INTRODUCTION
Heart failure with reduced ejection fraction (HFrEF) and resistant hypertension nevertheless represent urgent unmet needs in the discipline of cardiac disorders (Shim, 2020). Earlier studies report that overall mortality rates in congestive heart failure continue to be notably very high, accounting re-hospitalization of one-quarter of patients for cardiac failure (Dharmarajan et al., 2013) while 50% of patients demise within 5 years of heart failure diagnosis due to lack of pharmacological and non-pharmacological approaches that are enough to fulfill the clinical needs (Virani et al., 2020, Volpe and Gallo, 2021). None of the antihypertensive therapies developed in the last two decades have convincingly decreased morbidity or mortality in cardiac failure cases (Ponikowski et al., 2016). In current years, a remedy was developed for the first time by Novartis in the form of supramolecular complex of sacubitril and valsartan that captivated a great deal of research attention in medical care and benefit the patients diagnosed with guideline-defined heart failure, which includes both conditions that is, HFrEF and heart failure with preserved ejection fraction (Feng et al., 2012; Novartis, 2021).
Sacubitril-valsartan supramolecular complex (SVSC) comprises sacubitril (neprilysin inhibitor, NEPi) and valsartan (angiotensin receptor blocker, ARB) in equimolar ratio (Feng et al., 2007). It targets the key pathways that trigger heart failure: the autonomic nervous system, the renin–angiotensin–aldosterone system (RAAS), and the natriuretic peptide (NP) system (D’Elia et al., 2017). It exhibits dual inhibition mechanism of NEPi and ARB by simultaneously suppressing RAAS activation through blockade of angiotensin II type 1 receptor and enhancing vasoactive peptides including NP through inhibition of neprilysin, the enzyme responsible for their degradation (Kobalava et al., 2016). It is the first agent to be approved in a new class of drugs called angiotensin receptor NEPi (ARNI) and first heart failure therapy with an Food and Drug Administration (FDA)-approved indication for patients with signs and symptoms of cardiac failure (Solomon et al., 2019).
In 2005, a retrospective study report indicated that about 46% of unexpected cardiac death took place between midnight and 6 a.m., particularly in people with obstructive sleep apnea as the cardiovascular functions were closely associated to circadian rhythms (Gami et al., 2005). To overcome such challenges, the concept of chronotherapy (Smolensky and Portaluppi, 1999) need to be adapted to develop controlled onset oral drug delivery system (Stevens and MacDougal, 2014) that meets the requirements of nocturnal and prewake up drug delivery to target organs at appropriate time in cardiovascular conditions (Muller et al., 1985). This approach associated with bedtime dosing therapy of antihypertensives can minimize the cardiovascular risk of sudden death occurring early hours in the morning (Hermida et al., 2011). From the past to present decades, a wide range of chronotherapeutic formulation approaches like compression-coated tablets CCTs; Gunsel, 1980, pulsatile capsule device (Ross et al., 2000), compressed multiple unit dosage forms (pellets, granules, and mini-tablets) (Lopes et al., 2006), 3D-printed core-shell pulsatile tablets (Li et al., 2022), etc. were developed. Among all dosage forms, CCTs are the easiest to manufacture which is less time-consuming and lessen laborious coating or granulation steps. Compression coating of tablets also protects the hygroscopic drug from moisture and maintains the stability of the product.
Aim of the present investigation is to design a chronotherapeutic compression-coated formulation of SVSC to be taken at bedtime (around 10 p.m.), enabling 5–6 hours lag time prior to drug release to attain the required Cmax. This approach may be beneficial for patients with morning surge.
CCTs were manufactured as a “tablet within a tablet” with an inner core containing an Active Pharmaceutical Ingredients (API) coated by an outer layer of hydrophilic polymers that forms an erodible barrier. It prevents the drug release from the inner core during the lag time. Various natural polymers such as Khaya/Albizia gum (Odeku and Fell, 2005), Delinox regia gum/HPMC K 4M (Dabhi et al., 2010), xanthan gum/guar gum (Chickpetty et al., 2010), guar gum (Vemula and Bontha, 2013), Karaya gum (Latha et al., 2015), Anogeissus latifolia/ Gardenia gummifera (s), and thiolated ghatti gum (Puri et al., 2021) were formerly used as a coating polymers to delay the drug release from the core tablet during the lag time.
In the present research of development of chronotherapeutic CCT of SVSC, hupu gum (Kondagogu gum) and lannea gum in different ratios along with channeling agent were used as compression coating polymers to achieve lag time up to 5–6 hours, followed by immediate drug release from the inner core with Cmax to be achieved in 1–2 hours that is, around 4–6 a.m. as per the reported Tmax of SVSC which is around 0.5–2 hours (Ayalasomayajula et al., 2017) that matches with the time when clinical symptoms are aggravated. The applicability of the natural gums as compression coating polymers in chronotherapeutic drug delivery systems was investigated and the formulations were optimized up on characterization.
MATERIALS AND METHODS
Materials
SVSC is a gift sample from Dr. Reddy’s Laboratories Ltd., Hyderabad. Hupu gum and lannea gum were purchased from Girijan Cooperative Corporation, Visakhapatnam. Crospovidone, Prosolve, Lubritab, lactose, MCC, and talc used as disintegrant, diluent, lubricant, channeling agent, and glidant, respectively, were procured from local suppliers. The commercial immediate-release tablets of SVSC used for comparison in this study were Azmarda 50 mg tablets manufactured by Novartis Farma SpA (Italy) labeled to contain 24 mg of sacubitril and 26 mg of valsartan.
Preparation of optimized core tablets (OCTs)
Different precompression blends were prepared with SVSC using different disintegrants and diluents and were evaluated for flow properties and tableting characteristics. Based on these preliminary studies, Optimized Core Tablets (OCTs) were prepared by using Prosolve as diluent and crospovidone as disintegrant using direct compression technique. Dose of SVSC taken in the present investigation was 50 mg that contains 24 mg of sacubitril and 26 mg of valsartan. Drug, diluent, and disintegrant sufficient for a batch of 300 tablets were accurately weighed according to the composition shown in Table 1 and passed through BSS #60 sieve with an aperture size 250 μm, then taken into a poly bag and mixed for 5–10 minutes. Required quantities of talc and Lubritab pre-sieved through BSS #60 sieve were blended to the above homogenous mixture and mixed for 2 minutes. Flow property of the final blend was evaluated by angle of repose, Carr’s index, and Hausner’s ratio. The final blend was then compressed using 12 station rotary tablet compression machine (Rimek mini press-II MT) to form round flat tablets with a diameter of 6 mm and thickness 2.9 ± 0.4 mm.
Characterization of OCTs
The OCTs were characterized for hardness, friability, uniformity of weight, drug content estimation, wetting time, and disintegration time. Hardness and friability indicate the integrity and mechanical strength of tablets. Six core tablets were randomly selected and tested for hardness using tablet hardness tester (TH 1050 M, LABINDIA). Sixty-five core tablets (equivalent to 6.5 g) were collected and tested for friability using tablet friability tester (FT 1020, LABINDIA) at 25 rpm for 4 minutes (Indian Pharmacopoeia, 2018a). Twenty core tablets were weighed accurately to check the average weight and percentage weight variation for determination of uniformity of weight (Indian Pharmacopoeia, 2018b). For determination of content of active ingredients, randomly selected 20 core tablets were assayed in pH 6.8 phosphate buffer (Indian Pharmacopoeia, 2018c) and the amount of active ingredient per tablet was calculated by observing the UV absorbance at 253 nm using UV/VIS Spectrophotometer (UV 3,000+, LABINDIA) (Aleti and Murthy, 2022). Wetting time of core tablets was evaluated by measuring the time required for complete wetting of tablet placed on a piece of tissue paper (12 × 10.75 cm) folded twice and kept in a small Petri dish (internal diameter = 6.5 cm) containing 6 ml of pH 6.8 phosphate buffer (Koizum et al., 1997). Disintegration test was performed on randomly chosen 6 core tablets using pH 6.8 phosphate buffer in a tablet disintegration tester (DT 1000, LABINDIA) containing six cylindrical vessels with #10 sieve. The time taken for all the disintegrated particles to pass through the sieve was regarded as a disintegration time of the tablet and the average value was determined (Indian Pharmacopoeia, 2018d; Singh et al., 2015).
In vitro drug release of OCTs
In vitro drug release study of OCT was performed using USP XXIV dissolution test apparatus I (DISSO 2000, LABINDIA) at 37°C ± 0.5°C maintaining rotational speed of the basket at 75 rpm (FDA guidance for dissolution testing of sacubitril and valsartan tablets). An aliquot of 5 ml of dissolution samples was withdrawn at predetermined time intervals using a syringe fitted with a 0.45 µm pre-filter and immediately replaced with same amount of dissolution medium maintained at 37°C ± 0.5°C. The samples were analyzed spectrophotometrically at 253 nm for SVSC and the cumulative percentage drug released was calculated. The drug release studies for experimental formulation and immediate release marketed formulation (Azmarda 50) were performed in triplicate and the average values were compared graphically.
Preparation of CCTs
Evaluation of suitability of polymers for compression coating
The flow properties of hupu gum and lannea gum are measured and the results of angle of repose (>40), Carr’s index (>27), and Hausner’s ratio (>1.3) as reported in Table 2 indicated poor flow characteristics of natural polymers and therefore unsuitable for direct compression. Hence, wet granulation was proposed and flow activators like talc and magnesium stearate are added to the granules to improve the flowability.
Preparation of compression coating granules
The coating granules were prepared by wet granulation method using hydro alcoholic mixture of isopropyl alcohol (IPA) and water in the ratio of 70:30 as granulating agent. All the required formulation excipients indicated in Table 3 except talc and magnesium stearate were weighed accurately and mixed in a mortar using geometric dilution technique. The granulating agent was added sufficiently to form wet dough mass, that was passed through BSS #22 sieve with aperture size of 710 μm instantly to avoid rigid agglomerates up on evaporation of IPA. The granules obtained were dried for 1 hour at 45°C in a hot air oven. The dried granules screened through BSS #30 sieve with aperture size of 500 μm were lubricated with magnesium stearate and talc, then mixed for 5 minutes. The flow characteristics of coating granules were determined and found to be good as per the results given in Table 2, therefore used for compression coating of the core tablet.
Coating of core tablets by compression coating
The weight of coating granules sufficient to coat the core tablets uniformly was investigated in preliminary studies and revealed that 100 mg of coating granules was sufficient to coat the core tablets uniformly on all sides. For compression coating, initially half of the required quantity of granules to coat each tablet was taken into the die cavity and spread uniformly to make a powder bed. Core tablet earlier optimized was placed at the center on the polymer bed. Then, the remaining half of the coating contents were filled into the die and compressed with a 12 station rotary compression machine using circular flat punches to form a round CCT having the hardness in the range of 5–7 kg/cm2 and a diameter of 8 mm (Latha et al., 2015).
Evaluation of CCTs
The CCTs were evaluated for thickness, hardness, friability, uniformity of weight, and drug content estimation by the method described earlier in core tablets evaluation. Friability test was performed for 33 CCT from each batch that corresponds to the aforementioned conditions. The thickness of coating layer in CCT was evaluated by subtracting core tablet thickness from CCT thickness. The cross-section view of the CCT was also examined for uniformity in coating of core tablet.
In vitro drug release study of CCTs
The study was performed using the similar conditions described for core tablets except change in buffer and duration of dissolution. For evaluation of drug release from CCT, change in pH method was used in which the initial run was done in 0.1N HCl for first 2 hours followed by pH 6.8 phosphate buffer. Drug release studies of all the formulations were carried out in triplicate and average values were calculated. The results of cumulative percentage drug release and corresponding lag time are represented graphically for comparison.
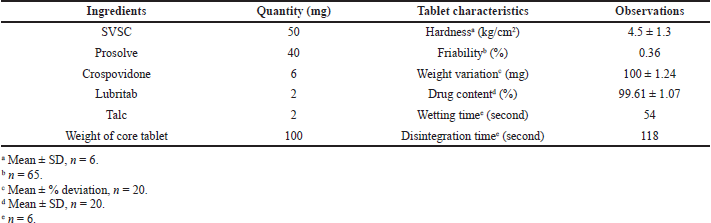 | Table 1. Composition and tablet characteristics of OCT. [Click here to view] |
 | Table 2. Flow properties of coating excipients and granules. [Click here to view] |
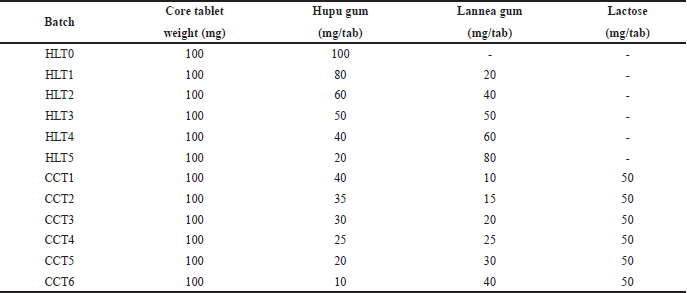 | Table 3. Composition of compression coated tablets. [Click here to view] |
Drug-excipient compatibility studies
Differential scanning calorimetry (DSC)
The drug excipient compatibility was examined by most common thermal technique DSC using Mettler DSC 821 (Mettler-Toledo, Greifensee, Switzerland) that generates data on melting endotherms and glass transitions with a small sample size of 4–5 mg. Hermetically sealed sample in an aluminum pan with a lid crimped on it was positioned on sample pan holder and empty aluminum pan on reference holder, was allowed to equilibrate for 1 minute and heated in an atmosphere of nitrogen (flow rate of 20 ml/minutes) over a temperature range from 30°C to 350°C with a heating rate of 10°C/minutes and the results were obtained as thermograms by using the STARe SW version 9.10 software (Songa et al., 2013).
Fourier transformed infrared spectroscopy (FTIR)
Drug excipient compatibility can also be detected by FTIR spectral studies by following the shift in vibrational or stretching bands of key functional groups. FTIR spectra were recorded on Alpha FTIR spectrophotometer (Bruker Optik GmbH, Ettlingen, Germany) using OPUS version 6.5 software. Samples were prepared by KBr pellet method by gently mixing 1 mg of the sample with 200 mg of KBr and the ratio of samples was selected as per the composition in the optimized formula. The spectra scanned over a wave number range of 4,000–400 cm−1. (Sunil et al., 2013).
Stability studies of optimized formulation
Stability of pharmaceutical product may be defined as the capability of a particular formulation in a specific container/package to remain within its physical, chemical, therapeutic, and toxicological specifications throughout its shelf life. Stability studies were performed as per International Council for Harmonisation (ICH) Guidelines, 2018.
Method
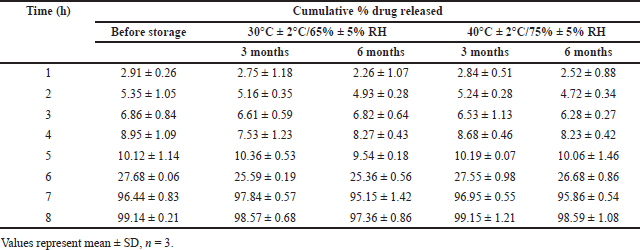
Samples of final optimized formulation CCT3 were sealed as two sets separately in hermetically sealed bottles and kept in humidity chambers maintained at 30°C/70% Relative humidity (RH) as per ICH guidelines for zone IV and accelerated testing conditions 40°C ± 2°C/75% ± 5% RH (ICH guidelines, 2018 and WHO, 2021). The samples stored were withdrawn after 3 and 6 months for analysis of tablet characteristics and in vitro dissolution performance. The results of tablet characteristics are mentioned in Table 5. The dissolution profile of samples was compared using similarity factor (f2) before and after storage in long-term and accelerated conditions, and the results are reported in Table 6.
RESULTS AND DISCUSSION
Preparation of OCTs
The initial trials made with different disintegrants and diluents revealed that Prosolve was giving excellent flow characteristics and required tablet characteristics as per the official requirements and tablet formulations with 6% crospovidone completed the drug release within 25 minutes in comparison with the commercial formulation. Also, the dissolution profile of experimental core tablets was comparable with immediate-release commercial formulation as shown in Figure 1 and f2 was found to be 83.71. Hence, the formula was selected as OCT composition as shown in Table 1, and used for compression coating.
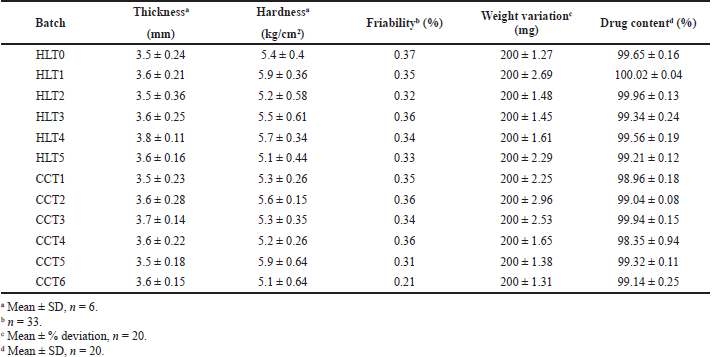 | Table 4. Tablet characteristics of compression coated tablets. [Click here to view] |
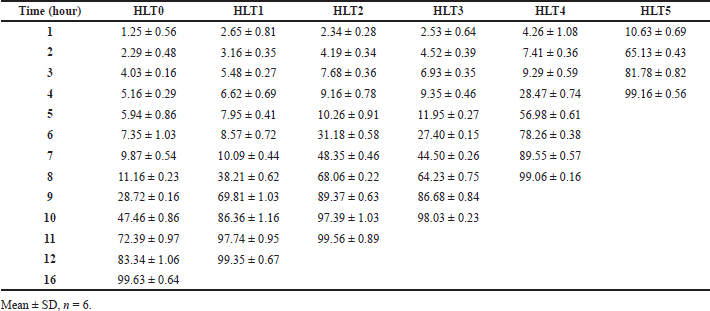 | Table 5. Cumulative % drug release of formulations HLT0-HLT5. [Click here to view] |
Characterization of OCTs
The core tablets formulated were evaluated for tablet characteristics and the results are reported in Table 1. The hardness of 4–5 kg/cm2 and friability below 0.5% indicated good mechanical strength of core tablets indicating their suitability for double compression during compression coating process. The tablets exhibited uniformity in weight and the drug content was found to be 99.61% ± 1.07% of the stated amount of SVSC (50 mg) indicating the uniform distribution of drug in the tablet. The wetting time and in vitro disintegration time were found to be 54 and 118 seconds, respectively. Thus, the core tablets complied with the requirements of official and other tests and subjected for preparation of CCTs.
Preparation of CCTs
The natural gums hupu gum and lannea gum selected for this study exhibited poor flow characteristics as per the reports indicated in Table 2. Hence granules of these gums were prepared by wet granulation technique and further flowability of granules was improved by addition of talc and magnesium stearate.
 | Table 6. Cumulative % drug release of formulations CCT1-CCT6. [Click here to view] |
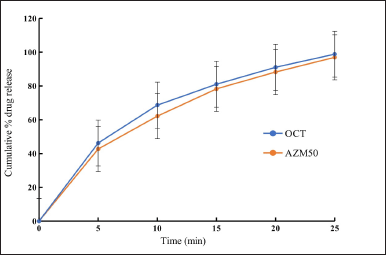 | Figure 1. Dissolution profile of optimized core tablet and marketed formulation (AZM50) (n = 6). [Click here to view] |
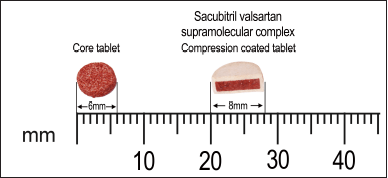 | Figure 2. Cross section of optimized compression coated tablet (CCT3). [Click here to view] |
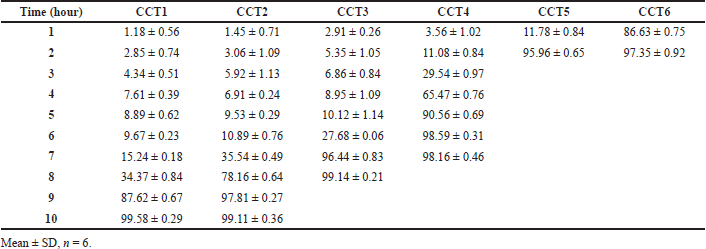
Initially core tablets were compression coated using pure hupu gum and pure lannea gum granules. In CCT with pure hupu gum (HLT0), lag time got extended beyond the contemplated 5 hours whereas, the CCTs prepared with pure lannea gum were not able to exhibit proper mechanical characteristics like friability and even the dissolution lag time was not extended beyond 2 hours hence, further studies with pure lannea gum were not carried out and drug release results were not reported. Further trials were performed with a combination of hupu gum and lannea gum. The following proposed formulations HLT1 (80:20), HLT2 (60:40), HLT3 (50:50), HLT4 (40:60), and HLT5 (20:80) were prepared using varying concentrations of hupu gum and lannea gum, respectively, in the coating composition, where the lag time was achieved but drug release was extended beyond 3 hours after lag time. Further studies were carried out for completing the drug release by incorporating pore forming hydrophilic channeling agent lactose to the coating composition by replacing part of hupu gum and lannea gum. The following ratios of hupu gum, lannea gum, and lactose are employed in the development of CCTs CCT1 (40:10:50), CCT2 (35:15:50), CCT3 (30:20:50), CCT4 (25:25:50), CCT5 (20:30:50), and CCT6 (10:40:50) as mentioned in Table 3.
Characterization of CCTs
The CCTs from all formulation batches were evaluated for tablet characteristics and represented in Table 4. The results of thickness, hardness, friability, uniformity of weight, and drug content indicated that all CCTs were uniform in weight and drug content with good mechanical integrity. Thickness of CCT ranges from 3.5–3.8 mm. The coat thickness was found to be 1 mm. The cross-section view of the CCT shown in Figure 2 also confirmed that coating was uniform on all sides of core tablet.
In vitro drug release study of CCTs
The results of dissolution profiles of all the CCT formulations containing SVSC were represented graphically in Figure 3. Lag time and corresponding drug release time of all formulations are shown in Figure 4. Formulations HLT0, HLT1, HLT2, HLT3, HLT4, and HLT5 prepared using varying ratios of hupu gum and lannea gum in the coat, exhibited lag time of 9, 8, 7, 6, 5, and 3 hours, respectively. Cumulative percentage drug release of formulation HLT0-HLT5 given in Table 5. Highest lag time was observed in formulations (HLT0, HLT1, and HLT2) containing higher concentration of hupu gum corresponding to its high swelling nature and lack of pores in the swollen polymer matrix. Formulations HLT3 and HLT4 achieved desired lag time of 5–6 hours but drug release was delayed extending up to 12 hours which failed to fulfill the objective of the present investigation. Though the core tablets release the drug at faster rate the diffusion path length has been increased and drug did not diffuse out of the swollen hydrophilic polymer matrix. The results of cumulative percentage drug release of formulation CCT1–CCT6 given in Table 6 indicated that the formulations CCT1, CCT2, CCT3, CCT4, CCT5, and CCT6 resulted in corresponding lag time of 7, 6, 5, 3, 2, and 1 hour, respectively, followed by burst release of the drug. The formulation CCT1 prepared with a comparatively higher proportion of hupu gum in the coat composition, resulted in lag time beyond the proposed time of 5–6 hours. Shorter lag time was observed with increasing concentration of lannea gum in coat composition due to the eroding nature of the gum hence, the formulations CCT4, CCT5, and CCT6 were not suitable to achieve predetermined lag time. Among all the formulations CCT2 and CCT3 achieved 5–6 hours lag time but maximum drug release within 1 hour was observed in CCT3 due to balanced mechanism of solvent penetration, diffusion, coat erosion, and dissolution of drug. During lag time, expansion of the swelling agent by water penetration followed by rupture/erosion of the swollen polymeric matrix due to stress created by swelling force takes place. The pores formed by channeling agent lactose cause rapid drug release from core tablets after lag time due to exposure of core material to aqueous fluids resulting in fast disintegration. Further, the drug was released by dissolution very quickly, suitable for reaching the peak plasma concentration (Cmax) at its Tmax. Hence, CCT3 CCTs formulated using hupu gum, lannea gum, and lactose in the ratio of 3:2:5 was considered optimum for chronotherapeutic drug delivery of drug.
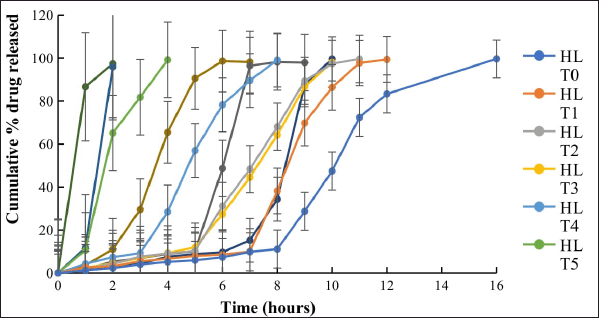 | Figure 3. Dissolution profiles of SVSC from compression coated tablets (n = 6). [Click here to view] |
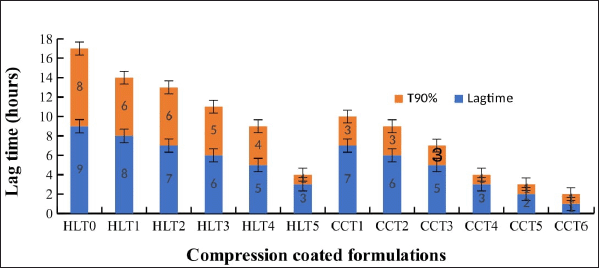 | Figure 4. Lag time and drug release time of all CCT formulations (HLT0–CCT6). [Click here to view] |
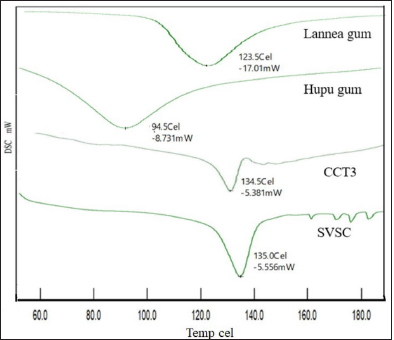 | Figure 5. DSC Thermograms of Lannea gum, Hupu gum, CCT3 and SVSC. [Click here to view] |
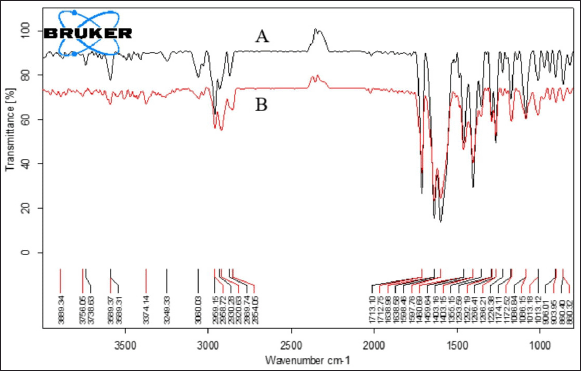 | Figure 6. FTIR spectra of (A) SVSC; (B) CCT3. [Click here to view] |
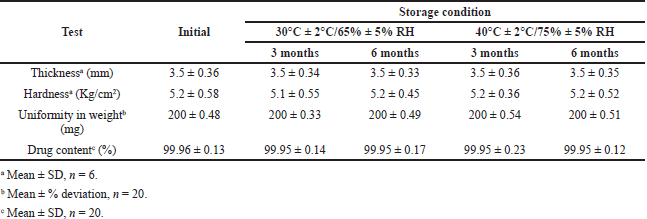 | Table 7. Stability studies of optimized compression coated tablets CCT3. [Click here to view] |
 | Table 8. In vitro dissolution profile of CCT3 tablets before and after stability studies. [Click here to view] |
Solid state characterization
Differential scanning calorimetry
The DSC thermograms of pure drug, hupu gum, lannea gum, and optimized CCT formulation are represented in Figure 5. The DSC thermogram of SVSC exhibited a sharp endothermic peak at 135°C corresponding to its melting point, indicating its crystalline nature. There is a very slight shift in the melting point of SVSC in the optimized formulation CCT3 to 134.5°C indicating that there was no significant drug-excipient interaction. Compared to pure drug SVSC, the melting peak in the thermogram of CCT3 formulation was broadened to some extent which may be due to changes in its crystalline form during compression.
Fourier transformed infrared spectroscopy
Characteristic bands of the SVSC in pure drug samples and optimized CCT3 formulation are shown in Figure 6. Pure SVSC showed characteristic bands at 1,595, 1,639, and 1,714 cm−1 corresponding to the hydrogen bonds and coordination bonds involved in crystal formation between valsartan and sacubitril (Liu et al., 2020). Optimized CCT3 formulation also exhibited the characteristic bands of SVSC with no additional bands observed in the spectra indicating retention of chemical identity of the drug. However, intensity of the bands corresponding to the drug was reduced or broadened in the formulation possibly due to the mixing with the polymers and addition of other excipients. The FTIR spectra data confirmed that hupu gum, lannea gum, and other excipients did not interfere with the performance characteristics of drug indicating their compatibility (Sunil et al., 2013).
Stability studies of optimized formulation
The results of stability studies mentioned in Table 7 indicated the absence of visible and physical change in the product withdrawn from humidity chambers under long-term and accelerated conditions. The results of drug release given in Table 8 were found to be similar for the product before and after storage confirmed by the similarity factor (f2) value of 88.64 and 83.96 in long-term or accelerated conditions, respectively. The results of the stability study also confirmed that the coating thickness and the polymers in coating mixture were intact in efficiently protecting the drug from external environment with percentage drug content and lag time unaltered indicating stability of the product as per ICH guidelines.
CONCLUSION
Development of CCTs using two natural gums hupu gum and lannea gum as compression-coating polymers was one of the successful approaches in bed time dosing of antihypertensives with chronotherapeutic drug delivery. The drug release from the developed chronotherapeutic compression-coated formulation would be in pulsatile manner with respect to time (symptoms aggravated) rather than rate hence it is more effective than conventional immediate release or sustained release dosage forms. The optimized final formulation (CCT3) showed lag time of 5–6 hours followed by burst release of drug reaching maximum concentration (Cmax) within 1 hour when the disease symptoms are at peak (i.e., during 4–6 a.m.), provided the medication was taken at around 10 p.m. Therefore, chronotherapeutic drug delivery of a synergistic antihypertensive drug like SVSC by development of CCTs using natural gums can provide an effective treatment therapy in reducing the risk of sudden cardiac death during early morning hours.
ACKNOWLEDGMENT
The authors sincerely acknowledge the support of Dr. Reddy’s Laboratories Ltd., Hyderabad for providing gift sample of drug.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation
REFERENCES
Aleti R, Murthy KVR. Development and validation of UV spectroscopic method for estimation of sacubitril and valsartan combination (LCZ696) in bulk and pharmaceutical tablet dosage forms. Res J Pharm Technol, 2022; 15(11):5232–8. CrossRef
Ayalasomayajula S, Langenickel T, Pal P, Boggarapu S, Sunkara G. Clinical pharmacokinetics of sacubitril/valsartan (LCZ696): a novel angiotensin receptor-neprilysin inhibitor. Clin Pharmacokinet, 2017; 56(12):1461–78. CrossRef
Bharathi A, Naik D. Formulation and in vitro evaluation of chronotherapeutic press-coated tablets of valsartan with natural controlled released gums. Eur J Pharm Med Res, 2019; 6(4):480–7.
Chickpetty SM, Basawaraj R, Kulkarni RV. Natural polysaccharides-based compression coated tablets for colon targeted delivery of naproxen for the treatment of colitis. Pharm Lett, 2010; 2(6):114–23.
Dabhi C, Randale S, Belgamwar V, Gattani S, Tekade A. Predictable pulsatile release of tramadol hydrochloride for chronotherapeutics of arthritis. Drug Deliv, 2010; 17(5):273–81. CrossRef
D’Elia E, Iacovoni A, Vaduganathan M, Lorini FL, Perlini S, Senni M. Neprilysin inhibition in heart failure mechanisms and substrates beyond modulating natriuretic peptides. Eur J Heart Fail, 2017; 19(6):710–7. CrossRef
Dharmarajan K, Hsieh AF, Lin Z, Bueno H, Ross JS, Horwitz LI, Barreto-Filho JA, Kim N, Bernheim SM, Suter LG, Drye EE, Krumholz HM. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. J Am Med Assoc, 2013; 309(4):355–63. CrossRef
Feng L, Godtfredsen SE, Karpinski P, Sutton PA, Prashad M, Girgis MJ, Hu B, Liu Y, Blacklock TJ, Novartis AG. Pharmaceutical combinations of an angiotensin receptor antagonist and a nep inhibitor. Canada Patent CA2590511A1.2007-05-18.
Feng L, Karpinski PH, Sutton P, Liu Y, Hook DF, Hu B, Blacklock TJ, Fanwick PE, Prashad M, Godtfredsen S, Ziltener C. LCZ696: a dual-acting sodium supramolecular complex. Tetrahedron Lett, 2012; 53(3):275–6. CrossRef
Gami AS, Howard DE, Olson EJ, Somers VK. Day–night pattern of sudden death in obstructive sleep apnea. N Engl J Med, 2005; 352(12):1206–14. CrossRef
Gunsel WC. Compression-coated and layer tablets, in pharmaceutical dosage forms: tablets. In: Lieberman HA, Lachman L (Eds.). Marcel Dekker, New York, NY, vol. 1, pp 187–224, 1980.
Hermida RC, Ayala DE, Mojón A, Fernandez JR. Bedtime dosing of antihypertensive medications reduces cardiovascular risk in CKD. J Am Soc Nephrol, 2011; 22(12):2313–21. CrossRef
aIndian Pharmacopoeia, Govt of India, Ministry of Health and Family Welfare, IPC, Ghaziabad, India, 2018a; 2.5.5:309.
Indian Pharmacopoeia, Govt of India. Ministry of Health and Family Welfare, IPC, Ghaziabad, India, vol. 2.5.3, p 308, 2018b.
Indian Pharmacopoeia, Govt of India. Ministry of Health and Family Welfare, IPC, Ghaziabad, India, vol. 2, p 1118, 2018c.
Indian Pharmacopoeia, Govt of India. Ministry of Health and Family Welfare, IPC, Ghaziabad, India, vol. 2.5.1, p 299–302, 2018d.
International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use guidelines on “Stability testing of active pharmaceutical ingredients and finished pharmaceutical products” Annex 10. WHO Technical Reports Series, No. 1010, 2018. Available via https://www.fda.gov/regulatory-information/search-fda-guidance-documents/q1ar2-stability-testing-new-drug-substances-and-products (Accessed 24 August 2019)
https://www.who.int/publications/m/item/stability-testing-of-active-pharmaceutical-ingredients-and-finished-pharmaceutical-products-stability-conditions-for-who-member-states-by-region- (Accessed 09 March 2021)
Kobalava Z, Kotovskaya Y, Averkov O, Pavlikova E, Moiseev V, Albrecht D, Chandra P, Ayalasomayajula S, Prescott MF, Pal P, Langenickel TH, Jordaan P, Rajman I. Pharmacodynamic and pharmacokinetic profiles of sacubitril/valsartan (LCZ696) in patients with heart failure and reduced ejection fraction. Cardiovasc Ther, 2016; 34(4):191–8. CrossRef
Koizumi K, Watanabe Y, Monita K, Utosuchi N. New method of preparing high porosity rapidly saliva soluble compressed tablets using mannitol with camphor, a subliming material. Int J Pharm, 1997; 152:127–31. CrossRef
Latha K, Srikanth VV, Sunil SA, Srinivasa NR, Uhumwangho MU, Shaik NB, Ramana Murthy KV. Applicability of gum karaya in the preparation and in vitro evaluation of losartan potassium as chronotherapeutic drug delivery system. J Sci Res, 2015; 7(1–2):65–74. CrossRef
Li R, Pan Y, Chen D, Xu X, Yan G, Fan T. Design, preparation and in vitro evaluation of core-shell fused deposition modelling 3D-printed verapamil hydrochloride pulsatile tablets. Pharmaceutics, 2022; 14(2):437. CrossRef
Liu XJ, Zhang Y, Wang XZ. Study on co-crystallization of LCZ696 using in situ ATR-FTIR and imaging. Crystals, 2020; 10(10):922. CrossRef
Lopes CM, Lobo JMS, Pinto JF, Costa P. Compressed mini-tablet as a biphasic drug delivery system. Int J Pharm, 2006; 323(1–2):93–100. CrossRef
Muller JE, Stone H, Turi ZG, Rutherford JD, Czeisler CA, Parker C, Poole WK, Passamani E, Roberts R, Robertson T. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med, 1985; 313(21):1315–22. CrossRef
Novartis Entresto granted expanded indication in chronic heart failure by FDA. 2021. Available via https://www.novartis.com/news/media-releases/Novartis Entresto granted expanded indication in chronic heart failure by FDA. (Accessed 16 April 2021)
Odeku OA, Fell JT. In-vitro evaluation of khaya and albizia gums as compression coatings for drug targeting to the colon. J Pharm Pharmacol, 2005; 57(2):163–8. CrossRef
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, ESC Scientific Document Group. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European society of cardiology (ESC) developed with the special contribution of the heart failure association (HFA) of the ESC. Eur Heart J, 2016; 37(27):2129–200. CrossRef
Puri V, Sharma A, Kumar P, Singh I, Huanbutta K. Synthesis and characterization of Thiolated Gum Ghatti as a novel excipient: development of compression-coated mucoadhesive tablets of domperidone. ACS Omega, 2021; 6(24):15844–54. CrossRef
Ross AC, Macrae RJ, Walther M, Stevens HN. Chronopharmaceutical drug delivery from a pulsatile capsule device based on programmable erosion. JPP, 2000; 52(8):903–9. CrossRef
Shim CY. Heart failure with preserved ejection fraction: the major unmet need in cardiology. Korean Circ J, 2020; 50(12):1051–61. CrossRef
Smolensky MH, Portaluppi F. Chronopharmacology and chronotherapy of cardiovascular medications: relevance to prevention and treatment of coronary heart disease. Am Heart J, 1999; 137(42):S14–24. CrossRef
Solomon SD, McMurray JV, Anand I, Ge J, Lam P, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM, Rouleau JL, van Veldhuisen DJ, Zannad F, Zile MR, Desai AS, Claggett B, Jhund PS, Boytsov SA, Comin-Colet J, Cleland J, Düngen D, Goncalvesova E, Katova T, Kerr Saraiva JF, Lelonek M, Merkely B, Senni M, Shah SJ, Zou J, Rizkala R, Gong J, Shi C, Lefkowitz MP, PARAGON-HF Investigators and Committees. Angiotensin-Neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med, 2019; 381(17):1609–20. CrossRef
Songa AS, Meka VS, Nali SR, Kolapalli VR. An in vitro and in vivo investigation into the suitability of compression coated tablets of indomethacin for the treatment of rheumatoid arthritis which follow circadian rhythms. Drug Dev Ind Pharm, 2013; 39(3):447–56 CrossRef
Singh S, Shyale SS, Karade P. Formulation and evaluation of orally disintegrating tablets of lamotrigine. Int J Pharm Sci Nanotechnol, 2015; 8(2):2881–8. CrossRef
Stevens H, MacDougal F. Controlled onset oral drug delivery: an opportunity for innovative life cycle management. American Pharmaceutical Review, 2014. Available via https://www.americanpharmaceuticalreview.com/Featured-Articles/163671-Controlled-Onset-Oral-Drug-Delivery-An-Opportunity-for-Innovative-Life-Cycle-Management/ (Accessed 19 June 2019)
Sunil SA, Srikanth MV, Rao NS, Murthy KV. Chronotherapeutic drug delivery from indomethacin compression coated tablets for early morning pain associated rheumatoid arthritis. Curr Drug Deliv, 2013; 10(1):109–21. CrossRef
USFDA Dissolution testing methods. Available via https://www.accessdata.fda.gov/scripts/cder/dissolution/dsp_getalldata.cfm (Accessed 24 October 2019)
Vemula SK, Bontha VK. Colon targeted guar gum compression coated tablets of flurbiprofen: formulation, development and pharmacokinetics. Biomed Res Int, 2013; 2013(6):287919; https://doi.org/10.1155/2013/287919 CrossRef
Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway W, Carson AP, Chamberlain AM, Chang R, Cheng S, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Khan S, Kissela BM, Knutson L, Kwan TW, Lackland T, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran E, Mussolino ME, Perak AM, Rosamond D, Roth GA, ampson KA, Satou GM, Schroeder EB, Shah SH, Shay M, Spartano NL, Stokes A, Tirschwell L, VanWagner LB and Tsao CW. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2020 update: a report from the American heart association. Circulation, 2020; 141(9):e139–596. CrossRef
Volpe M, Gallo G. Sacubitril/valsartan for heart failure with preserved ejection fraction and resistant hypertension: one shot for a double strike? Eur Heart J, 2021; 42(36):3753–5. CrossRef