INTRODUCTION
Aging is a process of decreasing physiological function that increases the risk of cell damage over time (Fontana et al., 2010). The increasing age of a cell will make its homeostatic mechanism weaker. However, cell aging often occurs earlier due to triggers such as reactive oxygen species (ROS), free radicals, and accumulation of advanced glycation end products (AGEs) (Gkogkolou and Böhm, 2012; Rattan, 2008). ROS are generated in the human body’s metabolism and act as signals (redox) in cellular regulation. However, when the amounts are not balanced, it will have harmful effects. The targets of ROS include DNA, lipid membrane integrity, and protein activity. In addition, the formation of cellular molecules of AGEs can undergo further oxidation resulting in many molecules of AGEs, including toxic ones. A strategy to reduce this damage is to use antiaging compounds (Prastya et al., 2020).
Antiaging compounds can be obtained from plants, one of which is from the leaves of the clove plant. The clove plant (Syzygium aromaticum L.) is a spice plant that belongs to the Myrtaceae family and comes from the Maluku Islands, Indonesia. Clove leaves are rich in metabolites, including eugenol, eucalyptol, caryophyllene, and α-cadinol (Bhuiyan, 2012). Previous studies reported that clove leaf extract has antiaging properties (Fauzya et al., 2019; Lesmana et al., 2021). The antiaging activity is considered inseparable from the presence of secondary metabolite active compounds produced in clove leaves. Secondary metabolites are not only produced by plants but also can be produced by endophytic bacteria that live in the plant tissues.
Endophytic bacteria can live in plant tissues without causing disease symptoms in their hosts. The ability to produce the same active compound as the host is thought to be the effect of a genetic exchange over a long period of evolution (Tan and Zou, 2001). Exploration of active compounds through plants directly requires a lot of biomass, so acquiring active compounds from endophytic bacteria is much faster and more efficient. Studies on the isolation of endophytic bacteria from clove leaves are still limited. Previously, only one report isolated endophytic bacteria from clove leaves and analyzed their extracellular metabolite activity as antioxidants (Triandriani et al., 2020). However, information on the antiaging activity of endophytic bacterial extracts from clove leaves at the cellular level is not yet available. Therefore, this study aims to determine the antiaging properties of the endophytic bacterial extract from clove leaves at the cellular level and identify endophytic bacterial isolates that produce these antiaging compounds based on analysis of the 16S rRNA gene. Schizosaccharomyces pombe was used as a model organism to determine the mode of action of endophytic bacterial extract in regulating antiaging properties. In this regard, the metabolite extracts of endophytic bacteria were assayed for their antioxidant activity in vitro and further evaluated for their antiaging activity through cell viability and mitochondrial activity assay using the model yeast.
MATERIALS AND METHODS
Sample collection and sterilization
Clove leaves samples were obtained from the Indonesian Medicinal and Aromatic Crops Research Institute, Bogor, Indonesia. The selected leaves were characterized as fresh, no disease symptoms, and medium to old aged (Razafimamonjison et al., 2016). Surface sterilization of the samples was carried out following Nurhelmi and Putri (2021) with modifications. The leaves were washed with sterile distilled water; then washed in 70% alcohol for 30 seconds, 2% sodium hypochlorite (NaOCl) for 2 minutes, and 70% alcohol for 30 seconds; and finally rinsed using sterile distilled water three times. A total of 0.1 ml of the last rinse water was spread on tryptic soy agar (TSA) media (17 g/l pancreatic digest of casein, 5 g/l peptic digest of soybean meal, 5 g/l NaCl, and 15 g/l agar) as a negative control test to prove the success of surface sterilization of the sample.
Isolation and purification of endophytic bacteria
The surface sterilized clove leaves were ground using 0.9% NaCl in a ratio of 1:6 (g: ml). The tissue extract from the grinding results was incubated for 3 hours at ±27°C. The tissue extract was diluted with 0.9% NaCl to a dilution of 10−1 and 10−2 (Costa et al., 2012). Each dilution was spread on nutrient agar (NA) (5 g/l tryptone peptone, 2.5 g/l yeast extract, 1 g/l glucose, 15 g/l agar), TSA, and Luria Bertani Agar (LBA) (10 g/l tryptone, 5 g/l yeast extract, 10 g/l NaCl, and 15 g/l agar) which contained 0.2 mM H2O2 as oxidative stress treatment (Sarima et al., 2019). The cultures were incubated at ±27°C for 3 days. Each growing colony was selected and purified using the same isolation media. All endophytic bacteria were then characterized through Gram staining (O’Toole, 2016).
Hemolysis assay
The hemolysis assay was carried out by using 5% sheep blood agar (Thermo Fisher Scientific). Endophytic bacteria were grown on sheep blood agar and incubated at ±27°C for 24 hours (Kalambhe et al., 2016). Klebsiella pneumoniae was used as positive control bacteria. Endophytic bacteria that did not show hemolytic activity were selected as isolates for further analysis.
Molecular identification of bacteria isolates based on 16S rRNA gene
The genomic DNA of bacteria isolates was extracted using Quick-DNA™ Fungal/Bacterial Miniprep (Zymo Research, Irvine, USA), following the manufacturer’s guidelines. 16S rRNA gene was amplified using the primer set designed by Marchesi et al. (1998), 63F (5’-CAGGCCTAACACATGCAAGTC-3’) and 1387R (5’-GGGCGGWGTGTACAAGGC-3’). Each PCR reaction was conducted in a total volume of 50 μl, containing 25 μl GoTaq Green® Master Mix 123 (Promega), 5 μl of forward primer (10 pmol), 5 μl of reverse primer (10 pmol), 4 μl DNA template (100 ng/μl), and nuclease-free water. A negative PCR reaction was prepared as above but without DNA template. The PCR conditions used were predenaturation at 94°C for 5 minutes, denaturation at 94°C for 30 seconds, annealing at 55°C for 45 seconds, elongation at 72°C for 1 minute 45 seconds, and after elongation at 72°C for 10 minutes. PCR was performed in 35 cycles. The PCR products were visualized using 1% agarose gel at 75 V for 30 minutes and sequenced in First Base (Applied Biosystems 3730xl) (Selangor, Malaysia). The 16S rRNA gene sequences generated were compared to the sequences in the National Center for Biotechnology Information (NCBI GenBank) database through the BLAST program (http://www.ncbi.nlm.nih.gov). The parameter thresholds for the species identification included the identity value (≥99% identity), query cover (≥99%), and e value of <10-5. The phylogenetic tree construction was carried out using MEGA 11 software and bootstrap analysis of 1,000× repetitions was carried out using the neighbor-joining method.
Cellular antioxidative stress response assay
The second screening uses the spot assay by following the method of Pudjas et al. (2022) with modifications. Each colony selected in the hemolysis assay was cultured and measured to OD625 = 1. Then, the culture was serially diluted to 10−5. Approximately 2 µl of each dilution was spot on the respective growth media containing the oxidative stress of H2O2 in several higher concentrations than before (4, 6, and 8 mM). Endophytic bacterial isolates that showed higher viability growth were used for the subsequent analysis.
Determination of the stationary phase of selected endophytic bacteria
Determination of the stationary phase was referred to Mahjani and Putri (2020) with modifications. Selected endophytic bacteria were precultured in nutrient broth (NB) (add composition) for 12 hours at ± 27°C. Then, preculture was transferred to a new NB medium as the main culture at initial OD625 = 0.01 in two replications. The main culture was incubated in a shaker incubator at 27°C (100 rpm). The measurement of the OD625 value was carried out every 4 hours until the OD625 value was stable. The initial stable value of OD625 was considered the stationary phase’s starting point.
Extraction of endophytic bacterial extracellular metabolites
The extraction of the extracellular metabolites was according to Kim et al. (1997) and Utami and Putri (2020) with modifications. Selected endophytic bacteria were prepared as previously described in the determination of the stationary phase. The volume of the main culture used was 3,000 ml. Then, the main culture was incubated in a shaking incubator for 28 hours at ±27°C. The product was centrifuged for 30 minutes at 3.000 × g and 4°C to separate the supernatant from the cell mass. The supernatant was filtered through filter paper and concentrated with a rotary evaporator at 50°C to obtain a thick supernatant (a paste). The concentrated supernatant was macerated using three solvents (ethyl acetate, ethanol, and methanol) in a dark bottle with a ratio of 1:10 concentrated supernatant: solvent. The concentrated supernatant was macerated using a solvent with graded polarity. First, ethyl acetate solvent was used, maceration was carried out in a dark bottle, and every 12 hours, it was replaced with new ethyl acetate three times. The residue (insoluble concentrated supernatant) from the previous solvent was added to the second solvent (ethanol), and maceration was carried out with the same steps. Lastly, methanol solvent was added to the residue from the second solvent, and maceration was carried out with the same steps. During the extraction process, stirring was carried out using a shaking incubator. The extract was separated from the residue. Then the solvent was evaporated using a vacuum rotary evaporator. The extract in the form of pasta was stored at 4°C until it was used.
Antioxidant activity
The antioxidant activity was determined using 2,2-Diphenyl-1-picrylhydrazyl (DPPH) radicals described by Yasser et al. (2020) with modifications. 1.5 ml of endophytic bacterial extracts at various concentrations (300, 600, 900, 1,200, and 1,500 µg/ml) were added with 1.5 ml reagent DPPH solution (125 µM diluted in ethanol) (HiMedia, Mumbai, India), respectively. The sample solution was homogenized and incubated at ±27°C in the dark room for 30 minutes. The absorbance value was measured at a wavelength of 517 nm using a 7205 UV/Visible spectrophotometer (Stone, Staffordshire, UK). Ascorbic acid (AA) (add commercial brand) was used as a positive control. Antioxidant activity was expressed in percentage of DPPH radical scavenging activity as measured using the formula:
Sample absorbance was the mixture of sample (endophytic bacterial extract or AA) and DPPH reagent, sample control absorbance was the mixture of endophytic bacterial extract or AA and ethanol, blank absorbance was the mixture of ethanol and DPPH, and blank control absorbance was ethanol.
Cell viability analysis (spot test)
Cell viability analysis was carried out to determine the effect of the endophytic bacterial extract in maintaining the survival of S. pombe. The yeast S. pombe was precultured in liquid yeast extract supplement (YES) medium with 3% (w/v) glucose for 12 hours at ± 27°C. Then, preculture was transferred to a new liquid YES medium as the main culture at initial OD625 = 0.05. The endophytic bacterial extract was added to the main culture in several concentrations. The variation concentration of the endophytic bacterial extract used in this assay was based on the IC50 value of the DPPH assay, including 1/10×, 1/5×, 2/5×, and 4/5 × IC50. These concentrations are equated in units of µg/ml to 25, 50, 100, and 200. Yeast without extract treatment was used as a negative control, while yeast in liquid YES medium with 0.3% (w/v) glucose (calorie restriction) was used as a positive control. Each treatment was carried out in two repetitions. The spot test was carried out in 1, 7, and 11 days of incubation (Prastya et al., 2019). Each culture was harvested and adjusted to OD600 = 1 using a sterile medium and serially diluted to a 10-−4 dilution. Then, each dilution was spot on solid YES medium and incubated at ±27°C for 3 days.
Mitochondria activity assay
Mitochondria activity was assayed using a rhodamine B probe (Sigma-Aldrich, St. Louis, MO). The main culture of S. pombe yeast treated with endophytic bacterial extract was incubated for 24 hours at ±27°C. The main culture was harvested by centrifugation at 5,000 × g for 1 minute to obtain cell pellets. The pellet was washed with 0.05 M phosphate buffer pH 7.4, and rhodamine B probe 100 nM was added. Incubation was carried out for 30 minutes in a dark condition (Lesmana et al., 2021). Fluorescence as a response to mitochondria activity of yeast cells was observed using a fluorescence microscope Olympus BX51.
Liquid chromatography-mass spectrometry (LC-MS/MS) analysis
The endophytic bacterial extract was prepared as described previously on the extraction of endophytic bacterial extracellular metabolites. Bioactive compounds that act as antiaging were identified by LC-MS/MS with Xevo G2-Xs QTof column. H2O with 0.1% formic acid (A) and acetonitrile with 0.1% formic acid (B) was used as mobile phase. The gradient elution was designed from 0–1 minute (5% B), 1–11 minutes (5%–100% B), 11–14 minutes (100% B), and 14–17 minutes (5% B) at a flow rate of 0.3 ml/minute. Electrospray ionization was operated in positive ionization mode with desolvation temperature and capillary of 500°C and 2 kV, respectively. The mass spectrometer was operated with a scanning range of 100–1,200 m/z. PubChem matched the spectrum and compound fragmentation to obtain bioactive compound predictions.
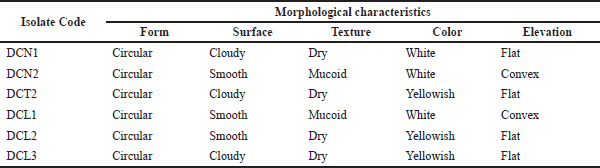 | Table 1. Morphological characteristics of endophytic bacterial isolates from clove leaves. [Click here to view] |
RESULTS
Endophytic bacteria from clove leaves (S. aromaticum L.)
Six isolates of endophytic bacteria were isolated and purified from the leaves of S. aromaticum. The six isolates were obtained from the growth medium of NA (2 isolates), LBA (3 isolates), and TSA (1 isolate). The isolates were then characterized morphologically (Table 1). Gram staining showed that all clove leaf endophytic bacteria cell shapes that had been isolated were bacilli and Gram-positive (Fig. 1).
Molecular identification of endophytic bacteria based on the 16S rRNA gene
The 16S rRNA gene sequences of the six endophytic bacteria isolates were similar to the three genera groups: Bacillus, Niallia, and Cytobacillus (Table 2). Sequences of 6 isolates were deposited in GenBank (www.ncbi.nlm.nih.gov). Based on the phylogenetic tree, isolates DCN1 and DCT2 were placed in the same cluster as Bacillus cereus JCM 2152, isolate DCL3 was placed in the same cluster as Bacillus paranthracis MCCC 1A00395, isolate DCL2 was placed in the same cluster as Cytobacillus kochii strain WCC4582, and isolates DCN2 and DCL 1 were placed in the same cluster as N. nealsonii strain DSM 15077 (Fig. 2).
Hemolytic activity
All endophytic bacterial isolates from clove leaves did not have hemolytic activity ((γ)-hemolysis). It is characterized by the absence of a clear zone and no color change in the media. Klebsiella pneumoniae as positive control showed a clear zone around the colony ((β)-hemolysis) (Fig. 3).
Response towards stronger oxidative stress treatment (second screening)
Five isolates of endophytic bacteria (DCN1, DCN2, DCL1, DCL2, and DCL3) showed higher cell viability against H2O2-induced oxidative stress up to 8 mM. Meanwhile, isolate DCT2 could only survive against H2O2-induced oxidative stress for up to 6 mM (Fig. 4). DCN1 grew with spot thickness up to a dilution of 10−5. Therefore, DCN1 was selected as the most potential isolate in producing antioxidant agents which elicit antiaging properties.
DCN1 turbidity growth curve
The OD625 value of DCN1 was determined to predict the start of the stationary phase, characterized by a stable OD625 value. These data were used to determine harvesting time to obtain secondary metabolites. Based on the results, DCN1 isolate began to stabilize at OD625 = ±3 at 24–44 hours (Fig. 5). Therefore, harvesting of secondary metabolites was chosen at 28 hours.
Extracellular metabolites extraction
The yield percentage of DCN1 extract using ethyl acetate, ethanol, and methanol was variable (Table 3). The highest yield was obtained from methanol extract at 38.083%, followed by ethanol extract (7.180%) and ethyl acetate extract (1.423%).
Antioxidant activities
The three extracts produced using different solvents and concentrated supernatant showed different percentage inhibition at two concentrations (1,000 and 25 µg/m). These two concentrations became the basis for determining the most potential extract as an antioxidant and antiaging agent. Ethyl acetate extract of isolate DCN1 (EAN) was the most active extract because the inhibition was higher than 60% at the concentration of 1,000 μg/ml (Table 4).
Antiaging analysis based on cell viability
EAN could promote the life span of S. pombe yeast cells up to day 11. EAN (50 µg/ml) showed higher viability on day 11 compared to negative control (without extract treatment). It is worth noting that EAN resulted in the best yeast viability with a concentration of 200 µg/ml (Fig. 6). However, all extract treatment exhibited a lower effect on yeast cell viability compared to the positive control treatment (calorie restriction, 0.3% glucose-containing medium).
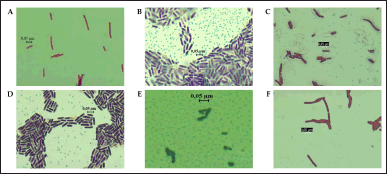 | Figure 1. Gram staining of six clove-endophytic bacterial isolates shows Gram-positive character. A. DCN1, B. DCN2, C. DCT2, D. DCL1, E. DCL2, and F. DCL3. [Click here to view] |
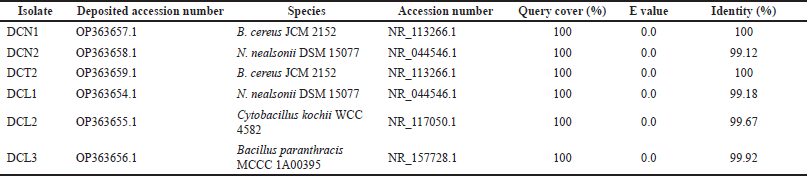 | Table 2. Taxonomic identification of 16S rRNA gene sequences from endophytic bacterial isolates against GenBank database using BLASTN program. [Click here to view] |
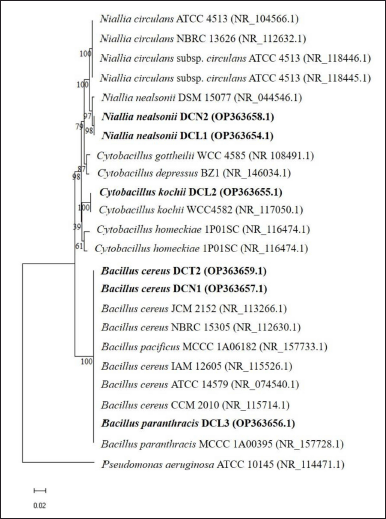 | Figure 2. The phylogenetic tree shows the position of the 16S rRNA gene sequence of six endophytic bacterial isolates from clove leaves among the reference species (reference species was obtained from the GenBank database) using the neighbor-joining method. The phylogenetic tree was constructed using MEGA 11 with a bootstrap of 1000 replications and the bar scale showed a genetic distance of 0.02. [Click here to view] |
LC-MS/MS data analysis
As EAN showed the most significant antiaging properties, we further analyzed the chemical content of this particular extract. Based on LC-MS/MS data, EAN was found to have five dominant compounds, including gallic acid (m/z 170.0817), epigallocatechin (m/z 305.1030), kaempferol (m/z 286.1437), kaempferol 3-o-(6”-malonyl-glucoside) (m/z 535,2717), and rutin (m/z 607.2936) (Fig. 7).
Yeast mitochondrial activity
EAN treatment induced yeast mitochondrial activity in the log phase with 200 and 100 µg/ml concentrations. This concentration significantly increased the mitochondrial membrane potential, as indicated by the intense fluorescence intensity (Fig. 8). Similar results were also observed in the calorie restriction treatment as the positive control, in contrast to the negative control which did not show fluorescence.
DISCUSSION
Naturally, plant growth is closely linked to the accompanying microorganisms known as endophytes. This microorganism includes bacteria, fungi, and actinomycetes. Endophytic bacteria can live in plant tissue without causing disease symptoms for more than part of the plant life cycle. Thus, endophytic bacteria can produce secondary metabolites, as well as plants. This ability is obtained through their evolutionary adaptation in the host plant through phenomenal horizontal gene transfer (Filip and Skuza, 2021). The number and types of endophytic bacteria that can be isolated from plant tissue are directly influenced by the type of growth medium selected, plant compartment, soil type, and plant growth conditions (Riva et al., 2022; Waheeda and Shyam, 2017). In this study, we used different types of growth media as a strategy to increase the chances of successfully isolating diverse endophytic bacteria from clove leaves.
In this study, endophytic bacteria from clove leaves were identified molecularly using the 16S rRNA gene. Bacillus cereus DCN1 and B. cereus DCT2 isolates showed the closest homology to B. cereus JCM 2152 with 100% identity. The isolates N. nealsonii DCN2 and N. nealsonii DCL1 showed the closest homology to N. nealsonii DSM 15077 with 99.12% and 99.18% identity, respectively. Cytobacillus kochii DCL2 isolate showed the closest homology to C. kochii WCC 4582 with 99.67% identity. Bacillus paranthracis DCL3 showed the closest homology to B. paranthracis MCCC 1A00395 with 99.92% identity. Phylogenetically, Bacillus, Niallia, and Cytobacillus are genera belonging to Firmicutes. Previous studies reported that B. cereus, as an endophytic bacterium, was isolated from the stem of Artemisia annua (Zheng et al., 2016). Meanwhile, N. nealsonii was reported as an endophytic bacterium isolated from tomato plant stems by the name Bacillus nealsonii (Fu et al., 2020). Furthermore, C. kochii was reported as a herbicide-tolerant endophytic bacterium isolated from rice plant stems by the name B. kochii (Rangjaroen et al., 2019). The nomenclature change of the species B. nealsonii and B. kochii was recently proposed based on phylogenetic, molecular, and protein data collection evidence into two new genera, namely Niallia and Cytobacillus (Gupta et al., 2020; Patel and Gupta, 2019).
Interestingly, DCN1 was the isolate with the best capacity to survive against H2O2-induced oxidative stress. Endophytic bacteria density and spot thickness on solid media were the basis for determining the isolate tolerance to H2O2 stress. Normally, H2O2 is produced and required in the physiological processes of cells (Gill and Levine, 2013). Meanwhile, excess H2O2 can cause damage to DNA, lipids, and proteins, interfering with these cellular components in carrying out their functions. The use of H2O2 stress in this screening test causes an imbalance in the number of free radicals faced by endophytic bacterial cells. The concentration of H2O2 stress in this study was high, up to 8 mM. Previous studies reported that the concentration of 6 mM H2O2 was already toxic and inhibited the growth of Escherichia coli and Pseudomonas spp. after 36 hours and 12 hours, respectively (Nur et al., 2014). It has been reported that bacteria possess two transcription factors (OxyR and PerR) which are highly reactive to the presence of H2O2. OxyR is a member of the LysR family that regulates the KatG gene (catalase-peroxidase), an enzyme involved in the defense system against oxidative stress. PerR is a member of the ferric uptake repressor that regulates enzymes and proteins involved in oxidative stress response, including KatA (catalase), AhpC (alkyl hydroperoxide reductase), and MrgA (DNA binding protein) (Zamocky et al., 2008). In addition, the H2O2-induced oxidative stress defense of endophytic bacteria from clove leaves can also be caused by the production of active compounds with antioxidant potential. Previous studies reported endophytic bacterial extract from clove leaves containing Pyrazine, an alkaloid compound. This particular extract has antioxidant activity with 68.90% ± 0.94% inhibition (Triandriani et al., 2020).
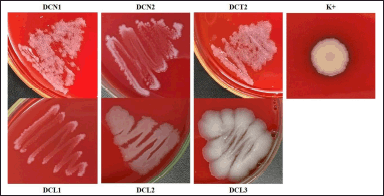 | Figure 3. Hemolytic activity of endophytic bacterial isolates using blood sheep agar. The bacterial colonies were incubated at ± 27°C, for 24 hours. Klebsiella pneumoniae was used as a positive control (K+). [Click here to view] |
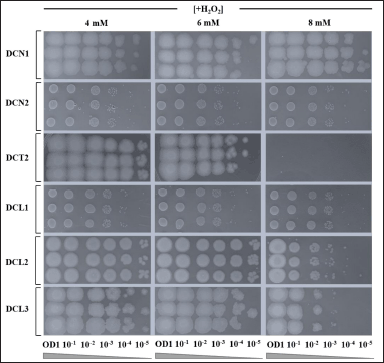 | Figure 4. The ability of endophytic bacteria to survive against strong H2O2-induced oxidative stress in vitro. Serial dilution of each bacterial culture was performed prior to spot assay. Spot assay was done in agar medium for each corresponding bacterial isolate containing 4, 6 and 8 mM H2O2. Each agar medium was then incubated for 24 hours at ±27°C. [Click here to view] |
Our study indicated that ethyl acetate was suitable for extraction of the metabolites with antioxidant properties from isolate DCN1. Indeed, EAN (extract yield = 1.423%) showed the most potent antioxidant activity based on the DPPH assay compared to other extracts. The IC50 value of the antioxidant activity of EAN was 262.27 ± 29.54 µg/ml. These results significantly differed from the positive control AA which was categorized as a very strong antioxidant (IC50 = 5.247 ± 0.30 µg/ml). The antioxidant activity of EAN showed 27× and 30× lower results than previous studies reported using clove leaves, ethanol fraction (IC50 = 9.80 ± 0.62 µg/ml), and n-hexane fraction (IC50 = 8.87 ± 0.75 µg/ml), respectively (Lesmana et al., 2021). The antioxidant activity of EAN was also 31× and 38× lower than clove buds, ethanol fraction (IC50 = 8.37 ± 0.99 µg/ml) and n-hexane fraction (IC50 = 6.88 ± 0.20 µg/ml), respectively (Lesmana et al., 2021). Triandriani et al. (2020) reported the scavenging activity of Staphylococcus epidermidis as a clove leaf endophyte relatively similar to EAN (68.90%) in the DPPH assay. Each antioxidant has its reaction mechanisms. Different values of antioxidant capacity may occur due to the different methods applied. In vitro analysis only shows a response to specific reaction systems, its correlation with in vivo analysis is uncertain (Paparella et al., 2020). The data of this study provide evidence to corroborate this, where EAN, which has low antioxidant activity in the DPPH assay (262.27 ± 29.54), can maintain the viability of the yeast S. pombe.
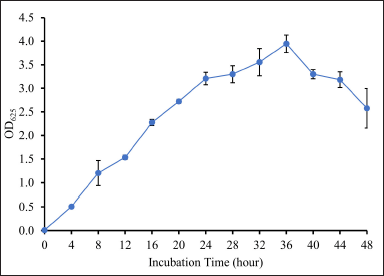 | Figure 5. Growth curve of isolate DCN1 based on turbidity assay. Isolate DCN1 was grown in NB medium and was incubated for 48 hours at 27°C. [Click here to view] |
To the best of our knowledge, there were no reports about the antiaging activity of endophytic bacterial extracts from clove leaves. Our data indicated that lower extract concentrations (below IC50) could maintain the viability of S. pombe up to day 11 of incubation. The EAN (50 µg/ml) in our assays maintained the viability of S. pombe compared to the treatment without extract. This concentration showed better results than the previously reported using clove leaf ethanol extract (100 µg/ml) and clove leaf n-hexane fraction (81 µg/ml), which maintained the viability of S. pombe up to days 9 and 11, respectively (Fauzya et al., 2019; Lesmana et al., 2021). This result was also better than the clove bud n-hexane fraction, which was reported not to exhibit antiaging properties in S. pombe (Lesmana et al., 2021). However, compared to the clove leaf ethanol fraction and the clove bud ethanol fraction, both were able to maintain the viability of S. pombe at lower concentrations of 30 and 8 µg/ml up to day 11 (Lesmana et al., 2021). Another study also reported that the ethanol fraction of clove leaves (25 µg/ml) could increase the cell viability of S. pombe up to day 11 (Anwar et al., 2021). Ethanol-derived clove bud extract (100 µg/ml) increased the viability of Saccharomyces cerevisiae cells following H2O2-induced oxidative stress (Astuti et al., 2019). Such different results may occur due to different types of samples and solvents. This study used an extracellular metabolite extract of endophytic bacteria from clove leaves with ethyl acetate as a solvent. Previous studies also reported endophytic bacteria extracts that can increase yeast life span from other sources. For instance, ethyl acetate extract of Bacillus aryabhattai HMD4 (250 µg/ml), isolated from Hoya multiflora, increased the growth rate of S. pombe (Pudjas et al., 2022). Ethyl acetate extract of PTR21 (500 µg/ml), isolated from sponge Petrosia sp., showed antiaging properties in S. pombe (Prastya et al., 2019).
 | Table 3. Yield percentage of DCN1 extracellular metabolite extract extracted with several organic solvents. [Click here to view] |
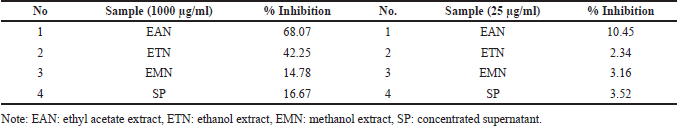 | Table 4. Preliminary assay of the antioxidant activity of all bacterial extracts using two different concentrations based on DPPH-method. [Click here to view] |
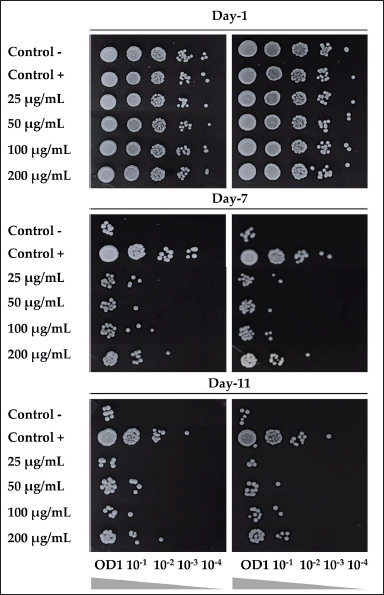 | Figure 6. The effect of ethyl acetate extract of isolate DCN1 (EAN) at various concentrations (25, 50, 100, and 200 µg/ml) on the viability of S. pombe yeast on days 1, 7, and 11 of incubation. Positive control was yeast grown on 0.3% glucose medium. Negative control was yeast grown on 3% glucose media without EAN treatment. [Click here to view] |
Calorie restriction has been known as antiaging treatment in various animal models (Fontana et al., 2010). In this study, we used calorie restriction (glucose 0.3% in medium) as a positive control for antiaging potential assays. Roux et al. (2006) reported that S. pombe inactivated Pka1 and Sck2 signal transduction under low glucose levels. Both signals are related to nutrient signaling pathways. The inactivation of the signal stimulates mitochondrial activity to produce energy and indirectly increase the expression of enzymes related to oxidative stress response (adaptive-mitochondrial ROS signaling), which extends the life span of S. pombe. Interestingly, our data showed that EAN stimulates mitochondrial activity at concentrations of 50–200 µg/ml. Lower concentrations were previously reported using the clove leaf and bud ethanol fractions of 10 µg/ml and 8 µg/ml, respectively (Lesmana et al., 2021). Interestingly, our data indicate better results from the clove leaf ethanol extract which was reported not to involve mitochondrial activity and the clove bud ethanol extract which increased mitochondrial activity at a concentration of 500 µg/ml (Astuti et al., 2019; Fauzya et al., 2019)
Based on liquid chromatography-mass spectrometry (LC-MS/MS) analysis, there were 5 dominant compounds in EAN, namely gallic acid, kaempferol, kaempferol 3-o-(6”-malonyl-glucoside), rutin, and (epi)-Gallocatechin. Lesmana et al. (2021) reported that gallic acid belongs to the hydroxybenzoic group of phenolic compounds, which has been reported as a constituent of clove bud ethanolic fraction that induces yeast life span by increasing mitochondrial activity. Gallic acid has also been reported as a constituent of endophytic bacterial extracts from the sponges Haliclona sp. and Petrosia sp., which also induce yeast life span by increasing mitochondrial activity (Prastya et al., 2019). Kaempferol is a flavonoid compound reported as a constituent of clove bud ethanol extract with strong antioxidant activity (IC50 = 0.037 µg/ml) (Rosarior et al., 2021). Kaempferol was also reported as a constituent in sacred lotus (Nelumbo nucifera ) stamen extract, which induces yeast life span by maintaining the mitochondrial function, along with gene expression (sir2 and sod2) and regulation of sirtuin (SIRT) and superoxide dismutase (SOD) enzyme activity (Tungmunnithum et al., 2022). Meanwhile, the bioactivity of kaempferol 3-o-(6”-malonyl-glucoside), rutin, and (epi)-Gallocatechin was reported from plants belonging to the same genus of S. aromaticum. Kaempferol 3-o-(6”-malonyl-glucoside) is a flavonoid compound reported as a constituent of the methanol extract of the bark of Syzygium cumini, which exhibits antioxidant activity (Wijayanti and Setiawan, 2018). Rutin (flavonoid compound) and (epi)-Gallocatechin (phenolic compound) were reported as constituents that facilitate the antioxidant activity of methanol extract from Syzygium aqueum leaves and methanol extract from S. cumini stem bark (Sobeh et al., 2018; Wijayanti and Setiawan, 2018). It is worth noting that plants in the same taxon correspond to similar metabolite content profiles (Liu et al., 2017).
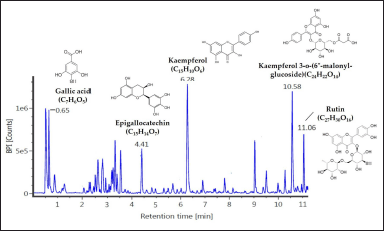 | Figure 7. The metabolite profiles of ethyl acetate of isolate DCN1 (EAN) extract which was revealed by LC-MS analysis. The dominant peaks were identified using a specific mass identity through the PubChem to determine the identity of the detected compounds. [Click here to view] |
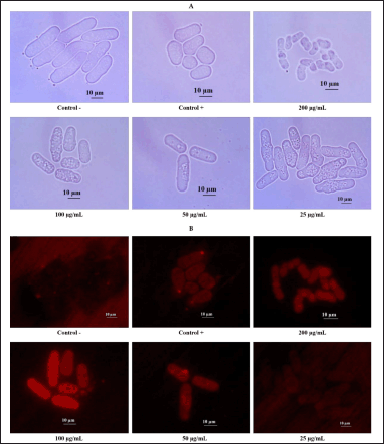 | Figure 8. The effect of ethyl acetate extract of isolate DCN1 (EAN) at various concentrations (25, 50, 100, and 200 µg/ml) on mitochondrial activity of yeast S. pombe. A: nonfluorescence mitochondrial observation, B: fluorescence mitochondrial observation. Positive control (Control +) was yeast grown on 0.3% glucose medium, while negative control (Control −) was yeast grown on 3% glucose media without EAN treatment. [Click here to view] |
CONCLUSION
Ethyl acetate extract of endophytic bacteria B. cereus DCN1 from clove leaves showed antioxidant and cellular antiaging activity. The mode of action of the extract was suggested via induction of mitochondrial activity, which is one of the organelles known to play a role in regulating cellular aging. The extract was predominantly rich in kaempferol, kaempferol 3-o-(6”-malonyl-glucoside), gallic acid, rutin, and (epi)-Gallocatechin. Gallic acid was previously reported to be a dominant component of the clove extract, which showed antiaging and antioxidant properties in yeast as a model.
AUTHORS’ CONTRIBUTIONS
All authors made substantial contributions to conception and design, data acquisition, analysis, and interpretation; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work. All the authors are eligible to authorship as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
ACKNOWLEDGMENTS
This research was funded by the Ministry of Education, Culture, Research and Technology of the Republic of Indonesia, which has funded this research through Scheme “Penelitian Dasar Kompetitif Nasional” (no. 001/E5/PG.02.00PT/2022)
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors declared no conflicts of interest in this study.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Anwar SA, Astuti RI, Solihin DD. Activity of ethanol-derived fraction of clove leaves and eugenol compound as antiaging agent in the yeast model organism Schizosaccharomyces pombe. Adv Biol Sci Res, 2021; 14:515–22; https://doi.org/10.2991/absr.k.210621.086.
Astuti RI, Listyowati S, Wahyuni WT. Life span extension of model yeast Saccharomyces cerevisiae upon ethanol derived- clover bud extract treatment. IOP Conf. Ser. Earth Environ Sci, 2019; 299:012059; https://doi.org/10.1088/1755-1315/299/1/012059.
Bhuiyan MNI. Constituents of the essential oil from leaves and buds of clove (Syzigium caryophyllatum (L.) Alston). African J Pharm Pharmacol, 2012; 6(16):1260–63; https://doi.org/10.5897/ajpp10.004.
Costa LE de O, Queiroz MV de, Chaer Borges A, Moraes CA de, Araújo EF de. Isolation and characterization of endophytic bacteria isolated from the leaves of the common bean (Phaseolus vulgaris). Brazilian J Microbiol, 2012; 43(4):1562–75; https://doi.org/10.1590/S1517-83822012000400041.
Fauzya AF, Astuti RI, Mubarik NR. Effect of ethanol-derived clove leaf extract on the oxidative stress response in yeast Schizosaccharomyces pombe. Int J Microbiol, 2019; 2019:1–7; https://doi.org/10.1155/2019/2145378.
Filip E, Skuza L. Horizontal gene transfer involving chloroplasts. Int J Mol Sci, 2021; 22:1–6; https://doi.org/10.3390/ijms22094484.
Fontana L, Partridge L, Longo VD. Extending healthy life span-from yeast to humans. Science 2010; 328:321–6; https://doi.org/10.1126/science.1172539.
Fu HZ, Marian M, Enomoto T, Hieno A, Ina H, Suga H, Shimizu M. Biocontrol of tomato bacterial wilt by foliar spray application of a novel strain of endophytic Bacillus sp. Microbes Environ, 2020; 35(4):1–11; https://doi.org/10.1264/jsme2.ME20078.
Gill T, Levine AD. Mitochondria-derived hydrogen peroxide selectively enhances T cell receptor-initiated signal transduction. J Biol Chem, 2013; 288(36):26246–55; https://doi.org/10.1074/jbc.M113.476895.
Gkogkolou P, Böhm M. Advanced glycation end products: keyplayers in skin aging? Dermatoendocrinol, 2012; 4(3):259–70; https://doi.org/10.4161/derm.22028.
Gupta RS, Patel S, Saini N, Chen S. Robust demarcation of 17 distinct Bacillus species clades, proposed as novel Bacillaceae genera, by phylogenomics and comparative genomic analyses: description of Robertmurraya kyonggiensis sp. nov. and proposal for an emended genus Bacillus limiting it only to the members of the subtilis and cereus clades of species. Int J Syst Evol Microbiol, 2020; 70(11):5753–98; https://doi.org/10.1099/ijsem.0.004475.
Kalambhe DG, Zade NN, Chaudhari SP, Shinde SV, Khan W, Patil AR. Isolation, antibiogram and pathogenicity of Salmonella spp. recovered from slaughtered food animals in Nagpur region of Central India. Vet World, 2016; 9(2):176–81; https://doi.org/10.14202/vetworld.2016.176-181.
Kim HS, Yoon BD, Lee CH, Suh HH, Oh HM, Katsuragi T, Tani Y. Production and properties of a lipopeptide biosurfactant from Bacillus subtilis C9. J Ferment Bioeng, 1997; 84:41–6; https://doi.org/10.1016/S0922-338X(97)82784-5.
Lesmana D, Andrianto D, Astuti RI. Antiaging properties of the ethanol fractions of clove (Syzygium aromaticum L.) bud and leaf at the cellular levels: study in yeast Schizosaccharomyces pombe. Sci Pharm, 2021; 89:1–13; https://doi.org/10.3390/scipharm89040045.
Liu K, Abdullah AA, Huang M, Nishioka T, Altaf-Ul-Amin M, Kanaya S. Novel approach to classify plants based on metabolite-content similarity. Biomed Res Int, 2017; 2017:1–12; https://doi.org/10.1155/2017/5296729.
Mahjani, Putri DH. Growth curve of endophyte bacteria Andalas (Morus macroura Miq.) B.J.T. A-6 isolate. Serambi Biol, 2020; 5(1):29–32; https://doi.org/10.24036/5692RF00.
Marchesi JR, Sato T, Weightman AJ, Martin TA, Fry JC, Hiom SJ, Dymock D, Wade WG. Design and evaluation of useful bacterium-specific PCR primers that amplify genes coding for bacterial 16S rRNA. Appl Environ Microbiol, 1998; 64(2):795–9; https://doi.org/10.1128/AEM.64.2.795-799.1998.
Nur I, Munna MS, Noor R. Study of exogenous oxidative stress response in Escherichia coli, Pseudomonas spp., Bacillus spp., and Salmonella spp. Turkish J Biol, 2014; 38(4):502–9; https://doi.org/10.3906/biy-1312-93.
Nurhelmi, Putri DH. Optimization of andalas leaf tissue surface sterilization (Morus macroura Miq.) with NaOCl for endophytic microbial isolation. Serambi Biol, 2021; 6(1):13–8; https://doi.org/10.2991/absr.k.200807.010.
O’Toole GA. Classic spotlight: how the gram stain works. J Bacteriol, 2016; 198(23):3128; doi: 10.1128/JB.00726-16.
Paparella M, Bennekou SH, Bal-Price A. An analysis of the limitations and uncertainties of in vivo developmental neurotoxicity testing and assessment to identify the potential for alternative approaches. Reprod Toxicol, 2020; 96(2020):327–36; https://doi.org/10.1016/j.reprotox.2020.08.002.
Patel S, Gupta RS. A phylogenomic and comparative genomic framework for resolving the polyphyly of the genus Bacillus: Proposal for six new genera of Bacillus species, Peribacillus gen. nov., Cytobacillus gen. nov., Mesobacillus gen. nov., Neobacillus gen. nov., Metabacillus gen. nov. and Alkalihalobacillus gen. nov. Int J Syst Evol Microbiol, 2019; 70(1):406–38; https://doi.org/10.1099/ijsem.0.003775.
Prastya ME, Astuti RI, Batubara I, Wahyudi AT. Antioxidant, antiglycation and in vivo antiaging effects of metabolite extracts from marine sponge-associated bacteria. Indian J Pharm Sci, 2019; 81(2):344–54; https://doi.org/10.4172/pharmaceutical-sciences.1000516.
Prastya ME, Astuti RI, Batubara I, Takagi H, Wahyudi AT. Natural extract and its fractions isolated from the marine bacterium Pseudoalteromonas flavipulchra STILL-33 have antioxidant and antiaging activities in Schizosaccharomyces pombe. FEMS Yeast Res, 2020; 20(3):1–14; https://doi.org/10.1093/femsyr/foaa014.
Pudjas NTG, Mubarik NR, Astuti RI, Sudirman LI. Antioxidant activity of endophytic bacteria derived from Hoya multiflora blume plant and their cellular activities on Schizosaccharomyces pombe. HAYATI J Biosci, 2022; 29(2):214–21; https://doi.org/10.4308/hjb.29.2.214-221.
Rangjaroen C, Lumyong S, Sloan WT, Sungthong R. Herbicide-tolerant endophytic bacteria of rice plants as the biopriming agents for fertility recovery and disease suppression of unhealthy rice seeds. BMC Plant Biol, 2019; 19(580):1–16; https://doi.org/10.1186/s12870-019-2206-z.
Rattan SIS. Increased molecular damage and heterogeneity as the basis of aging. Biol Chem, 2008; 389(3):267–72; https://doi.org/10.1515/BC.2008.030.
Razafimamonjison G, Boulanger R, Jahiel M, Ramanoelina P, Fawbush F, Lebrun M, Danthu P. Variations in yield and composition of leaf essential oil from Syzygium aromaticum at various phases of development. Int J Basic Appl Sci, 2016; 5(1):90–4; https://doi.org/10.14419/ijbas.v5i1.5614.
Riva V, Mapelli F, Bagnasco A, Alessio M, Borin S. A meta-analysis approach to defining the culturable core of plant endophytic bacterial communities. Appl Environ Microbiol, 2022; 88(6):1–10; https://doi.org/10.1128/aem.02537-21.
Rosarior VL, Lim PS, Wong WK, Yue CS, Yam HC, Tan S-A. Antioxidant-rich clove extract, a strong antimicrobial agent against urinary tract infections-causing bacteria in vitro. Trop Life Sci Res, 2021; 32(2):45–63; https://doi.org/10.21315/tlsr2021.32.2.4.
Roux AE, Quissac A, Chartrand P, Ferbeyre G, Rokeach LA. Regulation of chronological aging in Schizosaccharomyces pombe by the protein kinases Pka1 and Sck2. Aging Cell, 2006; 5(4):345–57; https://doi.org/10.1111/j.1474-9726.2006.00225.x.
Sarima, Astuti RI, Meryandini A. Modulation of aging in yeast Saccharomyces cerevisiae by roselle petal extract (Hibiscus sabdariffa L.). Am J Biochem Biotechnol, 2019; 15(1):23–32; https://doi.org/10.3844/ajbbsp.2019.23.32.
Sobeh M, Mahmoud MF, Petruk G, Rezq S, Ashour ML, Youssef FS, El-Shazly AM, Monti DM, Abdel-Naim AB, Wink M. Syzygium aqueum: a polyphenol- rich leaf extract exhibits antioxidant, hepatoprotective, pain-killing and anti-inflammatory activities in animal models. Front Pharmacol, 2018; 9(566):1–14; https://doi.org/10.3389/fphar.2018.00566.
Tan RX, Zou WX. Endophytes: a rich source of functional metabolites. Nat Prod Rep, 2001; 18(4):448–59; https://doi.org/10.1039/b100918o.
Triandriani W, Saputri DD, Suhendar U, Sogandi. Antioxidant activity of endophytic bacterial extract isolated from clove leaf (Syzygium aromaticum L.). J Agric Appl Biol, 2020; 1(1):9–17; https://doi.org/10.11594/jaab.01.01.02.
Tungmunnithum D, Drouet S, Hano C. Flavonoids from sacred lotus stamen extract slows chronological aging in yeast model by reducing oxidative stress and maintaining cellular metabolism. Cells, 2022; 11:1–15; https://doi.org/10.3390/cells11040599.
Utami LA, Putri DH. The effect of ethanol solvent concentration on antimicrobial activities the extract of Andalas endophytic bacteria (Morus macroura Miq.) fermentation product. Eksakta, 2020; 21(01):1–6; https://doi.org/10.24036//eksakta/vol21-iss1/210.
Waheeda K, Shyam K. Formulation of novel surface sterilization method and culture media for the isolation of endophytic actinomycetes from medicinal plants and its antibacterial activity. J Plant Pathol Microbiol, 2017; 8(2):1–10; https://doi.org/10.4172/2157-7471.1000399.
Wijayanti T, Setiawan DC. Secondary metabolites exploration in duwet (Syzygium cumini L.) stem bark using liquid chromatograph mass spectrometry method. Bioma J Ilm Biol, 2018; 7(2):1–15; https://doi.org/10.26877/bioma.v7i2.2757.
Yasser M, Rafi M, Wahyuni WT, Widiyanti SE, Asfar AMIA. Total phenolic content and antioxidant activities of buni fruit (Antidesma bunius L.) in moncongloe maros district extracted using ultrasound-assisted extraction. Rasayan J Chem, 2020; 13(1):684–9; https://doi.org/10.31788/RJC.2020.1315584.
Zamocky M, Furtmüller PG, Obinger C. Evolution of catalases from bacteria to humans. Antioxidants Redox Signal, 2008; 10(9):1527–47; https://doi.org/10.1089/ars.2008.2046.
Zheng LP, Zou T, Ma YJ, Wang JW, Zhang YQ. Antioxidant and DNA damage protecting activity of exopolysaccharides from the endophytic bacterium Bacillus cereus SZ1. Molecules, 2016; 21:1–15; https://doi.org/10.3390/molecules21020174.