INTRODUCTION
An active pharmaceutical ingredient (API) is often administered in solid form (Morissette et al., 2004). Its biological effect can be influenced by factors such as gastrointestinal physiological conditions, first-pass metabolism, and physicochemical properties, like solubility, stability, permeability, and dissolution rate, which can affect its bioavailability (Emami et al., 2018). Molecule organization in the solid component is important to determine the physicochemical properties. Crystal structure changes can be used as a strategy to improve physicochemical properties (Datta et al., 2004; Morissette et al., 2004).
APIs in crystalline form can exist as solvates, hydrates, polymorphs, salts, and co-crystals (Rodrigues et al., 2018; Shaikh et al., 2018). In general, co-crystals are solids formed by two or more different molecular and/or ionic compounds in the same crystal structure in a defined stoichiometric ratio (Holanda et al., 2019a; Kavanagh et al., 2019). A pharmaceutical co-crystal incorporates an API and a co-former that is a “generally recognized as safe” substance (Holanda et al., 2019b; Thakuria and Sarma, 2018).
The co-crystal molecules are organized in the crystal lattice through intermolecular interactions, such as hydrogen bonds, van der Waals forces, and π-stacking (Aitipamula et al., 2012; Rodrigues et al., 2018; Shaikh et al., 2018). API and its co-former interact through the supramolecular synthon that can be classified as heterosynthon, when different functional groups are combined, and homosynthon, when intermolecular interactions occur between identical groups. Homosynthons are energetically less favored and occur less frequently, with only 35% of co-crystals being formed by acid-acid interactions (Cherukuvada and Guru Row, 2014; Desiraju, 1995; Khan et al., 2009; Rodrigues et al., 2018; Shattock et al., 2008). Compared to other crystalline forms, co-crystals are more stable and, unlike salts, do not require proton transfer between their constituents (Barmpalexis et al., 2018; Fernandes et al., 2019; Jones et al., 2006).
Co-crystals can be used to improve the biopharmaceutical properties of API without changing the chemical structure and maintaining the pharmacological properties, mainly for drugs belonging to classes II and IV of the Biopharmaceutical Classification System (BCS) (Haneef and Chadha, 2017). They are potential solid forms for the delivery of dual drug therapy and can improve the pharmacokinetic profile and the bioavailability of API. Drug-drug co-crystals can simplify dosing regimens and improve treatment adherence. Furthermore, they offer a low-risk route and can be patented (Barmpalexis et al., 2018; Kavanagh et al., 2019; Wang et al., 2021).
Several methodologies can be used to synthesize pharmaceutical co-crystals, such as slow solvent evaporation, mechanochemical methods, antisolvent crystallization, supercritical fluid technology, and spray drying (Rodrigues et al., 2018). The most used methods for co-crystallization are slow solvent evaporation and mechanochemical methods (Kuminek et al., 2016).
Co-crystallization by mechanochemical methods occurs through the direct absorption of mechanical energy, which promotes the fracture of the crystalline lattice and increases the surface area, facilitating the interaction between molecules. In this technique, the components, in a defined stoichiometric proportion, are crushed using a mortar and pestle (manual grinding) or a ball mill (Rodrigues et al., 2018).
Grinding has been widely used in the formation of co-crystals. It comprises two techniques, solid-state grinding and the liquid-assisted grinding (LAG) method, in which a small amount of solvent is added, facilitating the diffusion of the co-former molecules in the crystal lattice of the API, which will accelerate the formation of the co-crystal (Sathisaran and Dalvi, 2018).
The LAG method provides greater control of crystal properties and is more effective than dry grinding, which is why it is chosen for co-crystal production (Rodrigues et al., 2018).
Gemfibrozil (GEM) (Fig. 1a) is a fibric acid derivative (Holanda et al., 2019a, 2019b). It is used for hypertriglyceridemia treatment by reducing triglyceride, low-density lipoprotein, and very low-density lipoprotein levels and increasing high-density lipoprotein levels (Prindiville et al., 2011; Wiggins et al., 2016). GEM belongs to class II of the BCS (which has low solubility and high permeability), and these characteristics can compromise absorption due to a lower dissolution rate (Holanda et al., 2019b).
Trans-cinnamic acid (TCA) (Fig. 1b) is an aromatic carboxylic acid found in nature that has low toxicity and high safety for pharmaceutical use and can be used as a co-former in the synthesis of co-crystals. TCA has antitumor, antioxidant, and antimicrobial activities (Chandra et al., 2019; Ekmekcioglu et al., 1998; Luo et al., 2020).
 | Figure 1. Molecular structure: (a) GEM and (b) TCA. [Click here to view] |
Co-crystals containing these substances can be formed through hydrogen bonds between the carboxylic acid groups of both components, forming supramolecular homosynthons. The objective of this work is to recrystallize the co-crystal GEM: TCA, evaluating its main characterization techniques and solubility in relation to the commercial GEM in its anhydrous form. Furthermore, it aims to confirm the cytotoxicity of Caco-2 cells.
MATERIALS AND METHODS
Synthesis by the LAG method
GEM 98.5% and TCA 99% were purchased from Sigma-AldrichTM (St. Louis, MO) and used without purification following analysis by the certificate of analysis.
GEM-TCA co-crystal in equimolar ratio 1:1 was successfully produced by the LAG method as described by literature (Devi et al., 2022; Holanda et al., 2019b). GEM and TCA, with a total mass of 50 mg using 10 μl of ethanol, were subjected to milling for 60 minutes at a frequency of 15 Hz in the Retsch® MM400 mill (Germany), consisting of a 10 ml stainless steel jug and two spheres of 7 mm in diameter inside. An amber bottle was used for storage and kept in a dry environment (Holanda et al., 2019b).
Characterization
The powder X-ray diffraction (PXRD) analyses were performed on Shimadzu® equipment, model XRD-7000 (Japan), with CuKα copper radiation (λ = 1.54060 Å), equipped with a scintigraphy detector. The current and voltage applied were 15 mA and 40 kV, respectively. These analyses were carried out at room temperature. The angular range was 5°–50° 2θ, in continuous mode (2°/minute with 0.02° step).
The API, co-former, and co-crystal infrared spectra were obtained using an Agilent Technologies® instrument, the Cary 660 Fourier-transform infrared spectroscopy (FTIR) (Australia), coupled in the ATR FTIR mode. The samples were analyzed in a solid-state form (powder) over a 400–4,000 cm−1 range.
The differential scanning calorimetry (DSC) TA Instruments® thermal analysis system, model 2910 (USA), was used to perform the measurements. Approximately 3.0 mg of samples were placed in the closed-aluminum pan. The samples were heated to 200°C at a heating rate of 10°C minute−1. The environment was an inert argon atmosphere with a 50 ml minute−1 flow rate.
The thermogravimetric analysis (TGA) curves were obtained using the TA Instruments® thermal analysis system, model 2050 (USA). Approximately 5 mg of samples was weighed and placed in an open platinum pan. The parameters were set at a heating rate of 10°C minute−1 and a flow rate of 50.0 ml minute−1 in an inert argon atmosphere.
In vitro solubility studies
The shake-flask method was used to determine equilibrium in accordance with the literature (Bhatia et al., 2021). GEM and GEM:TCA (1:1) co-crystals were added in excess into tubes containing 7 ml of USP buffered aqueous solutions at pH 1.2 (1.0 M hydrochloric acid), pH 4.5 (acetate), pH 6 .8 (phosphate), and pH 7.4 (phosphate) and FeSSIF media (Biorelevant Ltd®, UK) in triplicate according to the method outlined in the BCS guidelines (Barbosa et al., 2021; Reviewer Guidance, 1994).
The suspensions were shaken continuously in an LS 4600 Digital shaker (Alpax®, Brazil) at 100 rpm for 48 hours at a temperature of 37°C ± 1°C. After 24 and 48 hours, an aliquot of the supernatant was collected, and the same amount of buffer solution was added. The samples were filtered with 0.45 μm PES syringe filters (Biofil®, China), diluted in an appropriate solvent when needed, and analyzed using a validated chromatographic method. The pH of the media was measured at the beginning and end of the experiment using a pH meter, Kasvi® equipment, model K38-2014B (Brazil). The remaining solid was analyzed by PXRD. Statistical analysis was performed using the GraphPad Prism 5® program. Student’s unpaired t-test was applied to compare the solubility results.
GEM concentration was determined by the high-performance liquid chromatography method using Waters® chromatography, model 717 plus autosampler (USA), equipped with a UV-vis detector model 2487 Dual, Waters® (USA). An Eclipse Plus C18 reversed-phase chromatography column (250 × 4.6 mm, 5 μm, Agilent-Zorbax®, USA) was employed at 25°C. The injected volume was 20 μl and the absorption of GEM was recorded at 276 nm. The mobile phase consisted in the application of methanol (Synth®, Brazil) and 1% acetic acid (Synth®, Brazil) solution (80:20, v/v). Elution took place at a flow rate of 0.8 ml minute−1 for the first four minutes and thereafter at a flow rate of 1 ml minute−1. The method was validated by evaluating the parameters of linearity, accuracy, and precision, as described in the literature (Holanda et al., 2019b). To establish linearity, three calibration curves were prepared by appropriate dilutions of standard stock solution in the range 0.005–0.1 mg ml−1. The coefficient of linear correlation R2 for the calibration curves was calculated by the least squares method (Holanda et al., 2019b).
Culture cell
Caco-2 cells were grown in Eagle’s Minimum Essential Medium Lonza (Biomédica Ltda®, USA), supplemented with 20% fetal bovine serum (Vitrocell Embriolife®, Brazil), 100 IU ml−1 penicillin, and 100 μg ml−1 streptomycin (Vitrocell Embriolife®, Brazil), in an atmosphere of 5% CO2.
Cell viability studies
The colorimetric 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide test (MTT) method was used to verify the percentage of viable cells. Caco-2 cells were subcultured in 96-well plates (TPP®, Switzerland) for 7 days at a seeding density of 104 cells/well. The cells were treated with the substances GEM, TCA, and GEM:TCA co-crystal in concentrations of 10, 50, and 100 μM and DMSO 0.5% (v:v) for 3 hours. After that, the cells were incubated with 100 μl of solution (0.5 mg ml−1) of MTT (Sigma-Aldrich®, USA) in each well for 3.5 hours. The formazan crystals were diluted in DMSO. The plate was shaken for 5 minutes in an orbital shaker, and the absorbance was determined using a Synergy H1 microplate reader (BioTek®, USA) set at 540 nm.
Cell wells that received 0.5% DMSO (v/v) were used as vehicle control, and wells that did not receive treatment were used as controls. The experiment was carried out in three independent tests. The percentage of live cells was calculated in relation to the control group.
The statistical analysis was run using GraphPad Prism® (version 5), applying a one-way analysis of variance followed by Bonferroni’s tests. If the p-value was less than 0.05, the value was considered significant.
RESULTS AND DISCUSSION
Powder X-ray diffraction
PXRD analysis is essential to confirm the formation of co-crystals, as each crystalline solid has a unique diffraction pattern (Reutzel-Edens, 2011). The GEM:TCA co-crystal was compared to the API and co-former, and the results are shown in Figure 2.
According to the data shown in Figure 2, the GEM diffractogram shows peaks at 11.7°, 12.02°, 12.84°, 13.98°, 16.76°, 18.1°, 18.44°, and 24.34° 2θ. Furthermore, the TCA diffractogram shows peaks at 10.26°, 20.14°, 23.18°, 25.78°, and 29.9° 2θ. The presence of single peaks in the diffractogram of the new GEM co-crystal was indicated by black circles in the diffractogram and located at 8.28°, 13.0°, 13.3°, 15.24°, and 27.4° 2θ. In addition to the absence of peaks typical of GEM, such as the absence of a peak in 13.98° 2θ indicated by a black arrow, this indicates that the co-crystal was successfully formed, as a new diffraction pattern is being shown. The presence of new peaks is common among the crystals obtained by co-crystallization processes and can be used to verify the new crystalline phase; an example is the study that confirmed the formation of carbamazepine-cinnamic acid co-crystals by the emergence of a new diffraction profile (Shayanfar, 2013).
Fourier-transform infrared spectroscopy
Structural studies by FTIR were performed to confirm the success of co-crystallization. This technique is important because it identifies any change in the vibrational state of the molecule, such as displacement, disappearance, or change in the intensity of bands resulting from the interaction between the components of the co-crystal (Gorain et al., 2018; Nikoli? et al., 2019). Figure 3 shows the FTIR analysis.
According to the results presented in Figure 3 and according to the molecular structure of TCA and GEM presented in Figure 1, it is possible to observe that both molecules have carboxylic acid groups. The FTIR spectra of GEM, TCA, and the new co-crystal showed carbonyl stretching in the region of 1,704, 1,670, and 1,693 cm−1, respectively. This shift to regions of lower energy, observed in co-crystals, may be related to the new crystalline organization of the molecule; since, in this case, it is expected that the formation of the co-crystal between these two acids occurs through hydrogen bonds involving the carbonyl group, forming dimers or catemers. Intermolecular interactions between the API and the co-former caused the displacement of the carbonyl band, resulting in supramolecular homosynthons between the molecules and the formation of a new crystal structure. In the FTIR spectrum of telmisartan-saccharin co-crystals, the band referring to carbonyl stretching suffered a decrease in vibrational frequency, indicating the occurrence of interactions involving this group (Chadha et al., 2014). Another study pointed to the displacement of the carbonyl stretch as an indication of hydrogen bonds between carbamazepine and cinnamic acid (Shayanfar et al., 2013). The displacement of the carbonyl band observed in these co-crystals is similar to that observed in the GEM-TCA system.
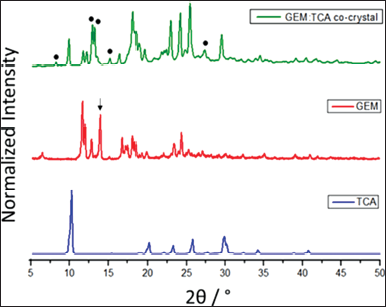 | Figure 2. PXRD patterns of GEM, TCA, and GEM:TCA co-crystal. The points represent the new diffraction peaks. [Click here to view] |
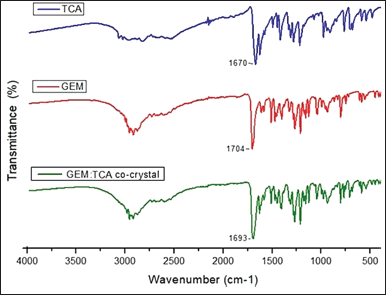 | Figure 3. The FTIR diagrams of TCA, GEM, and GEM:TCA co-crystal. [Click here to view] |
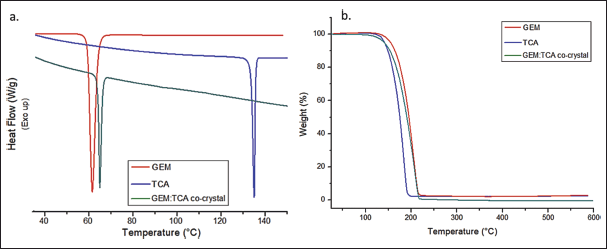 | Figure 4. Thermal analysis of GEM, TCA, and GEM:TCA co-crystal. (a) DSC curves and (b) TGA thermograms. [Click here to view] |
DSC AND TGA
Thermal analysis studies are presented in Figure 4.
Figure 4a presents the DSC study. The DSC curves of GEM and TCA showed an endothermic event with Tonset at 60.04°C and 133.76°C, respectively. These values are consistent with the melting points found in the literature for these substances (Stanton and Bak, 2008). The GEM:TCA co-crystal shows an endothermic event with Tonset at 64.56°C. This endothermic event is unique and presents a melting temperature between the melting temperatures of the pure substances, suggesting an interaction between the API and the co-former, resulting in the formation of the co-crystal (Karagianni et al., 2018). In addition, the DSC analysis eliminated the possibility of the solid being a eutectic mixture because eutectic mixtures must have a lower melting point than the isolated components. Therefore, this is strong evidence that the formation of the co-crystal occurred (Cherukuvada and Nangia, 2014).
Figure 4b shows the TGA analysis. TGA analysis certifies the purity of each sample. The TGA curves showed unique stages of mass loss after 100°C, which indicates that the analyzed substances are anhydrous and that the co-crystal does not contain any solvent in its crystalline structure. The TGA curves of GEM, TCA, and GEM:TCA co-crystal presented weight loss events at 99.34%, 97.48%, and 99.97%, respectively.
Solubility studies
The partial validation of the analytical methodology was carried out to demonstrate that the analytical method used to quantify GEM in samples from the solubility test is linear, exact, selective, precise, and reproducible, as established in the RDC 166/2017—ANVISA.
Linearity
The least squares method and linear regression analysis were performed to determine linearity. The coefficient of determination (R2) obtained was equal to 0.995. The regression line equation is as follows: y = 10,673,955.134x − 2,316.468 so that the slope was significantly different from zero, as seen in Figure 5. For the verification of the conformity of the regression model, residuals analysis was performed, as shown in Figure 6.
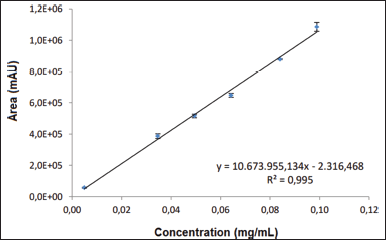 | Figure 5. Graphic representation of responses as a function of the concentration of analyte. [Click here to view] |
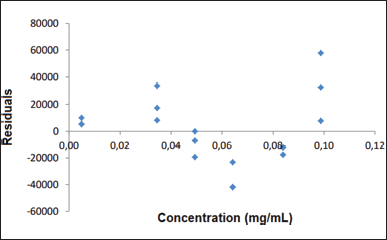 | Figure 6. Graphic representation of the distribution of residuals as a function of analytical response. [Click here to view] |
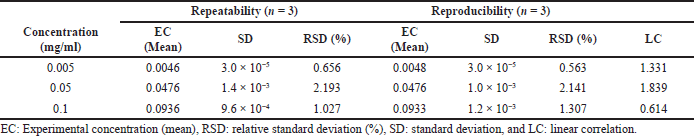 | Table 1. Evaluation of repeatability and reproducibility tests. [Click here to view] |
The Cochran test was performed and it was possible to verify that the calculated C value was lower than the tabulated C value, which represents the homoscedasticity of the data. It was assumed that y varies according to x so that the method is linear.
Precision
The precision of the method was determined by evaluating nine determinations, contemplating the low, medium, and high levels of the analytical range, with three replicates at each level.
The values of experimental means, standard deviation (SD), and relative SD (RSD) were calculated for the repeatability and reproducibility tests and are shown in Table 1.
The RSD (%) obtained was less than 5% for both criteria: repeatability and reproducibility, proving to be satisfactory. The linear correlation between the accuracies was demonstrated through the LC test.
Accuracy
Accuracy and recovery were verified from nine determinations at three different levels of concentration. Table 2 presents the average of the results obtained in this test.
Chromatograms
The chromatograms referring to the elution of GEM, ATC, and mobile phase, obtained by UV-vis detector, fixed at 276 nm, are shown in Figures 7–9, respectively. So it is evident that the retention time of the API and the co-former is different.
Solubility analysis
In vitro solubility testing can be correlated with absorption and distribution. It provides information that guides the choice of the best media for dissolution testing, which is essential for in vitro-in vivo correlations and pharmaceutical equivalence studies (Baka et al., 2008; Zhou et al., 2007). Solubility studies in media that simulate gastrointestinal fluids, such as FeSSIF, are important for predicting the behavior of pharmaceutical co-crystals in vivo (Lipert et al., 2015). The solubility results are described in Figure 10.
GEM and GEM:TCA co-crystal showed higher solubility in basic media (Fig. 10), as GEM is a weak monoprotic acid (pKa = 4.42) and its solubility increases when the pH is higher than its pKa (Holanda et al., 2019b). In all tested conditions, including FeSSIF media, the GEM:TCA co-crystal demonstrated lower solubility than GEM (p = 0.05).
The results presented in Figure 10 confirmed that the co-crystal is less soluble in acidic media when crystalline phase conversion was not observed and more soluble in basic media when a new diffraction pattern corresponding to the anhydrous form of GEM was observed. Therefore, through the solubility method, it can be observed that there is a correlation between the new structures found in the co-crystal, as there was a transition of crystalline phases evidenced in the diffractograms. Thus, it can be affirmed that there is the formation of a new co-crystal.
Some hypotheses describe reasons for the lower solubility of the co-crystal, such as higher lattice energy, lower solvation, and modification in the melting point of the new solid form due to strong hydrogen interactions or even to the recrystallization of the API (conversion to the more stable form) after the maximum dissolution of the co-crystal (Maheshwari et al., 2012; Rajesh Goud et al., 2014). The single crystal can help understand the exact reasons that led the co-crystal to be less soluble than the API and will constitute a research purpose for future studies.
Although cocrystal formation is normally used to improve solubility, it is noteworthy that a highly soluble cocrystal is not always desired (Rocha et al., 2016). There are works published in the literature demonstrating that the advantage of co-crystal for some drugs is the improvement of physical and chemical stability can be a strategy to be used to develop more stable and not necessarily more soluble products (Rocha et al., 2016).
In terms of drug delivery strategy, there are pharmacokinetic models whose main target would not be to focus on immediate and rapid release but rather to delay or prolong the release of the drug (Xuan et al., 2020). A promising strategy could be the use of less soluble cocrystals in delayed or prolonged release formulations without the need to use matrices or polymers with the pharmaceutical function of modulating drug release (Xuan et al., 2020).
Cell viability studies
Caco-2 cells are usually used to determine transcellular drug absorption through in vitro permeability assays. The first step in conducting this research is determining what quantities can be utilized safely in the cell viability assay (Liu et al., 2002). The MTT reagent can be used for this purpose since the reduction of MTT to formazan, through NADH, or other reducing molecules, is directly proportional to the number of living cells (Fernandes et al., 2012). In this work, the MTT reagent was used to determine the number of viable Caco-2 cells after exposure to substances GEM-ATC 1:1 (synthesized by the LAG), GEM, and ATC at concentrations of 10, 50, and 100 μM.
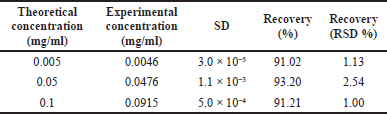 | Table 2. Accuracy and recovery testing. [Click here to view] |
 | Figure 7. Chromatogram referring to the GEM elution. [Click here to view] |
 | Figure 8. Chromatogram referring to the ATC elution. [Click here to view] |
 | Figure 9. Chromatogram referring to the mobile phase elution. [Click here to view] |
The cell viability assay showed that GEM, TCA, and GEM:TCA co-crystal at concentrations of 10, 50, and 100 μM under the conditions tested did not present a statistical difference (p < 0.05) compared to the control group (Fig. 11). A cytotoxic effect is only considered when the reduction in the number of viable cells is higher than 30% (Fernandes et al., 2012). Therefore, the combination of GEM and TCA is not cytotoxic to Caco-2 cells.
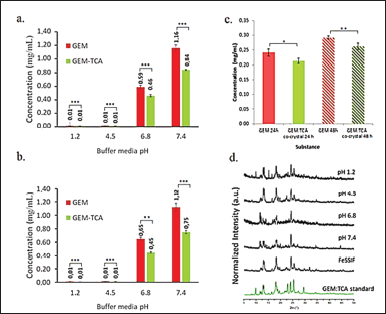 | Figure 10. Solubility graph comparing GEM and GEM:TCA co-crystal (a) after 24 hours and (b) after 48 hours in pH 1.2, 4.5, 6.8, and 7.4, (n = 3); (c) GEM and GEM:TCA co-crystal solubility comparison after 24 and 48 hours in FeSSIF media (n = 3); (d) PXRD of remaining GEM:TCA co-crystal powder after solubility assay in solutions buffer 1.2, 4.5, 6.8, and 7.4 and FeSSIF media and GEM:TCA co-crystal before this assay. [Click here to view] |
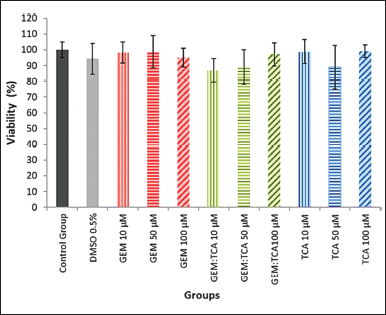 | Figure 11. Cell viability for Caco-2 cells after 3 hours of exposure to the substances GEM, TCA, and co-crystal GEM:TCA at 10, 50, and 100 μM and 0.5% DMSO (v/v) (vehicle control). There was no statistical difference (p < 0.05) between the groups. [Click here to view] |
CONCLUSION
The field of solid-state chemistry represents a great strategy for the characterization of the physicochemical properties and the efficiency of the co-crystal compared to pure API.
After analyzing the dataset obtained using the techniques of characterization (FITR, DSC, TGA, and PXRD) and comparing them to the reported results by other research groups in the literature, it was concluded that the GEM-ATC system, in an equimolar ratio of 1:1, was able to promote the formation of pharmaceutical cocrystal by the LAG method using ethanol through hydrogen bonds between the carboxylic acid groups between these two acids (GEM and TCA) forming dimers or catemers.
The systematic evaluation of the chromatographic method by the partial validation showed that the analytical method used is linear in the range of concentrations used in this study, precise, selective, and exact, in such a way that could be used to quantify the GEM present in the samples from the solubility test.
When evaluating the solubility and behavior of the GEM-TCA co-crystal, obtained by LAG, in buffered media (pH 1.2, 4.5, 6.8, and 7.4) and biorelevant media FeSSIF, it was possible to conclude that the co-crystallization process was able to decrease the solubility of the GEM.
The lower dissolution of these co-crystals is related to changes in intermolecular interactions, which resulted in a more stable crystalline structure and higher density; however, in terms of drug release, it may be an opportunity to use more stable and less soluble cocrystals for the design of delayed or prolonged release pharmaceutical formulations without the need to use matrices or polymers with a pharmaceutical function to modulate drug release.
The co-crystal did not prove to be cytotoxic to the cells studied, and the cell viability assay in cells Caco-2 revealed that TCA, GEM, and the GEM-TCA cocrystal did not significantly reduce the percentage of viable cells. In this way, the concomitant use of GEM and TCA can be tested in models using these cells in concentrations up to 100 μM.
The combination of these substances, through co-crystallization, can be pharmacologically favorable, assuming a possible synergistic effect, since GEM has a probable potential to soften the evolution of Alzheimer’s disease and that TCA has been shown to be able to reduce β-amyloid plaque (Chandra et al., 2019; Pahan et al., 2002).
ACKNOWLEDGMENTS
The authors wish to thank CNPq (process number 130444/2019-7) and the Extension and Research Support Fund of the University of Campinas (project number 3202/19) for their financial support. They are also grateful to Ms. Loredana Gomes from the Federal University of Piauí and Ms. Rafaella Peixoto from Fluminense Federal University for their contribution to the cell viability studies.
AUTHORS CONTRIBUTION
All authors have contributed significantly to the work presented here, and these authors contributed equally.
CONFLICTS OF INTEREST
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Aitipamula S, Banerjee R, Bansal AK, Biradha K, Cheney ML, Choudhury AR, Desiraju GR, Dikundwar AG, Dubey R, Duggirala N, Ghogale PP, Ghosh S, Goswami PK, Goud NR, Jetti RRKR, Karpinski P, Kaushik P, Kumar D, Kumar V, Mukherjee BMA, Mukherjee G, Myerson AS, Puri V, Ramanan A, Rajamannar T, Reddy CM, Rodriguez-Hornedo N, Rogers RD, Row TNG, Sanphui P, Shan N, Shete G, Singh A, Sun CC, Swift JA, Thaimattam R, Thakur TS, Thaper RK, Thomas SP, Tothadi S, Vangala VR, Variankaval N, Vishweshwar P, Weyna DR, Zaworotko MJ. Polymorphs, salts, and cocrystals: what’s in a name? Cryst Growth Des, 2012; 12(5):2147–52. CrossRef
Baka E, Comer JEA, Takács-Novák K. Study of equilibrium solubility measurement by saturation shake-flask method using hydrochlorothiazide as model compound. J Pharm Biomed Anal, 2008; 46(2):335–41. CrossRef
Barbosa TWL, Talmeli RMS, Junior HO, Doriguetto AC, de Araújo MB, Bonfilio R. Solid state characterization, solubility, intrinsic dissolution and stability behavior of allopurinol hydrochloride salt. Drug Dev Ind Pharm, 2021; 47(5):799–808. CrossRef
Barmpalexis P, Karagianni A, Nikolakakis I, Kachrimanis K. Preparation of pharmaceutical cocrystal formulations via melt mixing technique: a thermodynamic perspective. Eur J Pharm Biopharm, 2018; 131:130–40. CrossRef
Bhatia M, Kumar A, Verma V, Devi S. Development of ketoprofen-p-aminobenzoic acid co-crystal: formulation, characterization, optimization, and evaluation. Med Chem Res, 2021; 30:2090–102. CrossRef
Chadha R, Bhandari S, Haneef J, Khullar S, Mandal S. Cocrystals of telmisartan: characterization, structure elucidation, in vivo and toxicity studies. CrystEngComm, 2014; 16:8375–89. CrossRef
Chandra S, Roy A, Jana M, Pahan K. Cinnamic acid activates PPARα to stimulate lysosomal biogenesis and lower amyloid plaque pathology in an Alzheimer’s disease mouse model. Neurobiol Dis, 2019; 124:379–95. CrossRef
Cherukuvada S, Guru Row TN. Comprehending the formation of eutectics and cocrystals in terms of design and their structural interrelationships. Cryst Growth Des, 2014; 14(8):4187–98. CrossRef
Cherukuvada S, Nangia A. Eutectics as improved pharmaceutical materials: design, properties and characterization. Chem Commun, 2014; 50:906–23. CrossRef
Datta S, Grant DJW. Crystal structures of drugs: advances in determination, prediction and engineering. Nat Rev Drug Discov, 2004; 3:42–57. CrossRef
Desiraju GR. Supramolecular Synthons in crystal engineering—a new organic synthesis. Angew Chem Int Ed Engl, 1995; 34(21):2311–27. CrossRef
Devi S, Kumar A, Kapoor A, Verma V, Yadav S, Bhatia M. Ketoprofen-FA Co-crystal: in vitro and in vivo investigation for the solubility enhancement of drug by design of expert. AAPS PharmSciTech, 2022; 23:101. CrossRef
Ekmekcioglu C, Feyertag J, Marktl W. Cinnamic acid inhibits proliferation and modulates brush border membrane enzyme activities in Caco-2 cells. Cancer Lett, 1998; 128(2):137–44. CrossRef
Emami S, Siahi-Shadbad M, Adibkia K, Barzegar-Jalali M. Recent advances in improving oral drug bioavailability by cocrystals. BioImpacts, 2018; 8(4):305–20. CrossRef
Fernandes MB, Gonçalves JE, Scotti MT, de Oliveira AA, Tavares LC, Storpirtis S. Caco-2 cells cytotoxicity of nifuroxazide derivatives with potential activity against Methicillin-resistant Staphylococcus aureus (MRSA). Toxicol In Vitro, 2012; 26(3):535–40. CrossRef
Fernandes GJ, Rathnanand M, Kulkarni V. Mechanochemical synthesis of carvedilol cocrystals utilizing hot melt extrusion technology. J Pharm Innov, 2019; 14:373–81. CrossRef
Gorain B, Choudhury H, Pandey M, Madheswaran T, Kesharwani P, Tekade RK. Drug–excipient interaction and incompatibilities. Dosage form design parameters, Elsevier, pp 363–402, 2018. CrossRef
Haneef J, Chadha R. Drug-drug multicomponent solid forms: cocrystal, coamorphous and eutectic of three poorly soluble antihypertensive drugs using mechanochemical approach. AAPS PharmSciTech, 2017; 18:2279–90. CrossRef
Holanda BBC, Bannach G, Ramos Silva M, Eusébio MES, Castro RAE. Polymorphism of gemfibrozil: investigation by thermal and spectroscopic methods. Thermochim Acta, 2019a; 675:113–8. CrossRef
Holanda BBC, Alarcon RT, Gaglieri C, de Souza AR, Castro RAE, Rosa PCP, Tangerino DJA, Bannach. Thermal studies, degradation kinetic, equilibrium solubility, DFT, MIR, and XRPD analyses of a new cocrystal of gemfibrozil and isonicotinamide. J Therm Anal Calorim, 2019b; 136:2049–62. CrossRef
Jones W, Motherwell WDS, Trask AV. Pharmaceutical cocrystals: an emerging approach to physical property enhancement. MRS Bull, 2006; 31:875–9. CrossRef
Karagianni A, Malamatari M, Kachrimanis K. Pharmaceutical cocrystals: new solid phase modification approaches for the formulation of APIs. Pharmaceutics, 2018; 10(1):1–30. CrossRef
Kavanagh ON, Croker DM, Walker GM, Zaworotko MJ. Pharmaceutical cocrystals: from serendipity to design to application. Drug Discov Today, 2019; 24(3):796–804. CrossRef
Khan M, Enkelmann V, Brunklaus G. O−H···N Heterosynthon: a robust supramolecular unit for crystal engineering. Cryst Growth Des, 2009; 9(5):2354–62. CrossRef
Kuminek G, Cao F, Bahia de Oliveira da Rocha A, Gonçalves Cardoso S, Rodríguez-Hornedo N. Cocrystals to facilitate delivery of poorly soluble compounds beyond-rule-of-5. Adv Drug Deliv Rev, 2016; 101:143–66. CrossRef
Lipert MP, Roy L, Childs SL, Rodríguez-Hornedo N. Cocrystal solubilization in biorelevant media and its prediction from drug solubilization. J Pharm Sci, 2015; 104(12):4153–63. CrossRef
Liu Y, Peterson DA, Kimura H, Schubert D. Mechanism of cellular 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction. J Neurochem, 2002; 69(2):581–93. CrossRef
Luo R, Su L-Y, Li G, Yang J, Liu Q, Yang L-X. Activation of PPARA-mediated autophagy reduces Alzheimer disease-like pathology and cognitive decline in a murine model. Autophagy, 2020; 16(1):52–69. CrossRef
Maheshwari C, André V, Reddy S, Roy L, Duarte T, Rodríguez-Hornedo N. Tailoring aqueous solubility of a highly soluble compound via cocrystallization: effect of coformer ionization, pHmax and solute–solvent interactions. CrystEngComm, 2012; 14:4801–11. CrossRef
Morissette SL, Almarsson O, Peterson LM, Remenar JF, Read MJ, Lemmo AV, Ellis S, Cima MJ, Gardner CR. High-throughput crystallization: polymorphs, salts, co-crystals and solvates of pharmaceutical solids. Adv Drug Deliv Rev, 2004; 56(3):275–300. CrossRef
Nikoli? V, Ili?-Stojanovi? S, Petrovi? S, Ta?i? A, Nikoli? L. Administration routes for nano drugs and characterization of nano drug loading. Characterization and biology of nanomaterials for drug delivery, Elsevier, pp 587–625, 2019. CrossRef
Pahan K, Jana M, Liu X, Taylor BS, Wood C, Fischer SM. Gemfibrozil, a lipid-lowering drug, inhibits the induction of nitric-oxide synthase in human astrocytes. J Biol Chem, 2002; 277(48):45984–91. CrossRef
Prindiville JS, Mennigen JA, Zamora JM, Moon TW, Weber J-M. The fibrate drug gemfibrozil disrupts lipoprotein metabolism in rainbow trout. Toxicol Appl Pharmacol, 2011; 251(3):201–8. CrossRef
Rajesh Goud N, Khan RA, Nangia A. Modulating the solubility of sulfacetamide by means of cocrystals. CrystEngComm, 2014; 16:5859–69. CrossRef
Reutzel-Edens SM. Chapter 10. Analytical Techniques and strategies for salt/co-crystal characterization. In: Wouters J, Quéré L (Eds.). Drug discovery [Internet]. Royal Society of Chemistry, Cambridge, MA, pp 212–46, 2011. Available via http://ebook.rsc.org/?DOI=10.1039/9781849733502-00212 (Accessed 28 December 2021). CrossRef
Reviewer Guidance 1994. Validation of chromatographic methods. Center for Drug Evaluation and Research (CDER), pp 1–30.
Rocha ABO, Rocha AB, Kuminek G, Machado TC, Rosa J, Rauber GS, Borba PA, Siedler S, Stulzer HK, Cuffini SL, Rodríguez-Hornedo N, Cardoso SG. Cocrystals: a promising strategy in pharmaceutical sciences. Quím Nova, 2016; 39(09):1112–25.
Rodrigues M, Baptista B, Lopes JA, Sarraguça MC. Pharmaceutical co-crystallization techniques. Advances and challenges. Int J Pharm, 2018; 547(1–2):404–20. CrossRef
Sathisaran I, Dalvi S. Engineering cocrystals of poorly water-soluble drugs to enhance dissolution in aqueous medium. Pharmaceuticals, 2018; 10(3):1–74. CrossRef
Shaikh R, Singh R, Walker GM, Croker DM. Pharmaceutical cocrystal drug products: an outlook on product development. Trends Pharmacol Sci, 2018; 39(12):1033–48. CrossRef
Shattock TR, Arora KK, Vishweshwar P, Zaworotko MJ. Hierarchy of supramolecular synthons: persistent carboxylic acid·pyridine hydrogen bonds in cocrystals that also contain a hydroxyl moiety. Cryst Growth Des, 2008; 8(12):4533–45. CrossRef
Shayanfar A, Asadpour-Zeynali K, Jouyban A. Solubility and dissolution rate of a carbamazepine–cinnamic acid cocrystal. J Mol Liq, 2013; 187:171–6. CrossRef
Stanton MK, Bak A. Physicochemical properties of pharmaceutical co-crystals: a case study of ten AMG 517 co-crystals. Cryst Growth Des, 2008; 8(10):3856–62. CrossRef
Thakuria R, Sarma B. Drug?drug and drug?nutraceutical cocrystal/salt as alternative medicine for combination therapy: a crystal engineering approach. Cryst, 2018; 8(2):1–39. CrossRef
Wang X, Du S, Zhang R, Jia X, Yang T, Zhang X. Drug-drug cocrystals: opportunities and challenges. Asian J. Pharm. Sci., 2021; 16(3):307-317. CrossRef
Wiggins BS, Saseen JJ, Morris PB. Gemfibrozil in combination with statins—is it really contraindicated? Curr Atheroscler Rep, 2016; 18:1–18. CrossRef
Xuan B, Xuan B, Wong SN, Zhang Y, Weng J, Tong HH, Wang C, Sun CC, Chow SF. Extended release of highly water soluble isoniazid attained through cocrystallization with curcumin. Cryst Growth Des, 2020; 20(3):1951–60. CrossRef
Zhou L, Yang L, Tilton S, Wang J. Development of a high throughput equilibrium solubility assay using miniaturized shake-flask method in early drug discovery. J Pharm Sci, 2007; 96(11):3052–71. CrossRef