INTRODUCTION
Belzutifan (Fig. 1) is an anti-neoplastic that inhibits hypoxia-inducible factor 2α (HIF-2α) and is used to treat some malignancies that include renal cell carcinoma (RCC) linked with Von Hippel-Lindau (VHL) disease (Deeks, 2021). It is an autosomal dominant disease that can be identified by RCCs, pancreatic neuroendocrine tumors (pNET), Central nervous system (CNS) haemangioblastomas, and reproductive tract tumors. This condition is caused by dysfunctional mutations in the tumor suppressor VHL gene, which is found on chromosome 3, that causes the aggregation of a protein called a HIF, that enhances cellular proliferation and angiogenesis, and ultimately leads to carcinogenesis. Belzutifan binds to the Per-ARNT-Sim-B binding pocket to suppress HIF-2, which suppresses cellular proliferation and angiogenesis (Visweswaran and Pavithran, 2022). This drug was approved for the first time in the United States in August 2021 for treating the individuals having VHL condition who need treatment for concomitant RCC, pNET, or CNS haemangioblastomas but do not necessitate surgery. Patients tolerated Belzutifan well and has shown early anti-tumor efficacy in persons who had received extensive pre-therapy, indicating that HIF-2 suppression could provide an efficient treatment for clear cell RCC (Choueiri et al., 2021).
An intense literature review has revealed that only manufacturing process development, (Bottecchia et al., 2021; Chen et al., 2022; Peng et al., 2021; Pirnot et al., 2021; Pirnot et al., 2021; Salehi et al., 2021; Wang et al., 2021) and clinical trials (Fallah et al., 2022) has been reported since the approval of Belzutifan, and it is a newly approved drug and there is only one reported reverse phase-high performance liquid chromatography (RP-HPLC) method available (Sisindri and Darmamoorthy, 2022). Hence an initiative effort was made to develop and design an accurate, simple, rapid, precise, stability-indicating RP-HPLC for the quantification of Belzutifan in a pure and pharmaceutical dosage form. The developed method was validated according to the guidelines of International Conference on Harmonization (ICH) (Singh, 2015) and the degradation studies were performed as per ICH regulations (ICH, 2003).
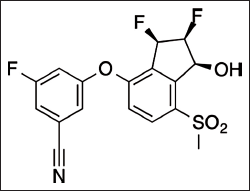 | Figure 1. Belzutifan structure. [Click here to view] |
MATERIALS AND METHODS
Chemicals
Belzutifan (API), Belzutifan tablets (Welireg), Acetonitrile, 0.1% Formic acid (98% Pure), Distilled water. All of the solvents and chemicals were of HPLC quality and obtained from Rankem Chemicals Pvt. Ltd.
Instruments
Electronic balance-Denver, Waters HPLC 2695 system with quaternary pumps, Ultrasonicator-BVK enterprises, photo diode array (PDA) detector and autosampler incorporated with Empower 2.O software, pH meter-BVK enterprises India, and Agilent C18 column (150 mm × 4.6 mm × 5 µ).
Selection of wavelength
Belzutifan in diluent (water and acetonitrile 50:50) was scanned in the range of 200–400 nm. The spectrum has shown 268 nm as a suitable wavelength for the present RP-HPLC method. Belzutifan has shown good absorption at this wavelength.
Chromatographic conditions
Isocratic elution mode was carried out during the experiment. The mobile phase includes Acetonitrile and 0.1% Formic acid in the ratio of 50:50 v/v. The degassing of effluents was done prior to the run at a flow rate of 1.0 ml/minute. The column temperature was maintained at 30°C temperature. The sample volume injected per run was 10 µl. The total run time was 5 minutes. The wavelength at which the effluents were detected is 268 nm using a PDA detector.
Standard solution preparation
Standard stock solution (200 µg/ml) was prepared by weighing 10 mg of Belzutifan accurately and by transferring it to a 50 ml volumetric flask. A three-fourth quantity of diluent was poured into the flask and sonicated for 5 minutes to eliminate the dissolved gases present if any and the remaining volume was brought up to the required level using diluent. 1 ml of the stock solution was pippetted out and transferred into a 10 ml volumetric flask after diluting it to the right concentration with diluent yielded a suitable dilution of 20 µg/ml of the working standard solution.
Sample solution preparation
The average weight of ten tablets was determined after they were weighed and powdered. The weight of the powder corresponding to one tablet was placed into a 100 ml volumetric flask. It was mixed with 50 ml of diluent and then sonicated for 25 minutes to eliminate the dissolved before adding more diluent to make a 200 µg/ml solution. Pipetting 1 ml of the previously mentioned sample stock solution into a 10 ml volumetric flask after diluting it to the right concentration with diluent yielded a suitable dilution of 20 µg/ml, which is used for routine analysis.
Buffer preparation
1,000 ml of HPLC grade water is used to dilute 1 ml of formic acid.
METHOD VALIDATION
As per ICH guidelines the developed method was validated for validating the analytical procedures. The validation parameters include system suitability, accuracy, linearity, limit of detection (LOD), limit of quantification (LOQ), precision, robustness and specificity were also performed.
System suitability
The appropriateness of the system was investigated by system suitability studies for each validation parameter by delivering six replicating injections of the reference solution. USP plate count, resolution, and tailing factor were all recorded as system suitability parameters. The area of results from 6 standard injections shouldn’t have a relative standard deviation (RSD) of greater than 2%.
Accuracy
Recovery studies were performed to evaluate the method’s accuracy by spiking various concentrations of pure drug in the analyte sample solutions of three different concentrations of standard having 50%, 100%, and 150% of pure drug. The percentage recovery and percentage RSD were measured to assess the accuracy. The acceptance limits of % recovery should be in the range 98%–102%.
Specificity
To verify the interferences in the optimized chromatogram, specificity was performed. By correlating the chromatograms of the placebo, blank and standard solution at working concentration, specificity was determined. The method has not shown any interfering peaks.
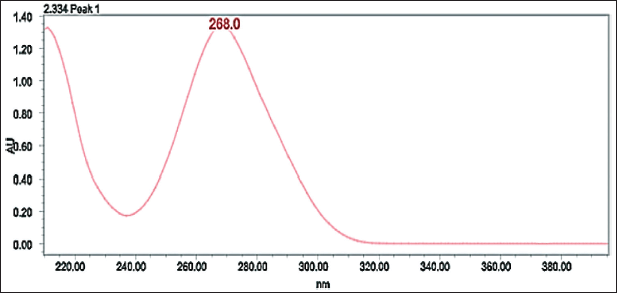 | Figure 2. UV spectra for Belzutifan. [Click here to view] |
Precision
The method and system precision were demonstrated by preparing the standard solution at a known concentration and six repeatable injections were given to ensure the repeatability of the proposed method. The intermediate precision was also performed by preparing six working sample solutions by injecting each solution six times. The area was noted, mean, standard deviation, and percentage of RSD were calculated. The results were satisfactory and below 2% as per the limit
Linearity
The linearity of the drug was studied by preparing serial dilutions in the range of 5–30 µg/ml. The correlation between peak area response and drug concentration was shown on a graph. It was observed to be linear for the specified drug concentration.
LOD and LOQ
Based on the response’s standard deviation and calibration curve’s slope, the LOD and LOQ can be estimated. The formulae given below can be used to calculate LOD and LOQ:
LOD = 3.3σ/S
LOQ = 10σ/S
where S is calibration curve of the slope and σ is the response of the standard deviation.
Robustness
The method robustness can be determined by changing some of the conditions like flow rate (±0.1 ml), temperature (±5°C), and mobile phase (±10%). In the duplicate manner, the samples were injected. No changes were observed in the system suitability specifications. So, it was claimed that the suggested procedure was robust.
Stability-indicating studies
These studies are performed at various stress conditions to describe the stability of the pure drug substance and are helpful in determining a suitable storage conditions. These studies include base, peroxide, acid, neutral hydrolysis, photo, and thermal degradation.
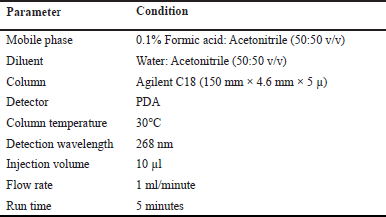 | Table 1. Optimized conditions for estimating Belzutifan. [Click here to view] |
Acid hydrolysis
1 ml of both stock solution of Belzutifan (200 µg/ml) and 1 N hydrochloric acid were pipetted into a 10 ml volumetric flask and then refluxed for 30 minutes at 60°C. Now required volume of 1 N NaOH was added and left for a few minutes to neutralize the solution. To determine the stability, the solution in the flask was diluted to 10 ml with diluent which was injected into the HPLC system and the chromatogram was recorded.
Base hydrolysis
1 ml of stock solution of Belzutifan (200 µg/ml) and 1 N sodium hydroxide were pipetted into a 10 ml volumetric flask and then refluxed for 30 minutes at 60°C. Now required volume of 1 N HCl was added and left for a few minutes to neutralize the solution. In order to record the chromatogram and evaluate the stability, the solution in the flask was diluted to a volume of 10 ml using diluent.
Peroxide hydrolysis
A 10 ml volumetric flask was filled with 1 ml Belzutifan (200 µg/ml) stock solution. It was treated with 20% H2O2 and then at 60°C it was refluxed for 30 minutes. To determine the stability, the solution in the flask was diluted to a volume of 10 ml using diluent and then injected into the HPLC apparatus.
Photo degradation
The stock solution of Belzutifan was placed in the UV chamber and exposed to UV light for 1 week at 200 W hour/m2 to study the drug’s photostability. To check the stability, the solution was diluted to 10 ml with diluent and injected into the HPLC apparatus to record the chromatogram.
Thermal degradation
A volumetric flask containing 1 ml of the standard drug solution was placed in an oven for 6 hours at 150°C. The solution was diluted with diluent to a volume of 10 ml before being injected into the HPLC system to determine the stability.
Neutral degradation
A 10 ml volumetric flask containing 1 ml of stock and water was used to study stress testing under neutral conditions by refluxing at 60°C for 6 hours. To determine the stability, the solution was injected into the HPLC system after being diluted to a volume of 10 ml with diluent.
RESULTS AND DISCUSSION
Method development
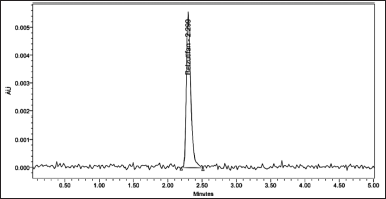
To develop a HPLC method for estimating the Belzutifan, the chromatographic conditions were optimized to produce the chromatogram with lesser analysis time (<5 minutes). Several trails have been conducted to develop the optimized method for Belzutifan estimation. Various mobile phase combinations like Methanol: Orthophosphoric acid (50:50 v/v) (yielded an inaccurate baseline), Water: Acetonitrile (50:50 v/v) (Peak splitting was observed), Acetonitrile: Orthophosphoric acid (60:40 v/v) (USP plate count of less than 2,000 was observed) have been tried but 0.1% Formic acid: Acetonitrile (50:50 v/v) has shown symmetrical peaks with better resolution. Based on the chemical nature of Belzutifan, the developmental trials were performed. During method optimization, the column was selected based on retention time, tailing factor, no. of theoretical plates, and shape of the peak. The mobile phase’s selection was done based on the polarity of the functional groups contained in Belzutifan. By considering all these aspects, the separation was carried out using Agilent C18 column (150 mm × 4.6 mm × 5 µ) with the mobile phase containing Acetonitrile and 0.1% Formic acid (50:50 v/v) with a flow rate of 1.0 ml/minute. 10 µl was the injection volume and the effluents from the column were detected at 268 nm (Fig. 2) using PDA detector. Belzutifan was eluted at a retention time of 2.314 minutes. The optimized conditions and chromatograms for blank, standard, and sample of belzutifan are given in Table 1 and Figures 3–5.
 | Figure 3. Chromatogram for blank. [Click here to view] |
Method validation
System suitability
Standard Belzutifan solution was injected six times, and their corresponding chromatograms were obtained. The theoretical plate count exceeded 2,000, the USP tailing was under 2, and the percent RSD was under 2%, according to observations. All of the requirements for system suitability were met and fall within acceptable ranges.
Linearity and calibration curve
Six different concentrations ranging from 5 to 30 µg/ml were prepared and linearity was estimated in a duplicate manner. The linearity equation for belzutifan was y = 89,099x + 9,790.4. For the estimated drug, the observed correlation coefficient was 0.999. For the calibration curve over the concentration range, the data have shown a good correlation. Thus, it was discovered that the current method was linear. The results are provided in Figure 6.
Accuracy
Three levels of accuracy samples were created using the usual addition method. Three doses were given at each level, and the mean % recovery was calculated. Belzutifan’s recovery rate was observed to be between 99.17% and 100.70%, which is within the acceptable ranges when evaluating the accuracy of the current suggested method discussed in this section. The results were provided in Table 2.
Precision
Six injections from a single standard stock solution were provided for system precision, and the regions were noted. Calculations were made for the average area, standard deviation, and % RSD. The %RSD for Belzutifan was 0.9%. As per the limit of precision, the system precision was acceptable. The result values for system precision were provided in Table 3.
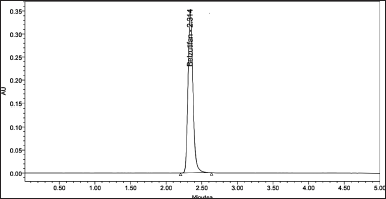 | Figure 4. Optimized chromatogram for sample. [Click here to view] |
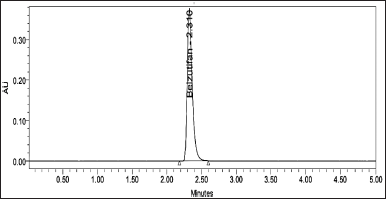 | Figure 5. Chromatogram for standard. [Click here to view] |
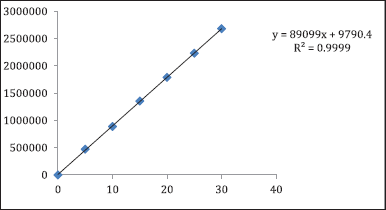 | Figure 6. Calibration curve for Belzutifan. [Click here to view] |
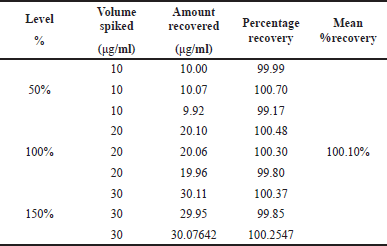 | Table 2. Data of accuracy for Belzutifan. [Click here to view] |
Six working solutions were created for intermediate and method precision, and numerous samples were taken from the sample stock solution. Each injection was given from a different working solution, and the areas were acquired. Calculations were made to determine the average area, standard deviation, and percent RSD. The %RSD for method precision of Belzutifan was 0.6% and for intermediate precision is 1.2%. Hence the method was observed to be precise, as the readings obtained are as per the limits. The data of method and intermediate precision were provided in Tables 4 and 5.
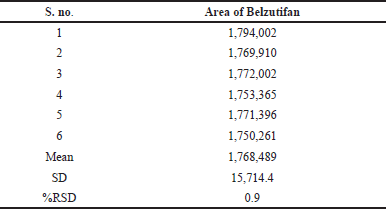 | Table 3. System precision data for Belzutifan. [Click here to view] |
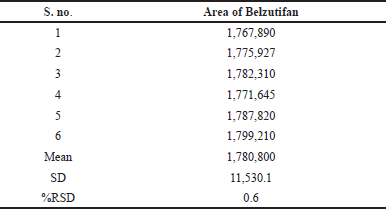 | Table 4. Method precision data for Belzutifan. [Click here to view] |
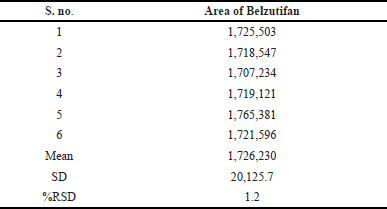 | Table 5. Intermediate precision data for Belzutifan. [Click here to view] |
LOD and LOQ
Belzutifan was found to have LODs and LOQs of 0.29 and 0.88, correspondingly. The data and chromatograms for LOD and LOQ were provided in Table 6 and Figures 7 and 8.
Some of the chromatographic conditions were altered, and the percent RSD values were calculated to determine the robustness.
Robustness
Some of the chromatographic conditions were altered, and the percent RSD values were calculated to determine the robustness. The conditions are temperature minus (27°C) and temperature plus (33°C), flow rate minus (0.9 ml/minute) and flow rate plus (1.1 ml/minute), mobile phase minus (55B:45A) and mobile phase plus (45B:55A). The percent RSD values are within the limits, and the system suitability parameters were not significantly impacted. Hence the method was said to be robust. The data for robustness is given the Table 7.
 | Table 6. LOD and LOQ data for Belzutifan. [Click here to view] |
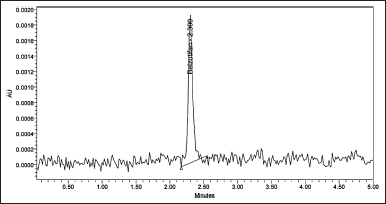 | Figure 7. LOD chromatogram of Belzutifan. [Click here to view] |
 | Figure 8. LOQ chromatogram of Belzutifan. [Click here to view] |
 | Table 7. Data of robustness for Belzutifan. [Click here to view] |
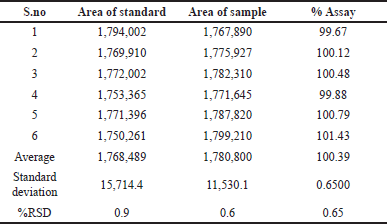
Assay
Test was done using the formulation (Welireg-100 mg). For Belzutifan, the typical percent assay is 100.39%. The data were provided in Table 8.
Specificity
The drug had a 2.314-minute retention time, it was discovered. At the drug’s retention time using this technique, there were no detectable interfering peaks in the blank or placebo. Hence the present approach was claimed to be specific. The chromatogram for blank and placebo were given in Figures 3 and 9 respectively.
 | Table 8. Data of assay for Belzutifan. [Click here to view] |
 | Figure 9. Placebo chromatogram for Belzutifan. [Click here to view] |
 | Table 9. Degradation data of Belzutifan. [Click here to view] |
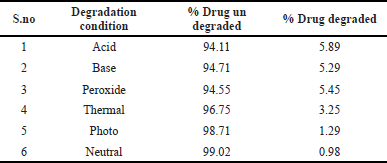
Stability-indicating studies
These studies are carried out to assess the stability of the drug under defined stress conditions. Studies are performed under the stress condition like acid, base, peroxide hydrolysis, photo, thermal and neutral degradation. The formulation was used in degradation experiments, and the degraded materials were injected. All results obtained from the assay of the injected materials are within the limits of degradation, however, an extra small peak has been appeared for peroxide degradation which is within the range. Studies on stress degradation’s findings were presented in Table 9 and the chromatograms were provided in Figures 10–15.
 | Figure 10. Acid chromatogram of Belzutifan. [Click here to view] |
 | Figure 11. Base chromatogram of Belzutifan. [Click here to view] |
 | Figure 12. Peroxide chromatogram of Belzutifan. [Click here to view] |
 | Figure 13. Thermal chromatogram of Belzutifan. [Click here to view] |
 | Figure 14. Photostability chromatogram of Belzutifan. [Click here to view] |
 | Figure 15. Neutral chromatogram of Belzutifan. [Click here to view] |
CONCLUSION
The present method was observed to be simple, specific, linear, precise, reliable, robust, and economical and validated according to the regulations of ICH Q2B. From the stability studies of the present analytical method, the degradation behavior of Belzutifan was known and it has been demonstrated that this method can successfully distinguish the degradation peak from analytical peak. Symmetrical peaks have been observed within lesser analysis time and the percent RSD values for all parameters were as per the limits for the developed method. This shows that the results and assay achieved by this approach are in good accordance. Hence the present analytical method developed can be used for the estimation of Belzutifan in bulk and pharmaceutical formulations and also for regular analysis in quality control purposes.
ACKNOWLEDGEMENT
The authors are thankful to Rankem chemicals for providing drug samples.
AUTHOR CONTRIBUTION
The first author D. Sravya has worked on all the research steps involved in the study. The second author was a mentor for the study.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Bottecchia C, Levesque F, McMullen J, Ji Y, Reibarkh M, Peng F, Reibarkh M, Tan L, Spencer G, Nappi J, Lehnherr D, Narsimhan K, Wismer M, Chen L, Lin Y, Dalby S. Manufacturing process development for belzutifan, part 2: a continuous flow visible-light-induced benzylic bromination. Org. Process Res Dev, 2021; 26(3):516–24.
Chen Z, Marzijarani N, Quirie S, Pirrone G, Dalby S, Wang T, Kim J, Peng F, Fine A. Manufacturing process development for belzutifan, part 3: completing a streamlined through-process with a safe and scalable oxidation. Org Process Res Dev, 2022; 26(3):525–32.
Choueiri T, Bauer T, Papadopoulos K, Plimack E, Merchan J, McDermott D, Michaelson MD, Appleman LJ, Thamake S, Perini RF, Zojwalla NJ, Jonasch E. Inhibition of hypoxia-inducible factor-2α in renal cell carcinoma with belzutifan: a phase 1 trial and biomarker analysis. Nat Med, 2021; 27(5):802–5.
Deeks E. Belzutifan: first approval. Drugs, 2021; 81(16):1921–7.
Fallah J, Brave M, Weinstock C, Mehta G, Bradford D, Gittleman H, Bloomquist E, Charalab R, Hamed S, Miller C, Dorff S, Chambers W, Mixter B, Dinin J, Pierce W, Ricks T, Tang S, Donoghue M, Pazdur R, Kordestani L, Ibrahim A, Beaver J. FDA approval summary: belzutifan for Von Hippel-Lindau disease-associated tumors. Clin Cancer Res, 2022; 28(22):4843–8.
ICH Guideline. Stability testing of new drug substances and products. Q1A (R2). Current Step, ICH, Paris, France, pp 1–24, 2003.
Peng F, Tan L, Chen L, Dalby S, DiRocco D, Duan J, Feng M, Gong G, Guo H, Jin L, Johnson H, Kim J, Le D, Lin Y, Liu W, Shen J, Wan Y, Xiao C, Xiang B, Xiang Q, Xu J, Yan L, Yang W, Ye H, Yu Y, Jun Z. Manufacturing process development for belzutifan, part 1: a concise synthesis of the indanone starting material. Org Process Res Dev, 2021; 26(3):508–15.
Pirnot M, Stone K, Wright T, Lamberto D, Schoell J, Lam Y, Schoell J, Zawatzky K, Wang X, Dalby S, Fine A, McMullen J. Manufacturing process development for belzutifan, part 6: ensuring scalability for a deoxyfluorination reaction. Org Process Res Dev, 2021; 26(3):551–9.
Salehi Marzijarani N, Fine A, Dalby S, Gangam R, Poudyal S, Behre T, Ekkati A, Armstrong B, Shultz C, Dance Z, Stone K. Manufacturing process development for belzutifan, part 4: nitrogen flow criticality for transfer hydrogenation control. Org Process Res Dev, 2021; 26(3):533–42.
Singh J. International Conference on Harmonization of technical requirements for registration of pharmaceuticals for human use. J Pharmacol Pharmacother, 2015; 6(3):185–7.
Sisindri D, Darmamoorthy G. Development and validation of a new analytical method for the determination of belzutifan in bulk and pharmaceutical dosage form. Int J Pharm Pharm Res, 2022; 25(1):384–94.
Visweswaran V, Pavithran K. Belzutifan: a narrative drug review. Curr Drug Res Rev, 2022; 14(2):88–95.
Wang T, Phillips E, Dalby S, Sirota E, Axnanda S, Shultz C, Pratiq P, Jacob H, Waldman, Alwedi E, Wang X, Zawatzky K, Chow M, Padivitage N, Weisel M, Whittington M, Duan J, Lu T. Manufacturing process development for belzutifan, part 5: a streamlined fluorination–dynamic kinetic resolution process. Org Process Res Dev, 2021; 26(3):543–50.