aureus, Salmonella pullorum, and Aspergillus flavus, Fusarium moniliforme, and Cladosporium species (Jaiswa et al., 1984). The C. orchioides extract prepared by steam distillation process showed antibacterial activity significantly against several Gram-positive bacteria (Staphylococcus epidermidis and S. aureus) and Gram-negative bacteria (Salmonella typhimurium, Pseudomonas aeruginosa, and Escherichia coli). Antiseptic properties make the extract ideal for preventing bacterial infections (Nagesh and Shanthamma, 2009).
With a minimum inhibitory concentration value of 49 g/ml, C. orchioides alcohol extract inhibited methicillin-resistant P. aeruginosa, whereas Gram-negative bacteria were not affected (Marasini et al., 2015). Curculigo orchioides leaf extracts contain phytochemical-loaded silver nanoparticles which are effective against S. aureus and P. aeruginosa, but less effective against Klebsiella pneumonia and E. coli (Perumal et al., 2017).
Anti-asthmatic activity
Goat tracheal chains and guinea pig ileum are relaxed by C. orchioides ethanol extract. Curculigo orchioides ethanol extract significantly reduced bronchoconstriction and passive paw anaphylaxis in guinea pigs, rats, and mice with haloperidol-induced catalepsy, suggesting an antiasthmatic effect (Pandit et al., 2008).
Anti-stress activity
In forced swimming and tail suspension tests, Curculigo orichioides ethanol extract at 200 mg/kg reduced immobility times. It increased mobility in actophotometer-based tests. Ethanolic extract of C. orchioides’ rhizomes increased resistance to heat [50 and 70 infrared (IR) units] in IR testing and rotarod testing. It provides strong evidence that ethanolic extracts have antistress activity (Chauhan et al., 2021).
Anti-cancer activity
Aqueous fresh root extract and methanolic dried root extract showed significant cytotoxicity activity on the human lung adenocarcinoma NCI-H-522 cancer cell line. The cytotoxicity may be attributed to the alkaloids and phenols present in it (Aloysius et al., 2020). A new chlorophenolic glucosides curculigines P, isolated from the dried rhizomes of C. orchioides showed the most potent inhibitory effect on 5α-reductase activity by a HaCaT-based bioassay and, hence, may be useful in benign prostatic hyperplasia (Deng et al., 2021).
Ethyl acetate fractions of C. orchioides Gaertn down regulated the levels of antiapoptotic Bcl-2 expression and upregulated the expression of apoptotic proteins caspase-3 and caspase-8 through an intrinsic ROS-mediated mitochondrial dysfunction pathway (Hejazi et al., 2018). The plant extract when administered in combination with cyclophosphamide enhanced the anticancer properties of cyclophosphamide and ameliorated its toxic side effects (Murali and Kuttan, 2015). Silver nanoparticles using C. orchioides rhizome extracts showed efficacy against human breast cancer cell line (MDA-MB-231) after 48 hours of incubation (Kayalvizhi et al., 2016). Polysaccharides from C. orchioides showed a significant anti-tumor effect on cervical cancer in vivo and in vitro by enhancement of immune function and induction of apoptosis (Xia et al., 2016).
Anti-gout activity
Two heterocyclic phenolic derivatives, orcinosides I and J, displayed xanthine oxidase inhibitory activities with IC50 values 0.25 and 0.62 mM, respectively. Hence, they may have anti-gout effect (Chen et al., 2017).
Anti-hypertensive activity
MECO root possesses antihypertensive activity by inhibiting angiotensin-converting enzyme in deoxycorticosterone acetate salt-induced hypertensive rats (Joshi et al., 2012).
Anti-malarial activity
Silver nanoparticles using C. orchioides rhizome extracts showed the highest mortality rate against the malarial vectors such as Anopheles subpictus and Culex quinquefasciatus (Kayalvizhi et al., 2016).
CONCLUSION
This review aims to summarize the existing phytochemistry and pharmacological activities of plant C. orchioides which is a perennial herb, belonging to the family Amaryllidaceae. The content gives a brief proof of the traditional uses of this plant. The plant is reported to show immuno-modulatory, anti-oxidant, hepatoprotective, neuroprotective nephrpprotective, anti-inflammatory, anti-gout, anti-arthritis, anti-cancer, anti-microbial, anti-bacterial, anti-malarial, anti-diabetic, anti-stress, and antihypertensive activities. These effects may be attributed to the anti-oxidant principles present in them. Many authors and researchers reported the phytochemical, pharmacological, and toxicological results which may provide suitable data for further scientific research.
AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Adefegha SA, Oyeleye SI, Oboh G. African crocus (Curculigo pilosa) and wonderful kola (Buchholzia coriacea) seeds modulate critical enzymes relevant to erectile dysfunction and oxidative stress. J Complement Integr Med, 2018; 15(4).
Agrahari AK, Panda SAK, Meher A, Padhan AR, Khaliquzzama M. Phytochemical screening of Curculigo Orchioides Gaertn. root tubers. J Chem Pharm Res, 2010; 2(2):107–11.
Aloysius KS, Sharanya K, Kini S, Milan GR, Hegde S. Phytochemical analysis of Curculigo orchioides and its cytotoxic effect on lung adenocarcinoma cancer cell line (NCI-H522). Med Plants, 2020; 12(3):400–4.
Asif M. A review on phytochemical and ethnopharmacological activities of Curculigo orchioides. J Pharm Sci, 2012; 39(3–4):1–10.
Bafna AR, Mishra SH. Immunostimulatory effect of methanol extract of Curculigo orchioides on immunosuppressed mice. J Ethnopharmacol, 2006; 104:1–4.
Bafna AR, Mishra SH. In vitro antioxidant activity of methanol extract of rhizomes of Curculigo orchioides Gaertn. ARS Pharm, 2005; 46:125–38.
Cao D, Zheng Y, Qin L, Han T, Zhang H, Rahman K, Zhang Q. Curculigo orchioides, a traditional Chinese medicinal plant, prevents bone loss in ovariectomized rats. Maturitas, 2008; 59:373–80.
Cao DP, Han T, Zheng YN, Qin LP, Zhang QY. Phenolic glycosides and lignans components in Curculigo orchioides Gaertn. Acad J Second Milit Univ, 2009; 30:194–7.
Cao S, Tian S, Bai M, Liu SY, Jia JJ, Miao MS. Effects of Curculigo orchioides total glucosides in mouse perimenopause model of related organization and organs morphology. Bangl J Pharmacol, 2016; 11:S72–81.
Chang WL, Lee SS. Norneolignan and phenols from Curculigo capitulata. Phytochemistry, 1998; 49(7):2133–6.
Chauhan NS, Shah K, Gupta PK. Studies on antistress activity of Curculigo orchioides Gaertn. Biomed Biotechnol Res J, 2021; 5(2):145.
Chauhan NS, Rao CV, Dixit VK. Effect of Curculigo orchioides rhizomes on sexual behaviour of male rats. Fitoterapia, 2007; 78:530–4.
Chauhan NS, Sharma V, Thakur M, Dixit VK. Curculigo orchioides: the black gold with numerous health benefits. Zhong Xi Yi Jie He Xue Bao, 2010; 8(7):613–23; doi:10.3736/jcim20100703
Chen QS, Chen WQ, Yang SY. Pharmacologic study of Curculigo orchioides Gaertn. Zhongguo Zhong Yao Za Zhi, 1989; 14:618–20.
Chen X, Zuo A, Deng Z, Huang X, Zhang X, Geng C, Li T, Chen J. New phenolic glycosides from Curculigo orchioides and their xanthine oxidase inhibitory activities. Fitoterapia, 2017; 122:144–9.
Deng XL, Zheng RR, Han ZZ, Gu LH, Wang ZT. New chlorophenolic glycoside from Curculigo orchioides and their activities on 5α-reductase. J Asian Nat Prod Res, 2021; 23(4):333–40.
Ding H, Gao G, Zhang L, Shen G, Sun W, Gu Z, Fan W. The protective effects of curculigoside A on adjuvant-induced arthritis by inhibiting NF-κB/NLRP3 activation in rats. Int Immunopharmacol, 2016; 30:43–9.
Dode PA, Wani NS, Deshmukh TA, Patil VR. Anti-inflammatory activity of hydrogel formulations of Curculigo orchioides Gaertn. rhizomes. Pharmacology, 2009; 2:1367–81.
Fang Z, Meng Y, Qian W, Si-Cheng W, Wei W, Chang-xiao L, Bin L, Cai-yun P. Research progress on chemical constituents and pharmacological activities of Curculigo orchioides. Chin Tradit Herbal Drugs, 2020; 51:2238–47.
Ge JF, Gao WC, Cheng WM, Lu WL, Tang J, Peng L, Li N, Chen FH. Orcinol glucoside produces antidepressant effects by blocking the behavioural and neuronal deficits caused by chronic stress. Eur Neuropsychopharmacol, 2014; 24:172–80.
Goyal P, Kabra A. A review on phytochemical and pharmacological profile on Curculigo orchioides. Plant Cell Biotech Mol Bio, 2020; 21:243–52.
Gulati V, Gulati P, Harding IH, Palombo EA. Exploring the anti-diabetic potential of Australian Aboriginal and Indian Ayurvedic plant extracts using cell-based assays. BMC Complement Altern Med, 2015; 15:8.
Hankey A. Ayurvedic physiology and etiology: Ayurvedo Amritanaam. The doshas and their functioning in terms of contemporary biology and physical chemistry. J Alternat Complement Med, 2001; 7(5):567–74.
Hejazi II, Khanam R, Mehdi SH, Bhat AR, Rizvi MM, Thakur SC, Athar F. Antioxidative and antiproliferative potential of Curculigo orchioides Gaertn in oxidative stress induced cytotoxicity: in vitro, ex vivo and in silico studies. Food Chem Toxicol, 2018; 115:244–59.
Jaiswa S, Batra A, Mehta BK. The antimicrobial efficiency of root oil against human pathogenic bacteria and phytopathogenic fungi. Phytopathology, 1984; 109:90–3.
Ji XH. Effect of Curculigo polysaccharide on immune function mice. Hai Xia Yao Xue, 2011; 23:33–35 [in Chinese].
Jiao L, Cao DP, Qin LP, Han T, Zhang QY, Zhu Z, Yan F. Antiosteoporotic activity of phenolic compounds from Curculigo orchioides. Phytomedicine, 2009; 16(9):874–81.
Jiao W, Chen X, Wang H, Lu R, Shao H. A new hepatotoxic triterpenoid ketone from Curculigo orchioides. Fitoterapia, 2013; 84:1–5.
Joshi UH, Solanki VR, Desai TR, Tirgar PR. Investigation of antihypertensive mechanism of Curculigo orchioides in DOCA salt induced hypertensive rats. Int J Phytopharmacol, 2012; 3(02):178–85.
Joy PP, Thomas J, Mathew S, Skaria BP. Curculigo orchioideds: a plant for health care. Indian J Arecanut Spices Med Plants, 2004; 6:131–4.
Karigidi KO, Olaiya CO. Antidiabetic activity of corn steep liquor extract of Curculigo pilosa and its solvent fractions in streptozotocin-induced diabetic rats. J Tradit Complement Med, 2020; 10(6):555–64.
Kayalvizhi T, Ravikumar S, Venkatachalam P. Green synthesis of metallic silver nanoparticles using Curculigo orchioides rhizome extracts and evaluation of its antibacterial, larvicidal, and anticancer activity. J Environ Eng, 2016; 142(09):C401600237.
Khare CP, 2007. Indian medicinal plants. Springer, Berlin/Heidelberg, Germany, p 22.
Kumar S, Dobos GJ, Rampp T. The significance of ayurvedic medicinal plants. J Evid Based Complement Alternat Med, 2017; 22(3):494–501.
Kushalan S, D’Souza LC, Aloysius K, Sharma A, Hegde S. Toxicity assessment of Curculigo orchioides leaf extract using Drosophila melanogaster: a preliminary study. Int J Environ Res Public Health, 2022a; 19(22):15218.
Kushalan S, Yathisha UG, Khyahrii SA, Hegde S. Phytochemical and anti-oxidant evaluation of in vitro and in vivo propagated plants of Curculigo orchioides. In Vitro Cell Dev Biol Plant, 2022b; doi:10.1007/s11627-021-10246-5
Lacaille-Dubois MA, Wagner H. A review of the biological and pharmacological activities of saponins. Phytomedicine, 1996; 2:363–86.
Lakshmi V, Pandey K, Puri A, Saxena RP, Saxena KC. Immunostimulant principles from Curculigo orchioides. J Ethnopharmacol, 2003; 89:181–4.
Li LY, Ma P, Yu Q, Wei YF, Zhong GY, Wang CH. Quality standards of Curculigo orchioides. Zhong Guo Yao Fang, 2011; 22:4068–71 [in Chinese].
Li N, Chen JJ, Zhou J. Note: Capitulatin B, A new eudesmane derivative from Curculigo capitulata, and revised assignment of 13C NMR data of 6α, 15α-epoxy-1β, 4β-dihydroxyeudesmane. J Asian Nat Prod Res, 2005a; 7(3):279–82.
Li N, Chen JJ, Zhao YX, Zhou J. Three new norlignans from Curculigo capitulata. J Asian Nat Prod Res, 2005b; 7(3):189–95.
Li RC, Zeng MY, Su YL, Wu CX. Effects of curculigoside on the behavior and hippocampal neuronal apoptosis of Alzheimer’s rat. Chin J Clin Pharmacol, 2019; 35:654–70.
Li SZ. Bencao Gangmu. Chongqing University Press, Chongqing, China, p 2, 1559.
Marasini BP, Baral P, Aryal P, Ghimire KR, Sanjiv N, Nabaraj D, Anjana S, Laxman G, Kanti S. Evaluation of antibacterial activity of some traditionally used medicinal plants against human pathogenic bacteria. BioMed Res Int, 2015; 2015:265425.
Liu Y, Guo Y, Gong S, Yuan M, Liu J, Li X, Wu Z, Guo L. Discrimination of Curculigo orchioides Rhizoma and Curculigo glabrescens Rhizoma using stable isotope and mineral element analyses coupled with chemometrics. Sci Rep, 2022; 12(1):12578.
Mehta BK, Bokadia MM, Mehta SC. Study of root oil compound fatty acids of Curculigo orchioides roots. Indian Drugs, 1980; 18(3):109–10.
Mehta J, Nama KS. A review on ethnomedicines of Curculigo orchioides Gaertn (Kali Musli): black gold. Int J Pharm Biomed Res, 2014; 1:12–6.
Miao M, Tian S, Guo L, Bai M, Fang X, Liu S. The effect of curculigoside on mouse model of perimenopausal depression. Saudi J Biol Sci, 2017; 24:1894–190.
Murali VP, Kuttan G. Curculigo orchioides Gaertn effectively ameliorates the uro-and nephrotoxicities induced by cyclophosphamide administration in experimental animals. Integr Cancer Ther, 2016; 15(2):205–15.
Murali VP, Kuttan G. Enhancement of cancer chemotherapeutic efficacy of cyclophosphamide by Curculigo orchioides Gaertn and its ameliorative effects on cyclophosphamide-induced oxidative stress. Integr Cancer Ther, 2015; 14:172–83.
Nagesh KS, Shanthamma C. Antibacterial activity of Curculigo orchioides rhizome extract on pathogenic bacteria. Afr J Microbiol Res, 2009; 3:5–9.
Nie Y, Dong X, He YJ, Yuan TT, Han T, Rahman K, Qin LP, Zhang QY. Medicinal plants of genus Curculigo: traditional uses and a phytochemical and ethnopharmacological review. J Ethnopharmacol, 2013; 147:547–63.
Niu C, Zhang ZZ, Yang LP, Zhai YY, Wang ZH. Chemical Constituents of Curculigo orchioides. Chem Nat Compd, 2020; 56:1–3.
Nwokonkwo DC. Antibacterial susceptibility of the constituents of ethanol crude extract and the neutral metabolite of the root of Curculigo pilosa Hypoxidaceae. Int J Chem, 2014; 6:19–23.
Pandit P, Singh A, Bafna AR, Kadam PV, Patil MJ. Evaluation of antiasthmatic activity of Curculigo orchioides Gaertn. rhizomes. Indian J Pharm Sci, 2008; 70:440–4.
Perumal V, Thamilchelvan K, Jinu U, Giovanni B, Natesan G. Enhanced antibacterial and cytotoxic activity of phyto-chemical loaded-silver nanoparticles using Curculigo orchioides leaf extracts with different extraction techniques. J Cluster Sci, 2017; 28:607–19.
Pratap GK. In vitro anti-cholinesterase activity and mass spectrometric analysis of Curculigo orchioides Gaertn. rhizome extract. Anal Chem Lett, 2020; 10:442–58.
Pratap GK, Shantaram M. A kinetic study of acetylcholinesterase inhibition by fractions of Oleo dioica Roxb. leaf and Curculigo orchioides Gaertn rhizome for the treatment of Alzheimer’s disease. EJMP, 2019; 30:1–12.
Ratnam KV, Ravishankar K, Priyabhandavi P. Evaluation of in vitro antioxidant activity of ethanolic root extract of Curculigo orchioides. Int J Res Pharm Chem, 2013; 3(2):364–9.
Sastri H (Ed.)2002. Ashtanga Hridayam. Chaukhambha Orientalia, Varanasi, India.
Selvaraj T, Agastian P. In vitro anticancer activity of ethyl acetate extract and green nanoparticles synthesized from Curculigo orchioides gaertn—an endangered medicinal. Int J Pharm Sci Res, 2017; 8:3030–8.
Saxena HO. Phytochemical screening and variation studies in secondary metabolite. Chem Sci Rev Lett, 2022; 11(42):151–8; doi:10.37273/chesci.cs205301412
Shen QP, Zeng DL, Zhou YT, Xia LG, Zhao YF, Qiao GY, Xu LY, Liu Y, Zhu ZY, Jiang XQ. Curculigoside promotes osteogenic differentiation of bone marrow stromal cells from ovariectomized rats. J Pharm Pharmacol, 2013; 65:1005–13.
Singla K, Singh R. Nephroprotective effect of Curculigo orchiodies in streptozotocin–nicotinamide induced diabetic nephropathy in wistar rats. J Ayurveda Integr Med, 2020; 11:399–404.
Surveswaran S, Cai YZ, Corke H, Sun M. Systematic evaluation of natural phenolic antioxidants from 133 Indian medicinal plants. Food Chem, 2007; 102:938–53.
Tacchini M, Spagnoletti A, Marieschi M, Caligiani A, Bruni R, Efferth T, Sacchetti G, Guerrini A. Phytochemical profile and bioactivity of traditional ayurvedic decoctions and hydro-alcoholic macerations of Boerhaavia diffusa L. and Curculigo orchioides Gaertn. Nat Prod Res, 2015; 29:2071–9.
Tan S, Xu J, Lai A, Cui R, Bai R, Li S, Liang W, Zhang G, Jiang S, Liu S, Zheng M, Wang W. Curculigoside exerts significant anti-arthritic effects in vivo and in vitro via regulation of the JAK/STAT/NF-κB signaling pathway. Mol Med Rep, 2019; 19:2057–64.
Thakur M, Chauhan NS, Sharma V, Dixit VK, Bhargava S. Effect of Curculigo orchioides on hyperglycemia-induced oligospermia and sexual dysfunction in male rats. Int J Impot Res, 2012; 24:31–7.
Thamilchelvan K, Ragavendran C, Kamalanathan D, Rajendiran R, Cherian T, Malafaia G. In vitro somatic embryo productions from Curculigo orchioides derived gold nanoparticles: synthesis, characterization, its biomedical applications, and their eco-friendly approaches to degradation of methylene blue under solar light irradiations. Environ Res, 2023; 216:114774.
Tian Z, Yu W, Liu HB, Zhang N, Li XB, Zhao MG, Liu SB. Neuroprotective effects of curculigoside against NMDA-induced neuronal excitoxicity in vitro. Food Chem Toxicol, 2012; 50:4010–5.
Tiwari RD, Misra G. Strutural studies of the constitutents of the rhizomes of Curculigo orchiodes. Planta Med, 1976; 29:291–4.
Umar AH, Ratnadewi D, Rafi M, Sulistyaningsih YC. Untargeted metabolomics analysis using FTIR and UHPLC-Q-Orbitrap HRMS of two Curculigo species and evaluation of their antioxidant and alpha-glucosidase inhibitory activities. Metabolites, 2021; 11:42.
Venkatesh P, Mukherjee PK, Nema NK, Bandyopadhyay A, Fukui H, Mizuguchi H. Mast cell stabilization and antihistaminic potentials of Curculigo orchioides rhizomes. J Ethnopharmacol, 2009; 126(3):434–6.
Venukumar MR, Latha MS. Antioxidant activity of Curculigo orchioides in carbon tetrachloride—induced hepatopathy in rats. Indian J Clin Biochem, 2002; 17(2):80–7.
Vijayanarayana K, Rodrigues RS, Chandrashekhar KS, Subrahmanyam EV. Evaluation of estrogenic activity of alcoholic extract of rhizomes of Curculigo orchioides. J Ethnopharmacol, 2007; 114:241–5.
Wang J, Zhao XL, Gao L. Anti-depressant-like effect of curculigoside isolated from Curculigo orchioides Gaertn root. Trop J Pharm Res, 2016; 15(10):2165–72.
Wang Y, Li J, Li N. Phytochemistry and pharmacological activity of plants of genus Curculigo: an updated review since 2013. Molecules, 2021; 26(11):3396.
Wang Y, Zhao L, Wang Y, Xu JL, Nie Y, Guo YH, Tong YT, Qin LP, Zhang QY. Curculigoside isolated from Curculigo orchioides prevents hydrogen peroxide-induced dysfunction and oxidative damage in calvarial osteoblasts. Acta Biochim Biophys Sin, 2012; 44(5):431–41.
Wang KJ, Li N, Wang H. New acetylenic norlignan compounds from rhizomes of Curculigo crassifolia. Molecules, 2008; 13(8):1696–701.
Wang X, Zhang M, Zhang D, Wang S, Yan C. An O-acetyl-glucomannan from the rhizomes of Curculigo orchioides: structural characterization and anti-osteoporosis activity in vitro. Carbohydr Polym, 2017; 174:48–56.
Wang X, Zhang M, Zhang D, Wang SM, Yan CY. Structural elucidation and anti-osteoporosis activities of polysaccharides obtained from Curculigo orchioides. Carbohydr Polym, 2019; 203:292–301.
Wang ZH, Gong XY, Zhou DJ, Xu PF, Huang M, Zhang QL, Meng YL, Niu C, Zhang YR. Three new chloro-phenolic glucosides from Curculigo orchioides Gaertn. Phytochem Lett, 2018; 26:9–11.
Wang ZH, Huang J, Ma XC, Li GY, Ma YP, Li N, Wang JH. Phenolic glycosides from Curculigo orchioides Gaertn. Fitoterapia, 2013; 86:64–9.
Wang ZH, Ma XC, Li GY, Niu C, Ma YP, Kasimu R, Huang J, Wang JH. Four new phenolic glucosides from Curculigo orchioides Gaertn. Phytochem Lett, 2014; 9:153–7.
Wu Q, Fu DX, Hou AJ, Lei GQ, Liu ZJ, Chen JK, Zhou TS. Antioxidative phenols and phenolic glycosides from Curculigo orchioides. Chem Pharm Bull, 2005; 53:1065–7.
Xia LF, Liang SH, Tang J, Huang Y, Wen H. Anti-tumor effect of polysaccharides from rhizome of Curculigo orchioides Gaertn on cervical cancer. Trop J Pharm Res, 2016; 15:1731–7.
Xu JP, Xu RS, Li XY. Four new cycloartane saponins from Curculigo orchioides. Planta Med, 1992; 58(02):208–10.
Xu JP, Dong QY. Chemical study on Curculigo orchioides II, the isolation and structural determination of new compound curculigine A. Chin Tradit Herb Drugs, 1987; 18:194–6 [in Chinese].
Xu JP, Xu RS. Phenyl glucosides Curculigo orchioides. Acta Pharm Sin, 1992; 27:353–7.
Yokosuka A, Sato K, Mimaki Y. Cycloartane glycosides from the rhizomes of Curculigo orchioides. Phytochemistry, 2010; 71(17–8):2174–81.
Yu Y, Li T, Wang X, Zhang M, Yu Q, Chen H, Zhang D, Yan C. Structural characterization and anti-osteoporosis activity of two polysaccharides extracted from the rhizome of Curculigo orchioides. Food Funct, 2022; 13(12):6749–61.
Zhang J, Li YD, Liu XM, Gao L, Zhang YP, Dang LZ. The study of terpeniods from Curculigo orchioides. J Yunnan Univ, 2019a; 41:367–71.
Zhang QL, Zhao L, Shen Y, He YQ, Cheng G, Yin M, Zhang QY, Qin LP. Curculigoside protects against excess-iron induced bone loss by attenuating Akt-FoxO1- dependent oxidative damage to mice and osteoblastic MC3T3-E1 cells. Oxid Med Cell Longev, 2019b; 2019:1–14.
Zhang Y, Ge JF, Wang FF, Liu F, Shi C, Li N. Crassifoside H improve the depressive-like behavior of rats under chronic unpredictable mild stress: possible involved mechanisms. Brain Res Bull, 2017; 135:77–84.
Zhou Y, Zhang L, Zhao LY, Zhang GY, Ma XQ, Ge DY, Wang CJ, Yen XZ. Experimental study of immunoregulative actions of xianmaopolysaccharid (XMPS) in mice. Shanghai J Immunol, 1996; 16:336–8.
Zhu CC, Wang KJ, Wang ZY, Li N. Chemical constituents from rhizomes of Curculigo breviscapa. Bull Korean Chem Soc, 2010; 31(1):224–6.
Zhu F, Wang J, Ni Y, Yin W, Hou Q, Zhang Y, Yan S, Quan R. Curculigoside protects against titanium particle-induced osteolysis through the enhancement of osteoblast differentiation and reduction of osteoclast formation. J Immunol Res, 2021; 2021:5707242.
Zhu FB, Wang JY, Zhang YL, Quan RF, Yue ZS, Zeng LR, Zheng WJ, Hou Q, Yan SG, Hu YG. Curculigoside regulates proliferation, differentiation, and pro-inflammatory cytokines levels in dexamethasone-induced rat calvarial osteoblasts. Int J Clin Exp Med, 2015a; 8:12337–46.
Zhu H, He J, Ye L, Lin F, Hou J, Zhong Y, Jiang W. Mechanisms of angiogenesis in a Curculigoside A-treated rat model of cerebral ischemia and reperfusion injury. Toxicol Appl Pharmacol, 2015b; 288:313–21.
Zuo AX, Shen Y, Zhang XM, Jiang ZY, Zhou J, Lü J, Chen JJ. Four new trace phenolic glycosides from Curculigo orchioides. J Asian Nat Prod Res, 2010; 12(1):43–50.
Zuo AX, Shen Y, Jiang ZY, Zhang XM, Zhou J, Lü J, Chen JJ. Two new triterpenoid glycosides from Curculigo orchioides. J Asian Nat Prod Res, 2012; 14(5):407–12.