INTRODUCTION
Ulcerative colitis (UC) is a long-term inflammatory disease of the large intestine and rectum. The character of UC appears as recurrent and transmitting mucosal inflammation beginning in the rectum and spreads to the proximal segment of the colon (Ungaro et al., 2017). The typical symptoms of UC include bloody diarrhea, fecal urgency, tenesmus, and lower abdominal pain (Collins and Rhodes, 2006). Chronic symptoms with an improper treatment may develop severity of the disease, and resulting in colorectal cancer (Jang et al., 2022). Extraintestinal manifestation (EIM) is frequently associated with UC, affecting organs outside the gastrointestinal tract, such as kidneys (Levine and Burakoff, 2011). Patients with inflammatory bowel disease (4%–23%) had kidney or urination problems (Ambruzs and Larsen, 2018). Kidney EIM includes nephrolithiasis, glomerulonephritis, tubulointerstitial nephritis, and amyloidosis (Levine and Burakoff, 2011). Sulfasalazine (SUL) is the most common treatment for mild to moderate UC (Ungaro et al., 2017). However, SUL can result in serious side effects, e.g., interstitial nephritis, tubular atrophy, kidney failure, and renal necrosis (Niknahad et al., 2017).
Garcinia mangostana Linn. (GM) is native to the tropical areas of Myanmar, Malaysia, the Philippines, and Thailand (Pedraza-Chaverri et al., 2008). α-Mangostin (MGS) was majorly found among significant xanthones taken from the GM pericarp (Ibrahim et al., 2016). GM pericarp extract possesses several beneficial properties such as antitumoral, antibacterial, antifungal, antioxidative, and anti-inflammatory activities. GM pericarp has been widely experimented with in rodent models (Pedraza-Chaverri et al., 2008).
Cytochrome P450 (CYP) is a super-enzyme responsible for metabolizing foods, drugs, and xenobiotic in the body. CYP constitutively expresses in various organs, such as the liver, kidneys, and colon. CYP in families 1–3, including Cyp1a1, Cyp1a2, Cyp2b9/10, Cyp2c29, Cyp2d9, Cyp2e1, Cyp3a11, and Cyp3a13, are majorly responsible for metabolism and biotransformation. Alteration of CYP expression profiles from dextran sulfate sodium (DSS) and GM pericarp might result in a risk of drug-drug and/or herb-drug interaction, following treatment failure or toxic effects (Court, 2013; Malki and Pearson, 2020). Thereby, this study aimed to examine the impacts of GM pericarp and MGS on the CYP expressions and inflammatory status in the colon and/or kidneys in DSS-induced UC mice.
MATERIALS AND METHODS
Chemicals and reagents
MGS (Cas No. 6147-11-1, >98% purity, Batch PR15092126) was obtained from Chengdu Biopurify Phytochemicals (Republic of China). SUL was a product of Sigma Chemicals (Saint Louis, MO). ReverTraAce® was from Toyobo® (Japan). Random primers and ribonuclease inhibitor were provided by Invitrogen® (Carlsbad, CA). Taq polymerase was obtained from Vivantis (Malaysia).
Preparation of the GM pericarp extract
GM was purchased from the Khon Kaen municipal market, Muang Khon Kaen, Thailand (April 2014) and deposited at the Herbarium of Faculty of Pharmaceutical Sciences, Khon Kaen University (KKU). The samples were identified as PANPB-GM 2014-001 by Prof. Waraporn Putalun, Department of Pharmacognosy and Toxicology, KKU, Thailand. GM pericarp was washed 2–3 times and dried at 50°C–60°C before grinding. The powdered GM pericarp was subjected to Soxhlet extraction with ethanol for 3 hours, filtered, evaporated, and lyophilized to achieve the ethanolic GM pericarp extract. MGS content of the extract was determined by a high-performance liquid chromatographic system, which consisted of a C18 column (Phenomenex® Luna 5 µm C18 100 Å, 4.6 × 250 mm, Waldbronn, Germany) coupled with an isocratic mobile phase of 85% acetonitrile in distilled water. The flow rate was 1 ml/minute and the UV detector was set at 244 nm (Tatiya-aphiradee et al., 2018). HPLC chromatograms of standard MGS and the extract are depicted in Figure 1. The MGS content of the ethanolic GM pericarp extract was 17.93% ± 0.08% dry weight (Tatiya-aphiradee et al., 2021a).
Animals and experimental design
Six-week-old male Institute of Cancer Research (ICR) mice weighing 25–30 g (from NELAC, KKU , Thailand) were acclimated in polycarbonate cages in an animal room of Faculty of Pharmaceutical Sciences, KKU, maintained at 25°C with a controlled 12 hours dark-light cycle under ad libitum feeding for 7 days. All animal handling and experimental protocols were implemented in accordance with the Institutional Animal Care and Use Committee of the Northeast Laboratory Animal Center, KKU (Approval No. IACUC-KKU-26/61) under supervision of a certified veterinary.
Mice were randomly divided into seven groups (N = 9 each); control, non-treatment, GM40, GM200, GM1,000, MGS, and SUL. UC was induced with intragastric administration of a 40 kDa-DSS solution (6 g/kg/day; 0.25 ml 21% DSS four times per day) for four consecutive days, as shown in Table 1 (Tatiya-aphiradee et al., 2020; Tatiya-aphiradee et al., 2021a). One day after the last treatment, the mice were euthanized with Zoletil® (100 mg/kg). The colon and kidneys were collected and kept at −80°C for further analysis.
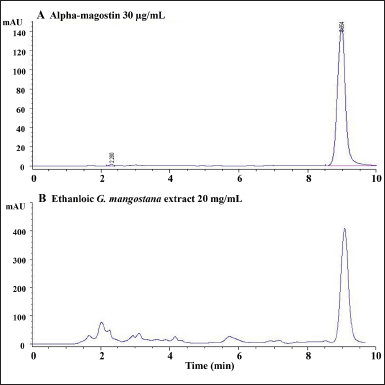 | Figure 1. HPLC chromatograms of A, α-mangostin 30 µg/ml and B, ethanolic GM pericarp extract 20 mg/ml. [Click here to view] |
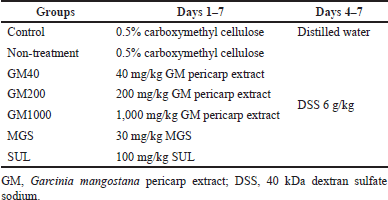 | Table 1. Animal treatment. [Click here to view] |
DSS-induced UC index determination
For disease activity index (DAI) determination, each mouse was placed in a cage without bedding for 15 minutes to examine the stool characteristics (Chassaing et al., 2014). Body weight, stool consistency, and the presence of blood in stool were recorded daily. Body weight loss, reduced stool consistency, and bleeding in stools were scored to determine the DAI (Tatiya-aphiradee et al., 2020; Xiao et al. 2013). One day after the last treatment, colons were collected for colon length measurement (Tatiya-aphiradee et al., 2020; Xiao et al., 2013) and colon tissue was embedded in a paraffin block and sectioned at 5-μm thickness for alcian blue staining. After the slide was rehydrated, it was soaked in 3% acetic acid at pH 2.5 for 3 minutes, followed by staining with alcian blue at pH 2.5 for 30 minutes and counterstaining with eosin for 5 minutes. All slides were covered with coverslips using a mounting medium (Tatiya-aphiradee et al., 2020). Images were collected at 1,000-fold magnification using an Olympus CX23-microscope and EP50 camera (Olympus, Tokyo, Japan).
Determination of mRNA expression by RT-qPCR
Total RNA was procured from the colon or kidney by guanidinium thiocyanate-phenol-chloroform technique (Nopwinyoowong et al., 2022) and reverse transcribed to cDNA using ReverTraAce®. cDNA of the CYP and inflammatory-associated genes, as well as the reference gene Gapdh was amplified by qPCR with specific primers (Table 2). mRNA expression was normalized to Gapdh and relative fold expression was calculated via ??Ct technique. The changes in Ct (?Ct) between the genes of interest and the mean of the reference (Gapdh) gene and the changes in ΔCt between the treatments and the control (ΔΔCt) were calculated. The fold change in expression between samples was calculated from 2−(??Ct) (Chatuphonprasert et al., 2020; Livak and Schmittgen, 2001).
Statistical analysis
The results are shown as mean ± standard deviation. One-way analysis of variance was carried out, followed by Tukey’s post hoc test (IBM SPSS ver. 26, Armonk, NY). A significance was considered at p < 0.05.
RESULTS AND DISCUSSION
DSS determines UC characters by their molecular weight, dosage, and the treatment period. DSS of 500 kDa failed to induce UC, while those lower molecular weights of 5 and 40 kDa for 7 days-treatment induced UC in BALB/c mice (Kitajima et al., 2000). DSS (5 kDa) caused mild UC, whilst DSS (40 kDa) developed more severity (Kitajima et al., 2000). Administration of 2%–5% DSS (36-50 KDa) to inbred mice for 4–9 days persuaded acute UC, whereas 15 days-period of 0.5% DSS led to chronic UC (Nishihara et al., 2006). Our previous study supported that 6g/kg of 40 kDa DSS for four consecutive days successfully induced UC in ICR mice, demonstrating weight loss, bloody stool, shortened colon, crypt erosion, mucin depletion, and inflammatory and mast cell infiltration (Tatiya-aphiradee et al., 2020; Tatiya-aphiradee et al., 2021a).
 | Table 2. Forward and reverse primers for qPCR. [Click here to view] |
GM pericarp extract protected against DSS-induced UC
The severity of UC was indicated by the DAI score (Xiao et al. 2013). The DAI scores were significantly higher after DSS administration for 4 days and all treatments, including GM pericarp extract, MGS, and SUL, reduced the DAI score (Fig. 2A). Likewise, the colons were significantly shortened after DSS induction (Fig. 2B). Interestingly, GM pericarp extract, MGS, and SUL restored the colon length comparable to the control. Distribution of mucin in colon tissue was examined by alcian blue staining (Fig. 2C). A significant pathological feature of DSS-induced UC is goblet cell loss, leading to mucin depletion (Tatiya-aphiradee et al., 2021a). The control demonstrated mucin-full crypt cavities (yellow arrows). The administration of DSS depleted the amount of mucin, as seen in the non-treatment group. Treatment with GM pericarp extract (40–1,000 mg/kg) and MGS preserved the colon tissue and restored the amount of mucin, while treatment with SUL was inferior. These findings reveal that GM pericarp extract and MGS potentially possess colon protective effects.
Effect of the GM pericarp extract and MGS on the mRNA expression of CYPs in the mouse colons
Typically, hepatic CYP enzymes are responsible for metabolism; nonetheless, intestinal CYPs likewise metabolized orally administered drugs and xenobiotics and contributed to the first-pass metabolism of oral medications (Xie et al., 2016). Colonic CYP profiles of UC patients were modified; expression of CYP2B6, CYP2E1, and CYP3A4 were slightly induced, while those of CYP2C9 and CYP1A1 were suppressed (Plewka et al., 2014).
Figure 1 showed DSS-modified colonic CYP mRNA profiles and the neutralizing effect of the GM pericarp extract and MGS. Cyp1a1, Cyp2b9/10, Cyp2c29, Cyp2d9, Cyp2e1, Cyp3a11, and Cyp3a13 expressions were significantly suppressed after DSS administration (Fig. 3A–G). The GM pericarp extract (40–1,000 mg/kg) up-regulated the CYP profiles to the levels comparable to control, except that of Cyp2b9/10. The lowest dose of GM pericarp extract (40 mg/kg) did not restore Cyp2b9/10 expression (Fig. 3B). MGS and SUL returned the CYP profiles equivalent to the GM pericarp extract.
In addition, Tatiya-aphiradee et al. (2021a) reported that DSS-induced UC suppressed mRNA expression of inflammatory-associated genes, namely Tnf-α, Mcp-1, Tlr-2, Icam-1, and Vcam-1, in mouse colons, by which these were up-regulated by the GM pericarp extract, leading to restoration of inflammatory revival in the colons. These observations suggested a relevance of inflammatory-associated genes and CYP profiles in the colon; up-regulating expression of inflammatory-associated genes down-regulating expression of CYP profiles. Likewise, GM pericarp extract reduced inflammation, causing an increase in CYP expression profiles. These findings revealed that CYP expression was inversely proportional to inflammation. IL-6 down-regulated orphan nuclear receptors, including constitutive androstane receptor (CAR), retinoid X receptor (RXR), and pregnane X receptor (PXR). In general, CAR regulated expression of Cyp2b9/10, Cyp2c29, Cyp3a11, and Cyp3a13 mRNAs, whilst RXR transactivated transcription of Cyp3a11 and Cyp3a13 and PXR associated with regulation of Cyp2c29, Cyp3a11, and Cyp3a13 (Down et al., 2007; Kusunoki et al., 2015). Albeit inflammation is corrected, IL-1β, IL-6, and TNF-α are lessened with meanwhile PXR and CAR normalization reinstated Cyp2b9/10, Cyp2c29, Cyp3a11, and Cyp3a13 expression (Kusunoki et al., 2015).
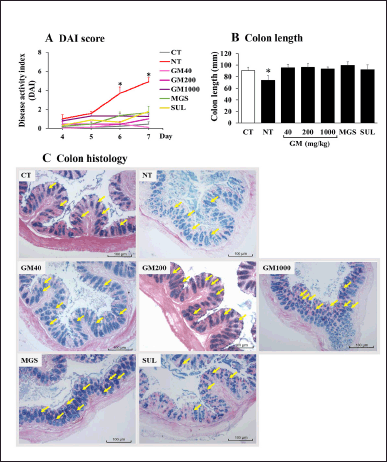 | Figure 2. Effects of GM pericarp extract on A, DAI, B, colon length, and C, colon histology. The results are expressed as mean ± SD (n = 9). *p < 0.05 VS CT (control). GM, Garcinia mangostana pericarp extract; MGS, α-mangostin; SUL, sulfasalazine. [Click here to view] |
Effect of the GM pericarp extract and MGS on the mRNA expression of CYPs and inflammatory-associated genes in the mouse kidneys
Kidneys comprise a large number of CYPs enzymes; 4%–20% of hepatic CYP protein contents (Gundert-Remy et al., 2014). DSS-induced UC caused renal injury and inflammation. Wistar rats daily received 5% DSS for 7 days showed an increase in creatinine, a biochemical parameter of renal function (Rtibi et al. 2016). Correspondingly, DSS extensively suppressed expression of renal CYP and inflammatory-associated genes (Fig. 4). Recovery of the CYP profiles was achieved after the GM pericarp extract, MGS, and SUL administration (Fig. 4A–E and G), excluding MGS-treated Cyp3a11 expression (s).
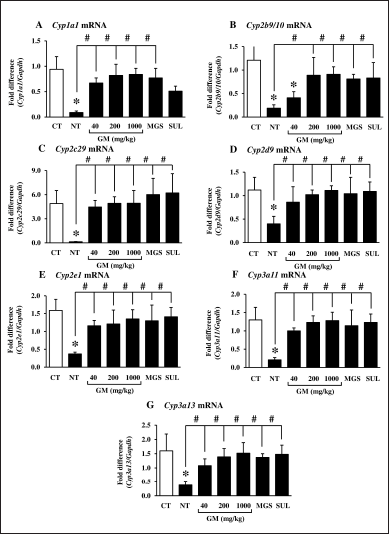 | Figure 3. Effects of GM pericarp extract and MGS on the mRNA expression of CYPs in the colons of DSS-induced UC mice. A, Cyp1a1; B, Cyp2b9/10; C, Cyp2c29; D, Cyp2d9; E, Cyp2e1; F, Cyp3a11; G, Cyp3a13. The results are expressed as mean ± SD (n = 9). *p < 0.05 VS CT (control), #p < 0.05 VS NT (non-treatment). GM, Garcinia mangostana pericarp extract; MGS, α-mangostin; SUL, sulfasalazine. [Click here to view] |
In addition, mRNA expression of inflammatory-associated genes, namely tumor necrosis factor-α (Tnf-α), monocyte chemoattractant protein-1 (Mcp-1), and nuclear factor kappa b (Nf-κb) was significantly induced after DSS administration (Fig. 5). The GM pericarp extract and MGS significantly returned those expressions to the levels comparable to the control. SUL extensively suppressed Tnf- α and Mcp-1 expression (Fig. 5A and B), while that of Nf-κb was slightly lessened and even significantly different to the control (Fig. 5C). TNF-α is a pro-inflammatory cytokine generated by T-lymphocyte, macrophage, and natural killer (NK) cell (Jang et al., 2021). Tnf- α is triggered by a foreign body or immune system, including B and T lymphocytes and fibroblast, to produce various inflammatory cytokines and mediators such as interleukin 1 (IL-1), IL-6, IL-8, and granulocyte monocyte colony-stimulating factor (Jang et al., 2021; Shanahan and Clair, 2002). MCP-1, a member of the C-C chemokine family, is induced by inducing cytokines, oxidative stress, or growth factor. Mcp-1 serves to regulate the migration and infiltration of monocyte, memory T lymphocyte, and NK cell in inflammatory response (Deshmane et al., 2009). Nf-κb is a transcription factor regulating the expression of critical inflammatory mediators, including IL-1, IL-6, IL-8, IL-12, Tnf-α, and cyclooxygenase-2 (Cox-2). Moreover, chronic inflammation stimulates Nf-κb to generate more inflammatory cytokines (Liu et al., 2017). Administration of 3.5% of DSS for 5 days increased neutrophil infiltration in kidney interstitium and induced expression of Il-6, interferon γ-induced protein 10 (Ip-10), Mcp-1, and Cox-2 in C57BL/6 mouse kidneys (Ranganathan et al., 2013). In consistency, the present study observed up-regulation of Tnf-α, Mcp-1, and Nf-κb expression, suggesting the occurrence of an inflammatory response.
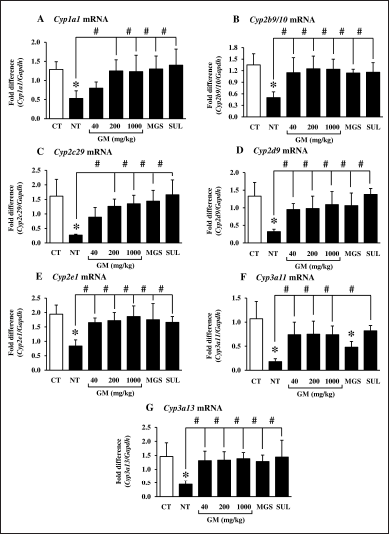 | Figure 4. Effects of GM pericarp extract and MGS on the mRNA expression of CYPs in the kidneys of DSS-induced UC mice. A, Cyp1a1; B, Cyp2b9/10; C, Cyp2c29; D, Cyp2d9; E, Cyp2e1; F, Cyp3a11; G, Cyp3a13. The results are expressed as mean ± SD (n = 9). *p < 0.05 VS CT (control), #p < 0.05 VS NT (non-treatment). GM, Garcinia mangostana pericarp extract; MGS, α-mangostin; SUL, sulfasalazine. [Click here to view] |
Conclusively, DSS-induced UC injured kidneys, leading to the alteration of renal CYP profiles and the inflammatory response. These findings suggested that suppression of renal CYP expression profiles resulted from the increased inflammation in the kidneys, by which the GM pericarp extract and MGS help to revive the inflammation. A decrease in the inflammation restored the CYP expression profiles, brought about normalizing CYP functions. Therefore, the GM pericarp extract is a potential candidate to develop as an alternative medicine for UC and kidney EIM therapy with a low risk of herb-drug interaction.
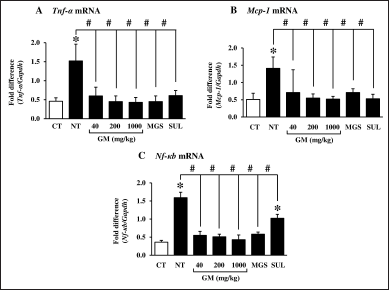 | Figure 5. Effects of GM pericarp extract and MGS on the mRNA expression of inflammatory-associated genes in the kidneys of DSS-induced UC mice. A, Tnf-α; B, Mcp-1; C, Nf-κb. The results are expressed as mean ± SD (n = 9). *p < 0.05 VS CT (control), #p < 0.05 VS NT (non-treatment). GM, Garcinia mangostana pericarp extract; MGS, α-mangostin; SUL, sulfasalazine. [Click here to view] |
CONCLUSION
UC induces EIM by inducing renal inflammation and affecting CYP expression profiles in the kidneys and colon. Promisingly, the GM pericarp extract and MGS down-regulated the expression of inflammatory-associated genes, including Tnf-α, Mcp-1, and Nf-κb in the mouse kidneys and normalized the expression of CYPs in the mouse kidneys and colon comparable to the standard drug SUL. Hence, it is worthwhile developing the GM pericarp extract for alternative therapy of UC and kidney EIM.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This project was financial supported by Research Group for Pharmaceutical Activities of Natural Products Using Pharmaceutical Biotechnology (PANPB), Faculty of Pharmaceutical Sciences, Khon Kaen University, Khon Kaen, Thailand.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The Institutional Animal Care and Use Committee of the Northeast Laboratory Animal Center, KKU (Approval No. IACUC-KKU-26/61) under supervision of a certified veterinary.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation
REFERENCES
Ambruzs JM, Larsen CP. Renal manifestations of inflammatory bowel disease. Rheum Dis Clin North Am, 2018; 44(4):699–714.
Chassaing B, Aitken JD, Malleshappa M, Vijay-Kumar M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr Protoc Immunol, 2014; 104(1):15–25.
Chatuphonprasert W, Jarukamjorn K, Kondo S, Nemoto N. Synergistic increases of metabolism and oxidation–reduction genes on their expression after combined treatment with a CYP1A inducer and andrographolide. Chemico-Biol Interact, 2009; 182(2-3):233–238.
Chatuphonprasert W, Nawaratt N, Jarukamjorn, K. Reused palm oil from frying pork or potato induced expression of cytochrome P450s and the SLCO1B1 transporter in HepG2 cells. J Food Biochem, 2020; 44(5):1–1.
Chatuphonprasert W, Sriset Y, Jarukamjorn K. Continuous consumption of reused palm oil induced hepatic injury, depletion of glutathione stores, and modulation of cytochrome P450 profiles in mice. Polish J Food Nutri Sci, 2019; 69(1):53–61.
Collins P, Rhodes J. Ulcerative colitis: diagnosis and management. Br Med J, 2006; 333(7563):340–3.
Court MH. Canine cytochrome P-450 pharmacogenetics. Vet Clin North Am Small Anim Pract, 2013; 43(5):1027–38.
Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (mcp-1): an overview. J Interferon Cytokine Res, 2009; 29(6):313–26.
Down MJ, Arkle S, Mills JJ. Regulation and induction of CYP3A11, CYP3A13 and CYP3A25 in C57BL/6J mouse liver. Arch Biochem Biophys, 2007; 457(1):105–10.
Gundert-Remy U, Bernauer U, Blömeke B, Döring B, Fabian E, Goebel C. Extrahepatic metabolism at the body’s internal-external interfaces. Drug Metab Rev, 2014; 46(3):291–324.
Ibrahim MY, Hashim NM, Mariod AA, Mohan S, Abdulla MA, Abdelwahab SI, Arbab IA. α-Mangostin from Garcinia mangostana L.: an updated review of its pharmacological properties. Arab J Chem, 2016; 9(3):317–29.
Jang DI, Lee AH, Shin HY, Song HR, Park JH, Kang TB, Lee SR, Yang SH. The role of tumor necrosis factor alpha (TNF-α) in autoimmune disease and current TNF-α inhibitors in therapeutics. Int J Mol Sci, 2021; 22(5):2719.
Jang J, Lee SH, Jeong S, Cho J, Kim HJ, Oh SH, Kim DY, Lee H-S, Park SH, Ye BD, Yang S-K, Kim KM. Clinical characteristics and long-term outcomes of pediatric ulcerative colitis: a single-center experience in Korea. Gut Liver, 2022; 16(2):236–45.
Kitajima S, Takuma S, Morimoto M. Histological analysis of murine colitis induced by dextran sulfate sodium of different molecular weights. Exp Anim, 2000; 49(1):9–15.
Kusunoki Y, Ikarashi N, Matsuda S, Matsukawa Y, Kitaoka S, Kon R, Tajima M, Wakui N, Ochiai W, Machida Y, Sugiyama K. Expression of hepatic cytochrome P450 in a mouse model of ulcerative colitis changes with pathological conditions. J Gastroenterol Hepatol, 2015; 30(11):1618–26.
Levine JS, Burakoff R. Extraintestinal manifestations of inflammatory bowel disease. J Gastroenterol Hepatol, 2011; 7(4):235–41.
Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Sig Transduct Target Ther, 2017; 2:17023.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using realtime quantitative PCR and the 2???CT method. Methods, 2001; 25:402–8.
Malki MA, Pearson ER. Drug-drug-gene interactions and adverse drug reactions. Pharmacogenomics J, 2020; 20(3):355–66.
Niknahad H, Heidari R, Mohammadzadeh R, Ommati MM, Khodaei F, Azarpira N, Abdoli N, Zarei M, Asadi B, Rasti M, Yeganeh BS, Taheri V, Saeedi A, Najibi A. Sulfasalazine induces mitochondrial dysfunction and renal injury. Ren Fail, 2017; 39(1): 745-753.
Nishihara T, Matsuda M, Araki H, Oshima K, Kihara S, Funahashi T, Shimomura I. Effect of adiponectin on murine colitis induced by dextran sulfate sodium. Gastroenterology, 2006; 131(3):853–61.
Nopwinyoowong N, Chatuphonprasert W, Tatiya-aphiradee N, Jarukamjorn K. Garcinia mangostana and α-mangostin revive ulcerative colitis-modified hepatic cytochrome P450 profiles in mice. Pak J Biol Sci, 2022; 25:843–51.
Pedraza-Chaverri J, Cárdenas-Rodríguez N, Orozco-Ibarra M, Pérez-Rojas JM. Medicinal properties of mangosteen (Garcinia mangostana). Food Chem Toxicol, 2008; 46(10):3227–39.
Plewka D, Plewka A, Szczepanik T, Morek M, Bogunia E, Wittek P, Kijonka C. Expression of selected cytochrome P450 isoforms and of cooperating enzymes in colorectal tissues in selected pathological conditions. Pathol Res Pract, 2014; 210(4):242–9.
Ranganathan P, Jayakumar C, Santhakumar M, Ramesh G. Netrin-1 regulates colon-kidney cross talk through suppression of IL-6 function in a mouse model of DSS-colitis. Am J Physiol Renal Physiol, 2013; 304(9):F1187–97.
Rtibi K, Selmi S, Jabri M-A, El-Benna J, Amri M, Marzouki L, Sebai H. Protective effect of Ceratonia siliqua L. against a dextran sulfate sodium-induced alterations in liver and kidney in rat. J Med Food, 2016; 19(9):882–9.
Shanahan JC, Clair WS. Tumor necrosis factor-alpha blockade: a novel therapy for rheumatic disease. Clin Immunol, 2002; 103(3):231–42.
Tatiya-aphiradee N, Chatuphonprasert W, Jarukamjorn K. Ethanolic Garcinia mangostana extract and α-mangostin improve dextran sulfate sodium-induced ulcerative colitis via the suppression of inflammatory and oxidative responses in ICR mice. J Ethnopharmacol, 2021a; 265:1–13.
Tatiya-aphiradee N, Chatuphonprasert W, Jarukamjorn K. Garcinia mangostana Linn. pericarp and alpha-mangostin ameliorate dextran sulfate sodium-induced hepatic injury in mice. J Physiol Pharmacol, 2021b;72(3):427–438.
Tatiya-aphiradee N, Chatuphonprasert W, Jarukamjorn K. Oxidative stress exacerbates dextran sulfate sodium-induced ulcerative colitis in ICR mice. Biologia. 2020; 75(11):2063–71.
Tatiya-aphiradee N, Nawanukraw C, Chatuphonprasert W, Jarukamjorn K. Determination of α-mangostin in Garcinia mangostana Linn. pericarp extract and its in vitro antioxidant capacity. Isan J Pharm Sci, 2018; 14(2):83–93.
Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet, 2017; 389(10080):1756–70.
Xie F, Ding X, Zhang QY. An update on the role of intestinal cytochrome P450 enzymes in drug disposition. Acta Pharm Sin B, 2016; 6(5):374–83.
Xiao HT, Lin Cy, Ho DH, Peng J, Chen Y, Tsang SW, Wong M, Zhang XJ, Zhang M, Bian ZX. Inhibitory effect of the gallotannin corilagin on dextran sulfate sodium-induced murine ulcerative colitis. J Nat Prod, 2013; 76(11):2120–5.