INTRODUCTION
Research involving the study of marine mollusks has exponentially increased over the last several decades as more is discovered about the vast array of health-promoting compounds found in marine mollusks. These compounds are a rich source for the growing market of nutraceuticals and novel pharmaceutical compounds as evidenced by the explosive growth of new marine organism-based products in the current market (Gad El-Karim et al., 2022; Kuppusamy and Ulagesan, 2016). The aquatic environment is the largest storage of natural molecules to be examined for beneficial drug activities (Gerwick, 1987; Ghareeb et al., 2020; Hamed et al., 2020). Marine organisms have proven useful in the development of anticancer compounds and secondary metabolite bioactive compounds, used to counter infectious diseases and inflammation (Agour et al., 2022; Ghareeb et al., 2020; Ibrahim et al., 2021). These developments strengthen the public perception of and subsequent demand for valuable products from the sea (Bhatnagar and Kim, 2010). Marine spineless creatures offer a prospective exporter of novel anti-infective medications (Bazes et al., 2009). The phylum mollusca, which consists of soft-bodied spineless creatures, is the second-biggest phylum in the animal realm and comprises a major part of the world’s marine invertebrate animal life. The bulk of the research related to the natural products from the phylum mollusca has been primarily focused on the fluffy body mollusk (Faulkner, 2019). Valuable information is provided through the investigations of antimicrobial ingredients of oceanic invertebrates which can contain additional antimicrobial compounds leading to the development of novel antibiotics; antimicrobial peptides are preferentially studied due to their wide application in both human and other animal systems (Boman, 1995). The increasing need for effective antimicrobials provides the opportunity for extensive investigation into bioactive compounds found in over 150,000 species of marine mollusks. Delving into a better understanding of the structural composition and bioactivity of the bioactive peptides discovered in marine mollusks gives the potential to enhance the health benefits of both nutraceutical and pharmaceutical agents through the development of novel supplements and medications useful in the treatment of a variety of illnesses. Trochus erithreus (Brocchi, 1821) are invertebrate marine mollusks belonging to the family Trochidae (Top Snails) characterized by their cone-shaped and pointed shape that resembles a spinning top. These mollusks help in growing algae and peak snail shells, just as turbans, characterized by a pale grayish-white color, tinted with blue, which fit in with mother-of-pearl. Also, this product has been used in an abundance of commercial products such as jewelry and buttons (Williams, 2010).
Increasing antibiotic resistance against existing antibiotics has created a crucial demand for the detection and acquiring novel antibiotics. The development of resistance to a broad spectrum of antimicrobial drugs is considered one of the most important challenges in healthcare today. The suboptimal treatment of microbial infections frequently leads to the recurrence of the infection (Kaczor et al., 2017; Simões et al., 2017) making the selection of specific targets critical for minimizing resistance (Silver, 2007). One of the pathways to overcoming resistance is the interaction of the antimicrobial agent with different enzymes using a variety of mechanisms that can improve efficacy (Tari et al., 2013). Based on this premise, the mechanisms of the newly isolated compounds were explored via the Molecular Operating Environment (MOE) program by using three bacterial enzymes: thymidylate kinase (TMK) which plays an important role in bacterial DNA synthesis; DNA gyrase B, which exists in most bacteria and is fundamental for bacterial DNA repetition and repair; and a subunit B of topoisomerase IV which decatenates bacterial DNA and helps in relaxing positive supercoils (Kawatkar et al., 2014). The three bacterial enzymes were used for investigation for their importance in regulating the topological status of bacterial DNA during replication utilizing their native ligands for comparison. A molecular docking study was conducted by the MOE program so as to explore the possible antimicrobial mechanism of the marine mollusk ingredients (1–7) and to foretell and record poses of protein–ligand binding via using the structure of the three bacterial objective enzymes.
This research work aimed to focus on the effect of extraction solvent on the chemical composition and beneficial peptides of marine molluscan. We also harvested T. erithreus-derived extracts in a bioprospecting manner in order to investigate the chemical composition as well as molecular docking and antioxidant, antibiofilm, antimicrobial, and cytotoxic activities in the various harvested extracts.
MATERIALS AND METHODS
Samples collection and extraction
The marine mollusks were assembled from Ain AlSokhna, Egypt, and identified (Abu-ElEinin et al., 2021). Soft parts were extracted separately with several organic solvents: ethanol, acetone, and ethyl acetate. Once extracted, the samples were tested for their total antioxidant competence and 2,2-diphenyl-1-picryl-hydrazyl (DPPH) free radical scavenging activity. Additional tests were performed to evaluate their antibacterial and antibiofilm activity (at a concentration of 500 µg/ml). Based on the antimicrobial testing, the most promising samples were further processed for cytotoxicity testing, Gas chromatography-mass spectrometry (GC-MS), and for molecular docking evaluation.
Antioxidant activity
DPPH free radical scavenging efficacy
The free radical scavenging antioxidant activity was estimated according to the reported procedures (Shirwaikar et al., 2006).
Estimation of total antioxidant capacity (TAC)
The TAC was evaluated according to the reported procedures (Ghareeb et al., 2016; Prieto et al., 1999).
Determination of total phenolic content (TPC)
The TPC was determined using Folin-Ciocalteu’s reagent according to the reported procedures (Kumar et al., 2008).
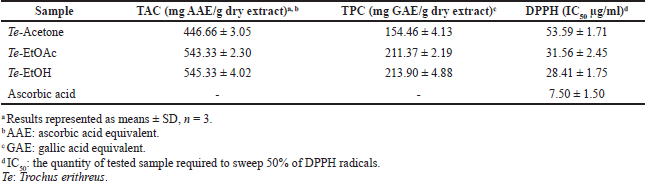 | Table 1. TAC, TPC, and antiradical efficacy of various tested extracts of Trochus erithreus. [Click here to view] |
Antimicrobial activity
The antimicrobial activity of the crude tested extracts was evaluated against six pathogenic microbial strains according to the reported procedures (Elkhouly et al., 2021a; Mayers et al., 2017).
Antibiofilm
The antibiofilm efficacy was assessed against four pathogenic microbes according to the reported procedures (Elkhouly et al., 2021b; Mayers et al., 2017).
Cytotoxicity [microculture tetrazolium (MTT) assay]
The cytotoxic activity was evaluated by using an MTT assay according to the reported procedures (Madkour et al., 2017).
Gas chromatography-mass spectrometry (GC-MS) analysis
GC-MS investigation was performed according to the previous procedures (Madkour et al., 2017).
Molecular docking
The docking profile of all compounds was obtained using the MOE-Dock/2014.09. Chemical skeletons of compounds (1–7) were drawn by using the builder button. Then, energy-minimization of the drawn compounds was done by the program. Docking of all compounds inside the active binding site of Thymidylate kinase, DNA gyrase B, and DNA topoisomerase IV/subunit B enzymes was then performed. During the docking process, water molecules were removed, and also missing H-atoms were reserved. The “Docking” tool was used to attain molecular docking. The operation was run with presumptive regulations. The top 30 poses, were recorded, and then, Generalized–Born Volume Integral/Weighted Surface Area (GBVI/WSA double integral) scoring task was utilized for scoring. The “Ligand Interactions” tool was utilized for the analysis of the obtained results by visualizing the ligand-protein interactions.
Statistical analysis
All data were submitted after completing triple measurements using mean ± SD that uses the Statistical Package for the Social Science software (version 20.1, Chicago, IL). Data are presented as mean ± SD or standard error of the mean. The limit for statistical significance was set at p > 0.05.
RESULTS
Antioxidant activity and TPC
DPPH and phosphomolybdenum tests were utilized to assess the antioxidant effect of different solvent extracts of T. erithreus. In the DPPH test, the IC50 values for the investigated extracts varied from 28.41 to 53.59 µg/ ml in comparison to ascorbic acid with IC50 = 7.50 µg/ml. The antiradical activities are in the arrangement: Te-EtOH ? Te-EtOAc ? Te-acetone. In the phosphomolybdenum test, the Te-EtOH extract exhibited high TAC with 213.90 mg AAE/g dry extract, followed by Te-EtOAc and Te-acetone with TAC values of 211.37 and 154.46 mg AAE/g dry extract, respectively. TPC values were coherent with TAC findings where Te-EtOH extract had the highest phenolic content being 213.90 ± 4.88 mg GAE/g extract while the Te-acetone fraction showed the lowest content (154.46 ± 4.13 mg GAE/g dry extract) (Table 1).
Antimicrobial activity
The antimicrobial potential of the T. erithreus extracts was measured using the MTP test. Findings disclosed that the Te-acetone extract exhibited noticeable antibacterial activity against Proteus vulgaris with a suppression proportion up to 67.93%, and mild antibacterial activity versus Escherichia coli and Klebsiella sp. while it demonstrated low antibacterial efficiency against Staphylococcus aureus and Pseudomonas aeruginosa. Additionally, the Te-EtOAc extract showed a moderate antibacterial effect on Klebsiella sp. and P. vulgaris and low antibacterial activity toward E. coli, P. aeruginosa, and S. aureus. The Te-EtOH showed very low antibacterial activity toward S. aureus and Klebsiella sp. The antifungal effect was observed toward Candida albicans and results indicated that both Te-EtOAc and Te-acetone demonstrated anticandidal efficacy (Table 2).
Antibiofilm
Using microtiter plates biofilm assays, the biofilm inhibition activity of the T. erithreus extract and pure compounds were measured using four microbial species (S. aureus, P. aeruginosa, E. coli, and B. subtilis). The antibiofilm outputs of the tested fractions showed that Te-EtOAc displayed a pronounced biofilm inhibition effect toward S. aureus with biofilm suppression proportion reaching 60%, while it showed moderate biofilm inhibition effect to B. subtilis with biofilm suppression proportion up to 40% and it showed very low biofilm inhibition effect to P. aeruginosa and E. coli in comparison with the untreated control. The biofilm activity of the Te-EtOAc was also assessed and results illustrated that a low biofilm inhibition effect was detected toward all tested microbes including S. aureus, P. aeruginosa, E. coli, and B. subtilis. Additionally, Te-EtOH showed only a moderate percentage of biofilm inhibition up to 40% against S. aureus, while no inhibition activity was detected against the remaining microbes (Fig. 1).
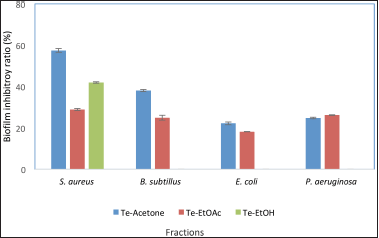 | Figure 1. Antibiofilm activity for different tissue extracts of Trochus erithreus. [Click here to view] |
 | Table 2. Antimicrobial activity of Trochus erithreus extracts. [Click here to view] |
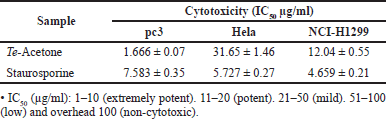 | Table 3. Cytotoxicity of Trochus erithreus acetone extract. [Click here to view] |
Cytotoxicity of Te-acetone extract
The antineoplastic effect of T. erithreus acetone extract on pc3, Hela, and National Cancer Institute (NCI)-H1299 cell lines was assessed through MTT. The IC50 value of acetone extract from T. erithreus was employed and effective doses were considered from the dose-response chart. Results of the cytotoxicity estimation against pc3, Hela, and NCI-H1299 cell line of the T. erithreus acetone extract are demonstrated in Table 3. The extract exhibited very strong efficacy versus the pc3 cell line achieving an IC50 value of 1.666 ± 0.07 µg/ml. On the contrary, the extract exhibited significantly less efficacy versus the NCI-H1299 cell line with an IC50 value of 12.04 ± 0.55 µg/ml. The criteria of cytotoxicity for the crude extract, as determined by the U.S. NCI, are an IC50 < 20 µg/ml in the initial test (Ghareeb et al., 2014; Shoeb et al., 2014). On the treatment with T. erithreus extract, Hela cells showed moderate cell death at a lower concentration of the extract when compared to staurosporine, the control drug (Fig. 2).
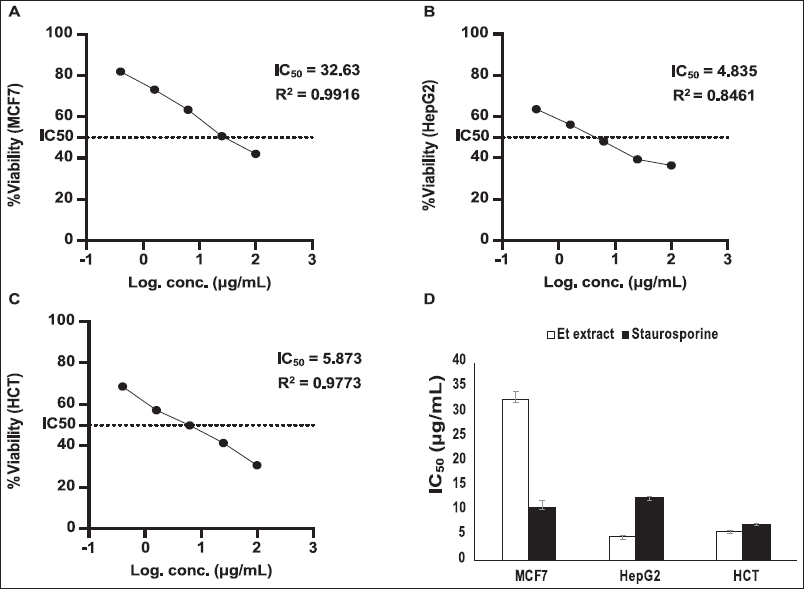 | Figure 2. Cytotoxicity of Te-acetone extract toward various human cancer cell lines after 24 hours of treatment. (A) MCF7, (B) HepG2, (C) HCT, (D) The comparison of average IC50 of the extract versus Staurosporine as a positive control. [Click here to view] |
GC-MS investigation of Te-acetone extract
GC-MS examination of Te-acetone extract comprises 45 components (Fig. 3). The whole peak areas of the determined constituents represent 96.53%, the predictions of the chemical structures of the determined components are mentioned in Table 1S. The principal identified compounds are bisabolol oxide A (24.63%), (E)-α-farnesene (7.89%), tricyclo[8.2.0.0(2,5)] dodeca-3,6,8,11-tetraene (5.41%), 2-[1-(4-cyano-1,2,3,4-tetrahydronaphthyl)]propanenitrile (4.21%), 9,12-octadecadienoic acid, methyl ester (3.47%), 3-tetradecyn-1-ol (3.03%), α-bisabolol-oxide-B (2.99%), bisabolone oxide (2.92%), cycloprop[a]indene, 1,1a,6,6a-tetrahydro (2.89%), and hexadecanoic acid, ethyl ester (2.46%), for which it constituted 59.9% of the overall peak areas (Fig. 4). The determination was attained by the use of computer search user-created reference libraries combined with mass spectra (Abdel-Wareth et al., 2019; Khalaf et al., 2021; Madkour et al., 2017; Shawky et al., 2019). The most predominant compound in the Te-acetone extract is bisabolol oxide A (24.63%). Bisabolol oxide A is a sesquiterpenoid that was reported to be the main contributor to the demonstration of numerous biological activities including antimicrobial (Sharifi-Rad et al., 2018), cytotoxic, and antileishmanial (Andrade et al., 2016) activities.
Molecular docking study
The molecular docking study started by downloading the three target enzymes: TMK (PDB: 4QGG), DNA gyrase B (PDB: 6F86), and DNA topoisomerase IV/subunit B (PDB: 4HZ5) from the protein data bank. The obtained results are displayed in Tables 4–6, Figures 5–7, and supplementary Figures 1S–15S. Interestingly, compounds 1, 3, 5, and 6 displayed the best binding modes in the active sites of the three examined enzymes whereas (E)-α-farnesene showed the least binding affinity forming arene-H interaction with Tyr100 amino acid while showing only hydrophobic interaction in both active sites of DNA gyrase B and DNA topoisomerase IV/subunit B.
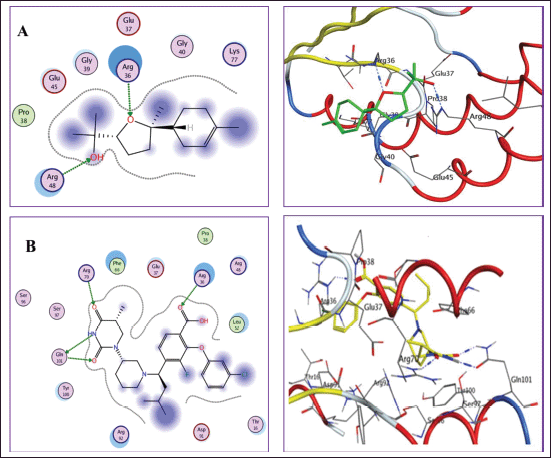
Docking into TMK active site (PDB: 4QGG)
Docking simulation of detected components (1–7) within the energetic location of TMK was performed on (PDB: 4QGG). Redocking of the cocrystallized ligand was first performed for validation, revealing a docking score value = −6.741 kcal/mol with a root-mean-square deviation (RMSD) of 1.69 Å. Two hydrogen bonds with the main residue Gln101, in addition to another two hydrogen bonds with Arg36 and Arg70, were observed (Fig. 5). Studied compounds showed binding energies with a range of values from −5.357 to −10.780 kcal/mol comparable to that of the ligand which was −10.741 kcal/mol. Compounds 1, 3, 5, and 6 were able to interact with the most important amino acids in the dynamic site, revealed by the docking results and their compulsory poses (Fig. 5 and Table 4). Compound 5 (α-bisabolol-oxide-B) revealed the best binding energy score = −10.780kcal/mol and also showed good binding interaction with the essential amino acids in the dynamic location via the development of two H-bounds with Arg36 and Arg48 residues.
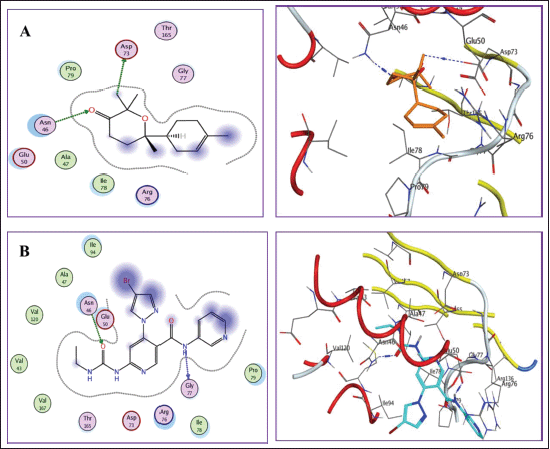
Docking into DNA gyrase B active site (PDB: 6F86)
Docking results revealed that all compounds showed good binding scores ranging from −8.341 to −12.021 kcal/mol relative to the ligand (Table 5). For justification, redocking of the cocrystallized ligand was performed showing a docking score value = −12.897 kcal/mol with an RMSD = 1.79Å. It formed H bonds with Asn46 and Gln77 amino acids. Additionally, bisabolone oxide was able to form two hydrogen bonds with Asn46 and Asp73 having a high energy score nearly equal to that of the ligand (Fig. 6).
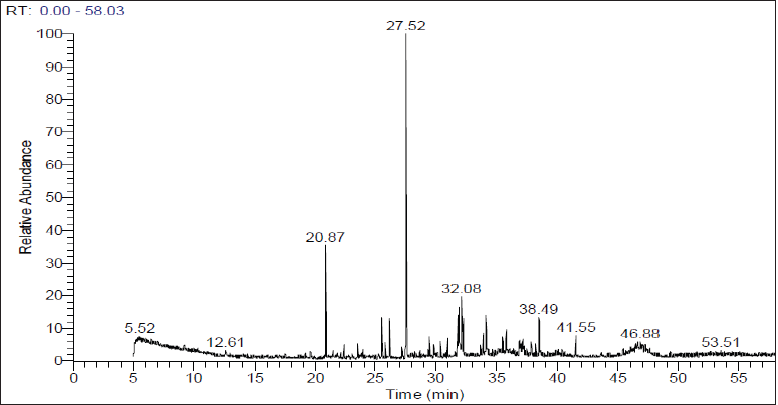 | Figure 3. GC-MS chromatogram of Te-acetone extract. [Click here to view] |
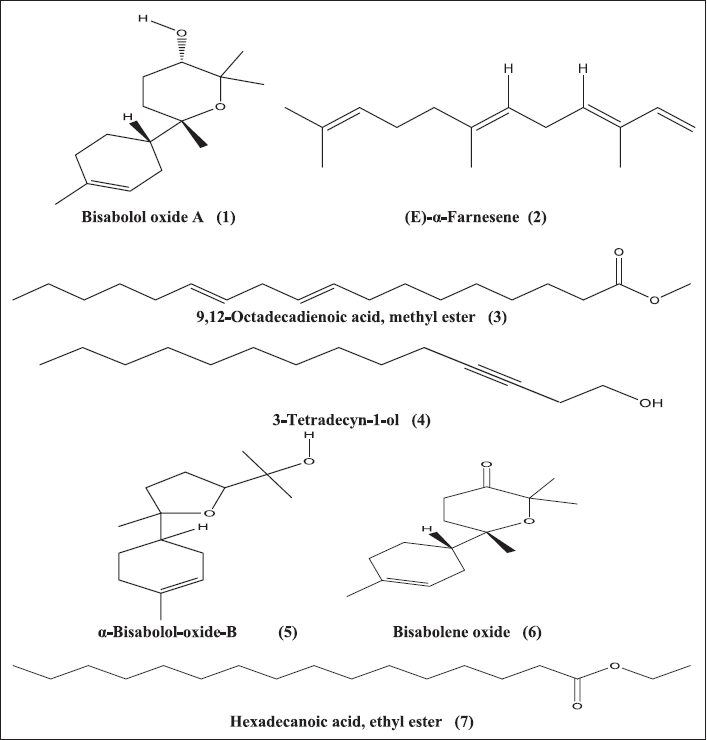 | Figure 4. Chemical structures of some major identified compounds in Te-acetone extract (1) Bisabolol oxide A (24.63%), (2) (E)-α- arnesene (7.89%), (3) 9,12-octadecadienoic acid, methyl ester (3.47%), (4) 3-tetradecyn-1-ol (3.03%), (5) α-bisabolol-oxide-B (2.99%), (6) bisabolene oxide (2.92%), and (7) hexadecanoic acid, ethyl ester (2.46%). [Click here to view] |
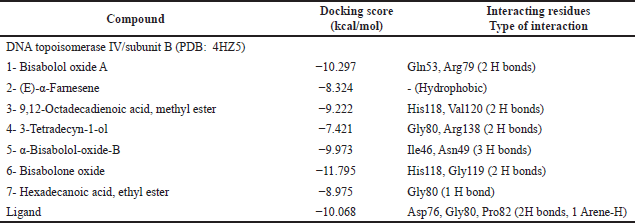
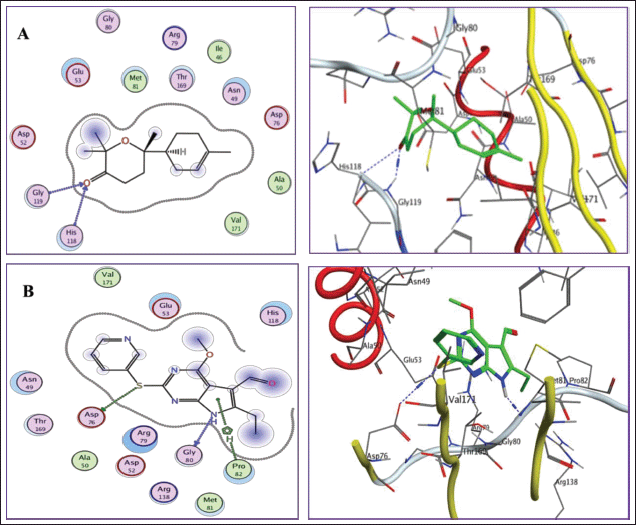
Docking into DNA topoisomerase IV/subunit B active site (PDB: 4HZ5)
The studied compounds were able to fit properly inside the active site of the enzyme forming hydrogen bonds with the key amino acids in the connecting location revealing binding energies ranging from −7.421 to −11.795 kcal/mol compared to the ligand = −10.068 kcal/mol (Table 6). Additionally, the RMSD value of the redocked cocrystallized ligand was 1.42Å. From the docking results, bisabolone oxide was theoretically the best DNA topoisomerase IV/subunit B inhibitor showing a binding score = −11.795 kcal/mol exceeding that of the ligand. The binding mode of bisabolone oxide revealed two hydrogen links with the amino acid residues His118 and Gly119 (Fig. 7).
DISCUSSION
Mollusca organisms in world research foundations are commonly used for diverse investigations, but lately, they have been renowned as possible sources of novel antibacterial and anticancer agents. This work aimed to identify and quantitate the capacity of antioxidant, antibacterial, and antineoplastic activity of the marine molluscan T. erithreus (Family: Trochidae) tissue extract. Solvent extraction is known as a widely used procedure to isolate bioactive compounds in research relevant to antioxidant screening. Solvent extraction uses nonpolar solvents primarily for extracting antioxidants (Moure et al., 2001). This study explicitly demonstrates significantly higher antioxidant activity compared to ascorbic acid TAC and TPC, which are two of the most important antioxidant parameters, showing a high potentiality of the extracts resignifying their ability as radical scavengers. The DPPH assay is superior regarding the cytotoxic activity shown by the other extracts, demonstrated by the high IC50 (53.59 ± 1.71 μg/ml) value noticed for the Te-acetone extract while the IC50 value of Te-EtOAc and Te-EtOH tissue extract were 31.56 ± 2.45 and 28.41 ± 1.75 μg/ml, respectively. This is in line with Nazeer and Naqash (2013) who reported that extracts taken after EtOAc and Et2O displayed greatly maximum DPPH radical scavenging efficacy than methanol extracts (controls) for both molluscan Loligo duvauceli and Donax cuneatus. Bae et al. (2012) indicated that when ascorbic acid was utilized as a positive control, it masked the DPPH radical by 93.02 % at 100 μg/ml, while their related IC50 values were 3.45 μg/ml.
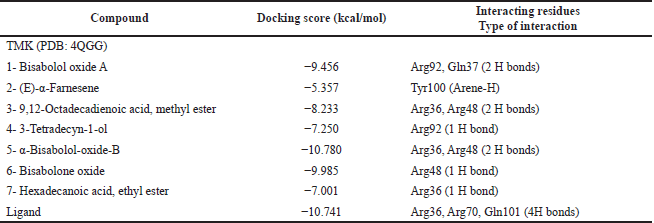 | Table 4. Docking scores of the major identified compounds in T. erithreus acetone extract into the active site of TMK (PDB: 4QGG). [Click here to view] |
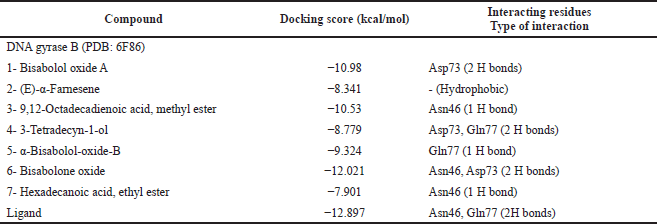 | Table 5. Docking scores of the major identified compounds in T. erithreus acetone extract into the active site of DNA gyrase B (PDB: 6F86). [Click here to view] |
 | Table 6. Docking scores of the major identified compounds in Trochus erithreus acetone extract into the active site of DNA topoisomerase IV/subunit B (PDB: 4HZ5). [Click here to view] |
 | Figure 5. The two-dimensional (left panel) and three-dimensional (right panel) suggested binding modes of α-bisabolol-oxide-B (A) and redocked ligand (B) within the binding pocket of Thymidylate kinase (PDB: 4QGG). [Click here to view] |
 | Figure 6. The two-dimensional (left panel) and three-dimensional (right panel) suggested binding modes of bisabolone oxide (A) and redocked ligand (B) within the binding pocket of DNA gyrase B (PDB: 6F86). [Click here to view] |
 | Figure 7. The two-dimensional (left panel) and three-dimensional (right panel) suggested binding modes of bisabolone oxide (A) and redocked ligand (B) within the binding pocket of DNA topoisomerase IV/subunit B (PDB: 4HZ5). [Click here to view] |
According to the WHO, antimicrobial resistance endangers the efficient obstruction and handling of a set of infections that is rapidly increasing, commonly produced by bacteria, parasites, viruses, and fungi. Antibiotic resistance emerges when pathogenic bacteria, viruses, fungi, and parasites have a weak response to antibiotics making infections more difficult to treat and increasing the risk of transmission, escalating the severity of diseases and risk of death. Antibiotic resistance also leads to persistent infections, which further increases the risk of spreading to others (Gueguen et al., 2006; Mitta et al., 1999). Antibacterial and antifungal effects have been formerly explained in the hemolymph of numerous molluscan species involving sea hares, sea slung, oysters, and mussels (Olicard et al., 2005; Roch et al., 2008; Zasloff, 2002).
In this study, the maximum zone of Te-EtOH extract was noted against S. aureus 31.32 ± 1.49 mm. Similar findings were obtained by Kuppusamy and Ulagesan (2016), while the maximum zone of Te-EtOAc extracts observed in P. aeruginosa, C. albicans, and Klebsiella sp. was 26.56 ± 5.88, 59.44 ± 0.22, and 51.16 ± 2.51 respectively. A similar finding was demonstrated by Anitha and Rose (2018) who reported that the EtOAc extract of the whole body showed a strong antimicrobial effect versus both Gram-positive and Gram-negative bacteria; antifungal activity was also detected from the extracts of various mollusks. Furthermore, there was a strong maximum zone of Te-acetone extract observed in P. vulgaris and E. coli which were 67.93 ± 4.86 and 49.87 ± 1.29, respectively. Similar findings were reported in Thais savignyi gastropod extracts from Egypt and the Persian Gulf (Ameri et al., 2017; Habib et al., 2022).
Bacterial biofilm is the total of microorganisms living inside extracellular polymeric materials taking place in the attachment stage of a biofilm to the surface (Jamal et al., 2018). This natural incident is believed as being an important supplier of nosocomial infections (Ziebuhr et al., 2006). The results indicated the defending capability effect of T. erithreus extract (Te-acetone, Te-EtOAc, and Te-EtOH) against the biofilm formation by many types of microbial species (P. aeruginosa, S. aureus, E. coli, and B. subtilis) with the variability from strong 60% (Te-acetone against S. aureus) to moderate 40% (Te-acetone against B. subtilis) to low (Te-acetone against P. aeruginosa and E. coli). The fact that the suppression of biofilm production by these extract constituents was seen in miscellaneous molluscan types and against microbial species that are generally not found in their living water (like S. aureus) is indicative of innate nonspecific immunity components found inside of it (Hinzmann et al., 2018). Moreover, identical research reports on mussels were stated by Estari et al. (2011) using Lamellidens marginalis and by Santhiya and Sanjeevi (2014) regarding Parreysia corrugata. Estari et al. (2011) examined tissue extracts from L. marginalis, which were diluted in numerous solvents.
GC-MS is used to identify the contents of volatile matter, long and branched-chain hydrocarbons, acids, alcohols, and esters. GC-MS study of acetone extract of T. erithreus confirms the occurrence of the above-mentioned bioactive constituents which could be accountable for the antioxidant, cytotoxic, and antimicrobial effects.
Many of these compounds have been structurally elucidated. Te-acetone extract comprises 45 compounds with total peak areas constituting 96.53%. According to the result, the main detected compounds are sesquiterpenoid bisabolol oxide A which has very strong anticancer activities against the human prostate cancer cell line (pc3), strong anticancer effect versus human big cell lung carcinoma cell line taken from the lymph node (NCI-H1299), and moderate anticancer against cervical cancer (Hela). Similar findings to these results (Cavalieri et al., 2009; Seki et al., 2011; Uno et al., 2016) indicated that bisabolol derivatives have been shown to exert antitumor, antioxidant, and antimicrobial activities. Murata et al. (2017) stated that the in vivo experiments revealed that bisabolol derivatives were found to successfully inhibit peritoneal dissemination and xenograft tumor growth of pancreatic cancer.
In the presented results, α-bisabolol-oxide-B revealed the best binding energy score (−10.780 kcal/mol) for TMK where the bisabolone oxide represented the best binding energy score for the other two enzymes DNA gyrase B and subunit B of topoisomerase IV (−12.897 and −11.795 kcal/mol, respectively) indicating that both compounds might have a good antibacterial activity compared to the other separated compounds from T. erithreus acetone extract in this investigation.
In light of the obtained results, we can conclude that T. erithreus have many types of chemically promising compounds that can be used in curative characteristics as a novel very strong antineoplastic drug for human prostate cancer and strong antineoplastic activity against human big cell lung carcinoma cell line taken from the lymph node, rather than the antioxidant, antibacterial, and antibiofilm activities. In addition, the molecular docking analysis disclosed that the studied compounds have a great attraction to the active sites of bacterial TMK, bacterial DNA gyrase B, and bacterial DNA topoisomerase IV/subunit B enzymes. Based on the above-mentioned findings, both α-bisabolol-oxide-B and bisabolone oxide could be considered promising inhibitors of the target bacterial enzymes used in this study and hopeful hits for the development of novel antimicrobial agents. Studies to investigate whether dietary intake of these marine mollusks can enhance health by acting on cellular pharmacological objectives are recommended.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ETHICAL APPROVAL
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abdel-Wareth MTA, Ghareeb AM, Abdel-Aziz SM, El-Hagrassi MA. Snailicidal, antimicrobial, antioxidant and anticancer activities of Beauveria bassiana, Metarhizium anisopliae and Paecilomyces lilacinus fungal extracts. Egypt J Aquat Biol Fish, 2019; 23:195–212.
Abu El-Einin AA, Gad El-Karim RM, Habib MR, Zayed KM, Ali REM. Identification of the gastropod snails and shells collected from Ain El-Sokhna region, Red Sea, Egypt. Egypt J Aquat Biol Fisher, 2021; 25:101–17.
Agour MA, Hamed AA, Ghareeb MA, Abdel-Hamid EAA, Ibrahim MK. Bioactive secondary metabolites from marine Actinomyces sp. AW6 with an evaluation of ADME?related physicochemical properties. Arch Microbiol, 2022; 204:537; doi:10.1007/s00203-022-03092-5
Ameri A, Shushizadeh MR, Bagher Nabavi SM, Espere F, Zarei Ahmady A. Antibacterial evaluation and biochemical characterization of Thais savignyi gastropod extracts from the Persian Gulf. Jundishapur J Nat Pharm Prod, 2017; 12:e13942.
Andrade MA, Azevedo CDS, Motta FN, Santos ML, dos Silva CL, Santana JM, de Bastos IMD. Essential oils: in vitro activity against Leishmania amazonensis, cytotoxicity and chemical composition. BMC Complement Altern Med, 2016; 16:444; doi:10.1186/s12906-016-1401-9
Anitha C, Rose SMB Antimicrobial activity of the hemolymph and whole body extract of a fresh water clam, Villorita cyprinoides (Gray, 1825). Int J Zool Appl Biosci, 2018; 3:151–6.
Bae H, Jayaprakasha GK, Crosby K, Jifon JL, Patil BS. Influence of extraction solvents on antioxidant activity and the content of bioactive compounds in non-pungent peppers. Plant Foods Hum Nutr, 2012; 67:120–8.
Bazes A, Silkina A, Douzenel P, Faÿ F, Kervarec N, Morin D, Berge JP, Bourgougnon N. Investigation of the antifouling constituents from the brown alga Sargassum muticum (Yendo) Fensholt. J Appl Phycol, 2009; 21:395–403.
Bhatnagar I, Kim SK. Immense essence of excellence: marine microbial bioactive compounds. Mar. Drugs, 2010; 8:2673–701.
Boman HG. Peptide antibiotics and their role in innate immunity. Annu Rev Immunol, 1995;13:61–92.
Cavalieri E, Bergamini C, Mariotto S, Leoni S, Perbellini L, Darra E, Suzuki H, Fato R, Lenaz G. Involvement of mitochondrial permeability transition pore opening in α-bisabolol induced apoptosis. FEBS J, 2009; 276:3990–4000.
Elkhouly HI, Hamed AA, El Hosainy AM, Ghareeb MA, Sidkey NM. Bioactive secondary metabolite from endophytic Aspergillus Tubenginses ASH4 isolated from Hyoscyamus muticus: antimicrobial, antibiofilm, antioxidant and anticancer activity. Pharmacogn J, 2021a; 13(2):434–42; doi:10.5530/pj.2021.13.55
Elkhouly HI, Sidkey NM, Ghareeb MA, El Hosainy AM, Hamed AA. Bioactive secondary metabolites from endophytic Aspergillus terreus AH1 isolated from Ipomoea carnea growing in Egypt. Egypt J Chem, 2021b; 64(12):7511–20; doi:10.21608/EJCHEM.2021.85908.4161
Estari M, Satyanarayana J, Kumar BS, Bikshapati T, Reddy AS, Venkanna L. In vitro study of antimicrobial activity in freshwater mussel (Lamellidens marginalis) extracts. Biol Med, 2011; 3:191–5.
Faulkner DJ. Chemical defenses of marine molluscs. In: Ecological roles of marine natural products. Cornell University Press, Ithaca, NY, pp 119–63, 2019.
Gad El-Karim RM, Habib MR, Ghareeb MA, Ali REM. Assessment of the antioxidant capacity of Lanistes carinatus tissue extract and its immune-boosting influence on Biomphalaria alexandrina against infection with Schistosoma mansoni. Egypt J Aquat Biol Fish, 2022; 26(4):361–76; doi:10.21608/EJABF.2022.249977
Gerwick WH. Drugs from the sea: the search continues. J Pharm Technol, 1987; 3:136–41.
Ghareeb MA, Hussein AH, Hassan MFM, Laila AR, Mona AM, Amal MS. Antioxidant and cytotoxic activities of Tectona grandis linn leaves. Int J Phytopharm, 2014; 5:143–57.
Ghareeb MA, Saad AM, Abdou AM, Refahy LAG, Ahmed WS. A new kaempferol glycoside with antioxidant activity from Chenopodium ambrosioides growing in Egypt. Orient J Chem, 2016; 32:3054–61.
Ghareeb MA, Tammam MA, El-Demerdash A, Atanasov AG. Insights about clinically approved and preclinically investigated marine natural products. Curr Res Biotechnol, 2020; 2:88–102; doi:10.1016/j.crbiot.2020.09.001
Gueguen Y, Herpin A, Aumelas A, Garnier J, Fievet J, Escoubas JM, Bulet P, Gonzalez M, Lelong C, Favrel P. Characterization of a defensin from the oyster Crassostrea gigas: recombinant production, folding, solution structure, antimicrobial activities, and gene expression. J Biol Chem, 2006; 281:313–23.
Habib MR, Hamed AA, Ali REM, Zayed KM, Gad El-Karim RM, Sabour R, Abu El-Einin HM, Ghareeb MA. Thais savignyi tissue extract: bioactivity, chemical composition, and molecular docking. Pharm Biol, 2022; 60(1):1899–914; doi:10.1080/13880209.2022.2123940
Hamed AA, Soldatou S, Qader MM, Arjunan S, Miranda KJ, Casolari F, Pavesi C, Diyaolu OA, Thissera B, Eshelli M, Belbahri L, Luptakova L, Ibrahim NA, Abdel-Aziz MS, Eid BM, Ghareeb MA, Rateb ME, Ebel R. Screening fungal endophytes derived from under-explored Egyptian marine habitats for antimicrobial and antioxidant properties in factionalised textiles. Microorganisms, 2020; 8:1617; doi:10.3390/microorganisms8101617
Hinzmann M, Bessa LJ, Teixeira A, Da Costa PM, Machado J. Antimicrobial and antibiofilm activity of unionid mussels from the north of Portugal. J Shellfish Res, 2018; 37:121–9.
Ibrahim AM, Hamed AA, Ghareeb MA. Marine, freshwater, and terrestrial snails as models in the biomedical applications. Egypt J Aquat Biol Fish, 2021; 25(3):23–38; doi:10.21608/EJABF.2021.172142
Jamal M, Ahmad W, Andleeb S, Jalil F, Imran M, Nawaz MA, Hussain T, Ali M, Rafiq M, Kamil MA. Bacterial biofilm and associated infections. J Chin Med Assoc, 2018; 81:7–11; doi:10.1016/j.jcma.2017.07.012
Kaczor AA, Polski A, Sobótka-Polska K, Pachuta-Stec A, Makarska-Bialokoz M, Pitucha M. Novel antibacterial compounds and their drug targets-successes and challenges. Curr Med Chem, 2017; 24; doi:10.2174/0929867323666161213102127
Kawatkar SP, Keating TA, Olivier NB, Breen JN, Green OM, Guler SY, Hentemann MF, Loch JT, McKenzie AR, Newman JV, Otterson LG, Martínez-Botella G. Antibacterial inhibitors of gram-positive Thymidylate Kinase: structure-activity relationships and chiral preference of a new hydrophobic binding region. J Med Chem, 2014; 57:4584–97; doi:10.1021/jm500463c
Kumar KS, Ganesan K, Rao PS. Antioxidant potential of solvent extracts of Kappaphycus alvarezii (Doty) Doty-An edible seaweed. Food Chem, 2008; 107:289–95.
Kuppusamy A, Ulagesan S. Antimicrobial activity of protein hydrolysate from marine molluscs Babylonia spirata (Linnaeus, 1758). J Appl Pharm Sci, 2016; 6:073–7.
Khalaf MO, Abdel-Aziz MS, El-Hagrassi AM, Osman AF, Ghareeb MA. Biochemical aspect, antimicrobial and antioxidant activities of Melaleuca and Syzygium species (Myrtaceae) grown in Egypt. J Phys Conf Ser, 2021; 1879:022–62; doi:10.1088/1742-6596/1879/2/022062
Madkour HM, Ghareeb MA, Abdel-Aziz MS, Khalaf OM, Saad AM, El-Ziaty AK, Abdel-Mogib M. Gas chromatography-mass spectrometry analysis, antimicrobial, anticancer and antioxidant activities of n-hexane and methylene chloride extracts of Senna italica. J Appl Pharm Sci, 2017; 7:23–32.
Mayers DL, Sobel JD, Ouellette M, Kaye KS, Marchaim D. Antimicrobial drug resistance: clinical and epidemiological aspects, Springer International Publishing AG, Cham, Switzerland, 2, 2017.
Mitta G, Hubert F, Noël T, Roch P. Myticin, a novel cysteine-rich antimicrobial peptide isolated from haemocytes and plasma of the mussel Mytilus galloprovincialis. Eur J Biochem, 1999; 265:71–8.
Moure A, Cruz JM, Franco D, Dom??nguez JM, Sineiro J, Dom??nguez H, Núñez MJ, Parajó JC. Natural antioxidants from residual sources. Food Chem, 2001; 72:145–71.
Murata Y, Kokuryo T, Yokoyama Y, Yamaguchi J, Miwa T, Shibuya M, Yamamoto Y, Nagino M. The anticancer effects of novel α-bisabolol derivatives against pancreatic cancer. Anticancer Res, 2017; 37:589–98; doi:10.21873/anticanres.11352
Nazeer RA, Naqash SY. In vitro antioxidant activity of two molluscs, Loligo duvauceli Orbigny and Donax cuneatus Linnaeus, by solvent extraction methods. Mediterr J Nutr Metab, 2013; 6:17–21.
Olicard C, Renault T, Torhy C, Benmansour A, Bourgougnon N. Putative antiviral activity in hemolymph from adult Pacific oysters, Crassostrea gigas. Antiviral Res, 2005; 66:147–52.
Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem, 1999; 269:337–41.
Roch P, Yang Y, Toubiana M, Aumelas A. NMR structure of mussel mytilin, and antiviral-antibacterial activities of derived synthetic peptides. Dev Comp Immunol, 2008; 32:227–38.
Santhiya N, Sanjeevi SB. Antibacterial activity of freshwater mussel Parreysia corrugata (Muller 1774) from lower Anaicut Reservoir India. Int J Pharm Life Sci, 2014; 5:3899–02.
Seki T, Kokuryo T, Yokoyama Y, Suzuki H, Itatsu K, Nakagawa A, Mizutani T, Miyake T, Uno M, Yamauchi K. Antitumor effects of α-bisabolol against pancreatic cancer. Cancer Sci, 2011; 102:2199–205.
Sharifi-Rad M, Nazaruk J, Polito L, Morais-Braga MFB, Rocha JE, Coutinho HDM, Salehi B, Tabanelli G, Montanari C, del Mar Contreras M, Yousaf Z, Setzer WN, Verma DR, Martorell M, Sureda A, Sharifi-Rad J. Matricaria genus as a source of antimicrobial agents: From farm to pharmacy and food applications. Microbiol Res, 2018; 215:76–88 doi:10.1016/j.micres.2018.06.010
Shawky TB, Nagah M, Ghareeb AM, El-Sherbiny MG, Moghannem AM, Abdel-Aziz M. Evaluation of antioxidants, total phenolics and antimicrobial activities of ethyl acetate extracts from fungi grown on rice straw. J Renew Mater, 2019; 7:667–82; doi:10.32604/jrm.2019.04524
Shirwaikar A, Prabhu KS, Samraj PI. In vitro antioxidant studies of Sphaeranthus indicus (Linn). Indian J Exp Biol, 2006; 44(12):993–6.
Shoeb HA, Madkour HM, Refahy LA, Mohamed MA, Saad AM, Ghareeb MA. Antioxidant and cytotoxic activities of Gmelina arborea ROXB. leaves. J Pharm Res Int, 2014; 4:125–44.
Silver LL. Multi-targeting by monotherapeutic antibacterials. Nat Rev Drug Discov, 2007; 6:41–55; doi:10.1038/nrd2202
Simões NG, Bettencourt AF, Monge N, Ribeiro IAC. Novel antibacterial agents: an emergent need to win the battle against infections. Mini-Rev Med Chem, 2017; 17; doi:10.2174/1389557516666160907151454
Tari LW, Trzoss M, Bensen DC, Li X, Chen Z, Lam T, Zhang J, Creighton CJ, Cunningham ML, Kwan B, Stidham M, Shaw KJ, Lightstone FC, Wong SE, Nguyen TB, Nix J, Finn J. Pyrrolopyrimidine inhibitors of DNA gyrase B (GyrB) and topoisomerase IV (ParE). Part I: Structure guided discovery and optimization of dual targeting agents with potent, broad-spectrum enzymatic activity. Bioorg Med Chem Lett, 2013; 23:1529–36; doi:10.1016/j.bmcl.2012.11.032
Uno M, Kokuryo T, Yokoyama Y, Senga T, Nagino M. α-Bisabolol inhibits invasiveness and motility in pancreatic cancer through KISS1R activation. Anticancer Res, 2016; 36:583–9.
Williams C. The nabobs of Berkshire. Goosecroft Publications, UK, 2010.
Zasloff M. Antimicrobial peptides of multicellular organisms. Nature, 2002; 415:389–95.
Ziebuhr W, Hennig S, Eckart M, Kränzler H, Batzilla C, Kozitskaya S. Nosocomial infections by Staphylococcus epidermidis: how a commensal bacterium turns into a pathogen. Int J Antimicrob Agents, 2006; 28:14–20.
SUPPLEMENTARY MATERIAL
Supplementary data can be downloaded from the journal’s website