INTRODUCTION
Tuberculosis is a contagious disease caused by Mycobacterium tuberculosis that normally disturbs lung functions. Nevertheless, this bacterial infection may also cause distress in many other organ systems such as the central nervous and lymphatic systems, liver, bones, and genitourinary and gastrointestinal tracts. Tuberculosis is communicable and is transmitted through respiratory droplets (Tasduq et al., 2007). The primary empiric treatment for tuberculosis comprises a four-drug regimen: Isoniazid (INH), Rifampicin (RIF), Pyrazinamide (PZA), and either Ethambutol (EMB) or Streptomycin (Sachin et al., 2009). Each drug has a unique mechanism of action and pharmacokinetic profile. This makes them susceptible to pharmacokinetic interactions when taken concomitantly. For instance, INH is a prodrug requiring activation by catalase-peroxidase (KatG) for the inhibition of bacterial cell wall synthesis by preventing the formation of mycolic acid (Unissa et al., 2011). Its metabolism and elimination exhibit interindividual variation depending on the acetylation phenotype (fast or slow). Therefore, drug–drug interactions (DDIs) with INH can also vary between individuals within a population. RIF, which inhibits bacterial DNA-dependent RNA polymerase, induces the metabolic pathway of several cytochrome P450 (CYP) enzymes such as CYP1A1, CYP1A2, CYP1B1, CYP3A4, and CYP2C9. Therefore, it can potentially result in DDI with a variety of other drugs.
Literature describes an alteration in the metabolic clearance of many drugs in the presence of RIF and its induction of CYP3A4. Furthermore, studies also demonstrate that fusidic acid strongly interferes with the pharmacokinetic activity of RIF (Marsot et al., 2017). However, few authors have proposed an alternative pathway for this interaction, leaving open the possibility that the interaction could be ascribed to the inhibitory effect of RIF on polypeptide 1B1, an organic anion transporter, of which it is also a substrate (Vavricka et al., 2002). Thus, more than one mechanism may be responsible for the effects of the DDI. The prodrug PZA (active form, pyrazinoic acid) targets membrane transport, affecting RNA and protein synthesis, and EMB inhibits arabinosyl transferase activity, which is required for mycobacterial cell wall synthesis. Neither drug is known to interact with CYP enzymes, but there have been reports about other modes of interaction (Nishimura et al., 2004; Venkatesan, 1992; Zhang et al., 2014, 2020).
A short-course antituberculosis (anti-TB) treatment, called directly observed treatment strategy, is a universally accepted treatment approach for carrying out TB case-finding and its cure. The disease and its treatment may pose undernutrition due to an increased metabolic load and decreased food intake. These nutritional insufficiencies may aggravate the disease or postpone the recovery by interfering with crucial immune functions (Grobler et al., 2017). Lately, various natural products derived from medicinal flora have been explored for their ability to enhance the bioavailability of the potential drugs. Herbal supplements like immunomodulators and micronutrients are consumed by patients, worldwide, to counteract nutritional insufficiencies. Immunomodulators are used to combat multiple infections that occur in tuberculosis and to counteract the side effects of modern therapies. It is a matter of concern when patients on an anti-TB regimen take concomitant therapies such as herbal supplements or adjuvants, whose DDI has not been established.
Immusante® is a polyherbal formulation from Himalaya Wellness Company (Bengaluru, Karnataka, India). It comprises Shanta (Prosopis glandulosa) and Lodhra (Symplocos racemosa). The formulation has a broad spectrum of properties including antioxidant, antimicrobial, as well as anti-inflammatory. It is an immunomodulator which is recommended in immunocompromised conditions, and it is taken as an adjuvant in many conditions, including cancer, tuberculosis, and several viral diseases. It acts as an immunomodulator that increases the chances of long-term disease-free survival. As tuberculosis and anti-TB drugs are known to suppress immunity, Immusante® is recommended as an adjuvant. Studies have suggested that the immunomodulatory activity of Immusante®, via immunotherapeutic cell-mediated and humoral mechanisms, could eradicate the opportunistic disease-triggering pathogens (Agraz et al., 2021; Firashathulla et al., 2016; Gangwar et al., 2021; Jayanthi et al., 2021). The pharmacokinetic interactions of such immunomodulators must be explored to ensure safety when taken as adjuvants with anti-TB drugs. This study was performed to rule out any pharmacokinetic interaction of anti-TB drugs with Immusante®.
Evaluations on herbal supplement-drug interactions are limited and inconclusive. Liquid chromatography with tandem mass spectrometry (LC-MS/MS) is a bioanalytical method used for the quantification of several biomolecules and also to study the pharmacokinetics of DDI (Long et al., 2020).
We developed an analytical method using the LC-MS/MS technique to investigate if there are any alterations in the pharmacokinetic parameters of the anti-TB drugs in the presence of Immusante®. The results of the study provide a comprehensive and scientifically in-depth perspective on the use of Immusante® as an adjuvant immunomodulator. The scheme was authenticated according to the latest European Medicines Agency (EMA) guidelines for endorsing bioanalytical methods (Le Blaye, 2011).
MATERIALS AND METHODS
Materials
Reference standards, EMB (100.0%) and PZA (99.9%) were purchased from TCI Chemicals (Tokyo, Japan); INH (99.0%) and RIF (99.9%) were purchased from Sigma Aldrich (Missouri, USA) and HiMedia Laboratories (Mumbai, India), respectively. Internal standards EMB-D4 (isotopic purity: 99.36%), INH-D4 (isotopic purity: 92.18%), PZA-D3 (isotopic purity: 96.23%), and RIF-D4 (isotopic purity: 95.23%) were purchased from Clearsynth Labs (Mumbai, India). LC-MS grade water, methanol, and acetonitrile were obtained from Thermo Fisher (Hanover Park, IL). The AKT-4 Tablet kit (Lupin) with a label claim of INH 300 mg, RIF 450 mg, EMB 800 mg, and PZA 750 mg was purchased from the local medical store.
Experimental animals
Inbred male Wistar rats weighing 280 ± 20 g were procured from the central animal house facility, R&D, Himalaya Wellness Company (Bengaluru, Karnataka, India). All animals were accommodated at customary settings of temperature 22°C ± 3°C, and the relative humidity was maintained at 40%–70%. Animals were subjected to light and dark cycles of 12 hours each before and throughout the investigations. The rats were provided standard rat feed pellet diet, ad libitum (from VRK Nutritional Solutions, Pune, India). They were also provided reverse osmosis-treated water (processed by Aquaguard Reviva -Eureka Forbes, Mumbai, India). The animals were randomized based on body weights prior to the study. The experimental procedures were permitted by the Institutional Animal Ethics Committee of Himalaya Wellness Company (Bengaluru, India) (Protocol no. 165/16). They were supported with humane care prescribed by the CPCSEA, The Government of India.
Chromatography and MS/MS
A mass spectrophotometer, API 2000 from Applied Biosystems/MDS SCIEX (AB Sciex, Foster, CA), additionally organized with an Electron Spray Ionization (ESI) source and a chromatographic system was used for the study. Batch attainment and data processing were monitored using the Analyst version 1.6.3 software. Analyte detection was done in both positive as well as negative ionization modes; however, the positive ionization mode provided better results. Chromatographic resolution of EMB, INH, PZA, RIF, and internal standards was achieved on an ACE C18 Excel (150 × 4.6 mm, 3 μm) column, sustained at 30°C using a gradient mobile phase comprising formic acid (0.1%) in water and acetonitrile (100%). Acetonitrile was ramped linearly from 5% to 90% in 3 minutes, held for 3 minutes at 90%, then reduced from 90% to 5% in 2 minutes, and re-equilibrated at 5% for 2 minutes. The MS was functioned in the multiple reaction mode (MRM) with positive electrospray ionization. A voltage of 5,500 V and a source temperature of 420°C were applied to achieve the ionization. The optimized source constraints for the analytes and internal standards, GS1 and GS2, were set at 50 and 60 psi, respectively. The compound parameters, declustering, entrance, and focusing potential, were 20, 10, and 400 V, respectively. The mass transition m/z (precursor ion > product ion) of EMB and EMB-D4 was 205.1 > 116.1 and 209.1 > 120.0, respectively. INH and INH-D4 were 138.1 > 121.1 and 141.9 > 124.9, respectively. PZA and PZA-D3 were 123.9 > 79.0 and 126.9 > 82.1, respectively, and RIF and RIF-D4 were 823.1 > 791.1 and 827.1 > 795.0, respectively.
Stock solutions, calibration, and quality control (QC) samples
The reference standard diluted with methanol (100 μg/ml) was considered as the stock solution. The subsequent serial dilutions (seven times) using methanol were considered as working solutions, having concentrations ranging from 0.50 to 40.0 μg/ml for EMB, INH, PZA, and RIF. Along with them, four QC working samples were considered using separately prepared stock solutions of EMB, INH, PZA, and RIF at 0.50–40.0 µg/ml. The internal standards were prepared from native reference standards at a final concentration of 10.0 µg/ml for INH-D4 and EMB-D4, 5.0 µg/ml for RIF-D4, and 50.0 µg/ml for PZA-D3. The prepared solutions were preserved at −20°C and brought to room temperature prior to their usage.
Pharmacokinetic investigations
A validated LC-MS/MS method of pharmacokinetic and drug-drug interaction studies was used to analyze the interaction of anti-TB drugs with Immusante® in rat plasma (Elgawish et al., 2019). Twelve Wistar rats (24 weeks old) were divided into two groups of six animals in each. Group-I rats received a cocktail of first-line anti-TB drugs, which is a combination of INH (84 mg/kg bwt), EMB (31 mg/kg bwt), PZA (156 mg/kg bwt), and RIF (47 mg/kg bwt). The dosages were selected based on the published literature and extrapolation of the human dose. Group-II rats received Immusante® (250 mg/kg of bwt po) in addition to the anti-TB drug cocktail. All the drugs were administered by oral gavage. The influence of Immusante® on the metabolism of the anti-TB drugs was evaluated by the coadministration of Immusante® along with the cocktail for 7 days. The drugs were suspended in water and administered orally. On day 7, all the animals received their respective treatments, and the blood samples were collected from a tail vein in heparinized vials at 0, 0.25, 0.50, 1, 2, 4, 6, 8, and 24 hours. These blood collection time points were selected based on the published literature and a pilot study. To calculate the pharmacokinetic parameters, the drug plasma concentration was log transformed and plotted on the Y-axis and time was plotted on the X-axis. The PK parameters like drug maximum concentration (Cmax), concentration peak time (tmax), area under the concentration-time curve (AUC), clearance (CL), half-life (t1/2), and volume of distribution (Vd) were derived.
Sample preparation
Plasma (50 µl) and internal standards (10 µl) were pipetted into Eppendorf tubes containing 40 µl of water and vortexed for 30 seconds. Methanol (300 µl) was added to precipitate the plasma samples, and then vortexed for several seconds and centrifuged at 3,000 rpm for 5 minutes at 15°C. The supernatant was collected and analyzed using the LC-MS/MS method. Linearity and selectivity, accuracy and precision, lower limit of quantification (LLOQ), extraction recovery, matrix effect, carryover, dilution integrity, and stability were the parameters considered for validating the LC-MS/MS method in accordance with the EMA guidelines.
A comparison between six blank plasma samples taken from individual sources; samples spiked with EMB, INH, PZA, and RIF at LLOQ; and the ones obtained from treated rats was performed for the selectivity analysis. The analyte responses for blank samples were less than 20% LLOQ of that of spiked samples. Subsequently after the upper limit of quantification (ULOQ) samples, the blank samples were analyzed to evaluate the carryover between the samples and were carried out in five cycles.
The calibration curves for EMB, INH, PZA, and RIF consisted of a blank with no analyte and internal standard, a zero calibrator with blank and internal standard, and seven non-zero calibrator levels, wrapping the quantitation array on three successive days. The linearity of every calibration curve was attained by assessing the concentration-response relationship (a ratio of peak to area of analytes/internal standard v/s analyte concentration) using a weighted (1/x2) least-squares linear regression. Non-zero calibrators should be ± 15% of the nominal concentrations, except at LLOQs (±20%). Accuracy and precision were evaluated across the quantitation range involving the performance analysis of EMB, INH, PZA, and RIF at the calibration curve and at LLOQ, low, medium, and high QCs. In three independent runs, the samples were analyzed at five replicates to investigate the accuracy and precision. The precision should be ±15% (except ±20% at LLOQ) and the accuracy should be ±15% of nominal concentration (except 20% at LLOQ).
At lower and higher QC concentrations, the peak areas between extracted and post-extracted spiked samples were compared to evaluate the recoveries of EMB, INH, PZA, and RIF. A ratio of peak to area between post-extracted samples and water-substituted samples at lower and higher QC concentrations was evaluated for matrix effects. The stability study was accomplished with triplicate QC samples at low and high concentrations, including bench-top, autosampler, and post-preparation stabilities. The method was deliberated to be stable when the accuracy at each level was ±15%
Statistical analysis
The pharmacokinetic parameter values were expressed in terms of mean ± standard error mean. The results were statistically analyzed by Student’s t-test using Prism GraphPad version 6.07 software (GraphPad Software Inc., San Diego, CA). p value <0.05 was considered statistically significant.
RESULTS
LC-MS/MS condition development
Each analyte dissolved in methanol was continuously infused for the optimized ionization and fragmentation. Positive ESI mode was selected, and in the first quadrupole (Q1) full-scan mode, protonated ions [M + H]+ were generated at m/z 205.1, m/z 138.1, m/z 123.9, and m/z 823.1 for EMB, INH, PZA, and RIF, respectively. Protein precipitation and liquid–liquid extraction techniques were chosen for a quick and proficient method for proteins and phospholipids removal. With the proposed method, we could achieve >85% recovery for all analytes.
Validation of bioanalytical method
Representative chromatograms of EMB, INH, PZA, and RIF in rat plasma are shown in Figure 1. The retention times were 1.71, 1.93, 2.99, and 4.30 minutes for EMB, INH, PZA, and RIF, respectively. Calibration curves over the quantification range 0.05–40 µg/ml for all analytes showed good linearity with r2 > 0.995. The means of regression equations obtained by least squared regression were y = 1.82x–0.00299 for EMB, y = 1.05x–0.00153 for INH, y = 2.07x–0.000374 for PZA, and y = 5.35x–0.00342 for RIF, where y represents the peak-area ratio of an analyte to internal standard and x represents analyte concentration. Table 1 provides the summary of precision and accuracies at four concentration levels of QC samples which were LLOQ (0.051 μg/ml), Lower QC (LQC, 0.086 μg/ml), Middle-QC (MQC, 20 μg/ml), and Higher QC (HQC, 30 μg/ml). The inter- and intra-batch precision (% RSD) was lower than 7.81 and 7.37, respectively, and the accuracy covered from 91.05% to 100.19% for intra-day and 93.05% to 106.67% for inter-day. The results indicated the reliability and reproducibility of the method.
The extraction recoveries were 99.70% ± 0.38% and 106.50% ± 1.38% for EMB, 103.41% ± 4.22% and 101.13% ± 2.24% for INH, 102.41% ± 3.12% and 96.74% ± 2.24% for PZA, and 92.77% ± 2.24% and 96.76% ± 1.12% for RIF over LQC and HQC sample levels. Matrix effect and extraction recovery of the internal standard were 99.70% ± 2.90%, 96.41% ± 3.11%, 98.88% ± 2.21%, and 99.41% ± 2.21% for EMB, INH, PZA, and RIF, respectively. As listed in Table 2, a series of stability testing demonstrated that both analyte and the internal standard showed no significant loss at the concentrations tested within the analysis period.
Application
The established and validated LC-MS/MS method was used to determine the plasma concentrations of EMB, INH, PZA, and RIF in rats. Exemplary plasma concentration-time profiles of anti-TB drugs (treated as control) and anti-TB drugs with Immusante® are shown in Figure 2. The pharmacokinetic parameters observed in the control anti-TB drugs remained unchanged compared with Immusante® treated animals, except for EMB and RIF, where Vd values were found to be greater than the control. However, it was not influenced significantly on the rest of the pharmacokinetic parameters.
Results are expressed as mean ± SEM. Unpaired t-test was used to compare groups. The minimum level of significance was considered p < 0.05. All the results are summarized in Table 3 and Figure 2.
DISCUSSION
Immusante®, a polyherbal formulation from Himalaya Wellness Company, is used to strengthen immunity in individuals who are prone to frequent infections and in immunocompromised patients. It primarily acts as an immunomodulator and is shown to reduce the disease manifestation in individuals by improving immunity. It may be recommended as an adjuvant to the individual suffering from tuberculosis, cancer and viral infections. However, no scientific studies have been performed to investigate interactions of the formulation with the pharmacokinetics of the first-line drug regimen used in tuberculosis therapy. The method reported is the first of its kind to study interactions based on the pharmacokinetics of Immusante® and first-line anti-TB drugs. Abundant literature illustrates how various enzymes and transporters play a key role in the absorption, distribution, metabolism, and excretion of first-line anti-TB drugs. Most drug interactions result from alterations in the metabolizing enzyme level, which either activate or inactivate the drugs or inhibit membrane transporters (Giacomini and Huang, 2013; International Transporter Consortium, et al., 2010). Both can significantly influence drug kinetics, affecting efficacy and safety in individuals practicing polypharmacy.
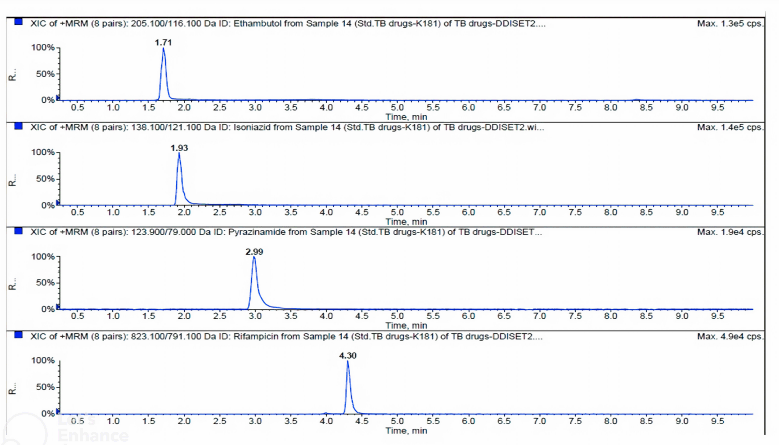 | Figure 1. Typical LC-MS/MS-MRM spectrum of EMB, INH, RIF, and PZA. [Click here to view] |
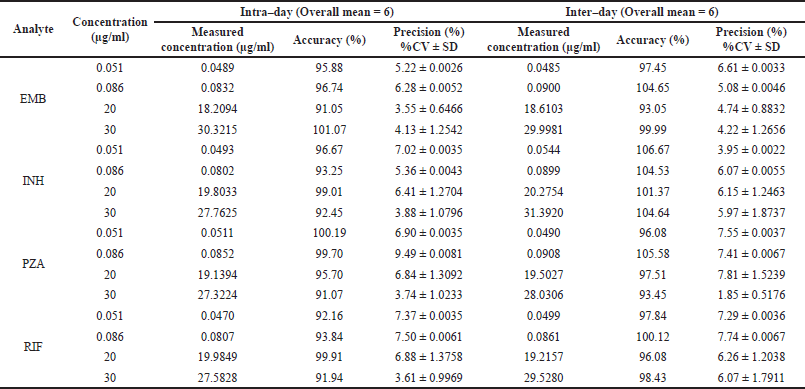 | Table 1. Intra-day and inter-day accuracy and precision of EMB, INH, PZA, and RIF measurements in rat plasma. [Click here to view] |
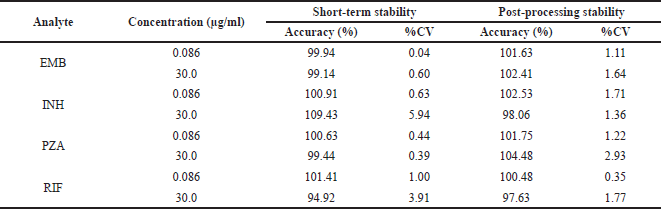 | Table 2. The stability of EMB, INH, PZA, and RIF in rat plasma under various storage conditions. [Click here to view] |
In this study, the LC-MS/MS method was developed and standardized to simultaneously determine the pharmacokinetic interactions of first-line anti-TB drugs in the presence of Immusante®. The simultaneous determination of the drugs in a single method was selected as it increases sample throughput and decreases the risk of analytical error (compared with methods for single analyte determination). Several trials were carried out to achieve the symmetric peak at a shorter run time for the simultaneous scrutiny of target compounds. Linear gradient elution was used under positive ionization mode with formic acid (0.1%) in water and acetonitrile (100%) as the mobile phase; this provided lesser background noise with appropriate retention time. The retention times around the analytes and internal standards were insignificant, indicating the specificity of the method. This analytical method is, therefore, free of potentially interfering substances, and carryover did not occur.
The demonstrated method is selective and sensitive to detect the lowest amounts of 0.035 µg/ml. The blank plasma extract did not show any interference, and no carryover was detected. The LLOQ was considered for each analyte as the lowest point of the calibration curve. The accuracy and precision results demonstrate the reliability and reproducibility of the method for the simultaneous quantification of EMB, INH, PZA, and RIF in rat plasma. The matrix effect data showed that ion suppression from the rat plasma was insignificant under existing conditions. The dilution integrity results demonstrate that a 10-fold dilution integrity is consistent for all the analytes, and the samples beyond the range of calibration curves can be obtained accurately after the dilution. This LC-MS/MS method was applied fruitfully to determine the plasma concentrations of EMB, INH, PZA, and RIF in rats. The pharmacokinetic parameters (except tmax) were logarithmically transformed before analysis.
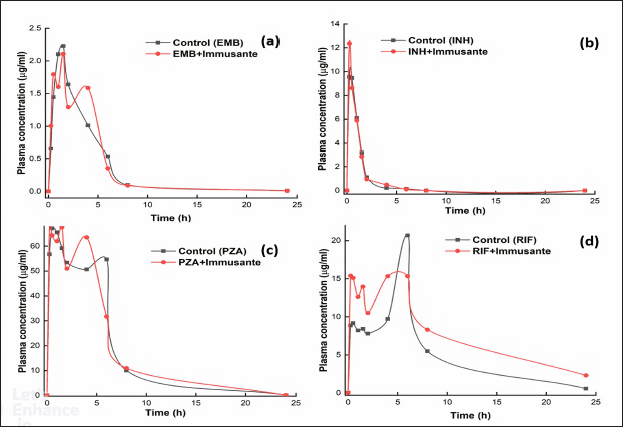 | Figure 2. Effect of Immusante® on plasma concentrations of anti-TB drugs. [Click here to view] |
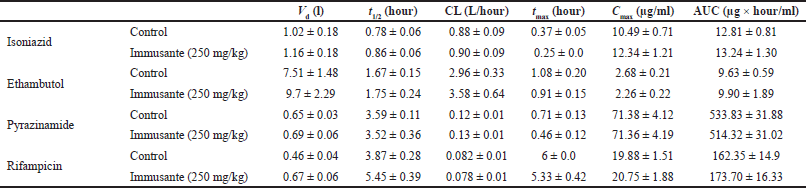 | Table 3. Effect of Immusante® on the pharmacokinetics of anti-TB drugs. [Click here to view] |
The pharmacokinetic parameters obtained from the study (Table 3) demonstrate Vd, t1/2, CL, tmax, Cmax, and AUC values both in control animals (that were on the anti-TB regimen alone) and in test animals (that were on anti-TB drugs + Immusante®) for 24 hours. The experimental pharmacokinetic parameters are comparable to the published data in the literature. Any significant alteration in these parameters would reveal possible interactions between the formulation and the regimen. However, in this study, no such significant alterations in the values of various pharmacokinetic parameters are seen, indicating that coadministration of Immusante® does not influence the pharmacokinetic profiles of the anti-TB drugs. Nevertheless, as Immusante® is a polyherbal formulation in which there can be more than one active constituent that could individually or synergistically elicit the immunomodulatory effect, the formulation does not alter the kinetics of the drugs. Although there was a slight difference in the AUC of PZA and RIF in the test group compared with the control, they are not significant. The anti-TB drugs absorption was not influenced with Immusante as neither Cmax nor tmax changes and Vd remains constant. It was observed that there were no enzyme induction/inhibition effects elicited by the formulation that would affect the metabolism of anti-TB drugs. However, the interactions with other anti-TB drugs to be confirmed by further experiments.
CONCLUSION FUTURE PERSPECTIVES
A robust LC-MS/MS method was established to simultaneously quantify EMB, INH, PZA, and RIF in the plasma of rats treated with AKT-4 tablets (which is a combination of anti-TB drugs). Immusante® was evaluated for its possible pharmacokinetic interactions with the anti-TB drugs. No significant change in the pharmacokinetic parameters of the drugs was seen in the presence of Immusante®. Thus, it can be concluded that Immusante® does not interact with the anti-TB drugs and can be recommended as an adjuvant to first-line anti-TB drug therapy.
Though analysis of single molecules is essential in clinical and preclinical analysis, simultaneous detection of two or more compounds is crucial when research related to drug development is concerned. The method described above efficiently saves time, cost-effective, and helps in establishing the mechanism of interaction with drugs. Simultaneous identification and quantification of targeted metabolite are the futuristic application of this analytical method. This study also opens avenue to further explore the interaction between the first line anti-TB drugs and other herbs/phytochemicals.
ACKNOWLEDGMENTS
The authors thank Himalaya Wellness Company for providing the research facility to carry out the study, and also the Scientific Publications Division of the company, for their editorial services.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This research work was funded by Himalaya Wellness Company, Bengaluru, India.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The experimental procedures were permitted by the Institutional Animal Ethics Committee of Himalaya Wellness Company (Bengaluru, India) (Protocol no. 165/16). They were supported with humane care prescribed by the CPCSEA, The Government of India.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Agraz AV, Rafiq M, Guru B, Madan MN, Anturlikar SD, Azeemuddin MM, Manjula SN. Evaluation of immunomodulatory activity of Immusante® in zebrafish. J Appl Biol Biotechnol, 2021; 9(3):7–2.
Elgawish MS, Soltan MK, Sebaiy MM. An LC-MS/MS spectrometry method for the simultaneous determination of Rosuvastatin and irbesartan in rat plasma: insight into pharmacokinetic and drug-drug interaction studies. J Pharm Biomed Anal, 2019; 174:226–34. CrossRef
Firashathulla S, Inamdar MN, Rafiq M, Viswanatha GL, Sharath Kumar LM, Babu UV, Ramakrishnan S, Paramesh R. IM-133N—a useful herbal combination for eradicating disease-triggering pathogens in mice via immunotherapeutic mechanisms. J Pharmacopuncture, 2016; 19(1):21–7. doi: 10.3831/KPI.2016.19.003. CrossRef
International Transporter Consortium, Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, Dahlin A, Evers R, Fischer V, Hillgren KM, Hoffmaster KA, Ishikawa T, Keppler D, Kim RB, Lee CA, Niemi M, Polli JW, Sugiyama Y, Swaan PW, Ware JA, Wright SH, Yee SW, Zamek-Gliszczynski MJ, Zhang L. Membrane transporters in drug development. Nat Rev Drug Discov, 2010; 9(3):215–36; doi: 10.1038/nrd3028. CrossRef
Jayanthi CR, Avinash HR, Sridhar S, Akhila K, Kumawat R. A randomized control study to evaluate the role of herbal immunomodulators in boosting the immunity and overall health of healthcare workers in COVID-19 wards: an exploratory, feedback clinical study. Asian J Pharm Clin Res, 2021; 14:138–42. CrossRef
Le Blaye O. The EMA guideline on bioanalytical method validation. EMA, London, UK, 2011.
Gangwar V, Garg A, Lomore K, Korla K, Bhat SS, Rao RP, Rafiq M, Kumawath R, Uddagiri BV, Kareenhalli VV. Immunomodulatory effects of a concoction of natural bioactive compounds—mechanistic insights. Biomedicines. 2021; 9(11):1522. CrossRef
Giacomini KM, Huang SM. Transporters in drug development and clinical pharmacology. Clin Pharmacol Ther, 2013; 94(1):3–9. doi: 10.1038/clpt.2013.86. CrossRef
Grobler L, Durao S, Van Der Merwe S M, Wessels J, Naude C E. Nutritional supplements for people being treated for active tuberculosis: a technical summary. S Afr Med J, 2016; 108(1):16–8. CrossRef
Long NP, Par S, Anh .H, Kim SJ, Kim HM, Yoon SJ, Lim J, & Kwon SW. Advances in liquid chromatography–mass spectrometry-based lipidomics: a look ahead. J Anal Test, 2020; 4:1–5. CrossRef
Marsot A, Ménard A, Dupouey J, Muziotti C, Guilhaumou R, Blin O. Population pharmacokinetics of rifampicin in adult patients with osteoarticular infections: interaction with fusidic acid. Br J Clin Pharmacol, 2017; 83(5):1039–47; doi: 10.1111/bcp.13178. CrossRef
Nishimura Y, Kurata N, Sakurai E, Yasuhara H. Inhibitory effect of antituberculosis drugs on human cytochrome P450-mediated activities. J Pharmacol Sci, 2004; 96(3):293–300. CrossRef
Sachin BS, Monica P, Sharma SC, Satti NK, Tikoo MK, Tikoo AK. Pharmacokinetic interaction of some antitubercular drugs with caraway: implications in the enhancement of drug bioavailability. Hum Exp Toxicol, 2009; 28:175–84. CrossRef
Tasduq SA, Kaiser P, Sharma SC, Johri RK. Potentiation of isoniazid-induced liver toxicity by rifampicin in a combinational therapy of antitubercular drugs (rifampicin, isoniazid and pyrazinamide) in Wistar rats: a toxicity profile study. Hepatol Res, 2007; 37(10):845–53. doi: 10.1111/j.1872-034X.2007.00129.x. CrossRef
Unissa AN, Selvakumar N, Narayanan S. Characterization of isoniazid-resistant mutant (S315R) of catalase-peroxidase, KatG, from Mycobacterium tuberculosis. Int J Med Sci Technol, 2011; 4(3):13–22.
Vavricka SR, Van Montfoort J, Ha HR, Meier PJ, Fattinger K. Interactions of rifamycin SV and rifampicin with organic anion uptake systems of human liver. Hepatology, 2002; 36(1):164–72. doi: 10.1053/jhep.2002.34133. CrossRef
Venkatesan K. Pharmacokinetic drug interactions with rifampicin. Clin Pharmacokinet, 1992; 22(1):47–65; doi: 10.2165/00003088-199222010-00005.
Zhang L, Zhao Y, Gao Y, Wu L, Gao R, Zhang Q, Wang Y, Wu C, Wu F, Gurcha SS, Veerapen N, Batt SM, Zhao W, Qin L, Yang X, Wang M, Zhu Y, Zhang B, Bi L, Zhang X, Yang H, Guddat LW, Xu W, Wang Q, Li J, Besra GS, Rao Z. Structures of cell wall arabinosyltransferases with the anti-tuberculosis drug ethambutol. Science, 2020; 368(6496):1211–9; doi: 10.1126/science.aba9102. CrossRef
Zhang Y, Shi W, Zhang W, Mitchison D. Microbiol Spectr, 2014; 2(4):1–2. CrossRef