INTRODUCTION
Neonatal sepsis (NS) is a blood infection in newborns less than 28 days old. There is no internationally recognized unified definition for NS. Currently, employed definitions vary (McGovern et al., 2020). Based on the time of presentation of sepsis after birth, NS is classified into early-onset sepsis (EOS): ‘occurring within 72 hours, and late-onset sepsis (LOS): occurring after 72 hours (Singh et al., 2022). Risk factors such as premature membrane rupture, very low birth weight (VLBW), prematurity, invasive medical procedures, poor intra- and postpartum hygiene, maternal pyrexia, and prolonged neonatal intensive care unit (NICU) stay are critically associated with both EOS and LOS (Leal et al., 2012). Many studies have shown a preponderance of Gram-negative bacteria (GNB) in EOS and Gram-positive bacteria (GPB) in LOS (Hornik et al., 2012). Global Burden of Disease study report (2016–17) mentions a global incidence of 1.3 million cases of NS per year with more than two hundred thousand sepsis-attributable deaths (Fleischmann et al., 2021).
World Health Organization (WHO) reported 2.4 million neonatal deaths globally, with one-third and three-fourths of mortality on the day of birth and within the first week of birth, respectively (WHO, 2020). Centre for Disease Dynamics, Economics and Policy (CDDEP), USA, reported that in 2019, sepsis caused nearly 1 million neonatal deaths globally within the first 4 weeks of life, of which 190,000 deaths were in India (CDDEP, 2012). NS, especially in VLBW populations, is significantly associated with increased complications of prematurity and adverse neurodevelopmental outcomes, which underscore the need for immediate diagnosis of NS and the commencement of antibiotic therapy. Antibiotics usage in NICUs is widespread, and numerous studies from high-income countries and low-income and middle-income countries (LMICs) have reported that 74% to 94% of neonates with negative blood cultures received antibiotics for suspected sepsis (Dadgostar, 2019; Thomson et al., 2021). The unnecessary administration of empiric antibiotics is an independent risk factor for the rapid emergence of antimicrobial resistance (AMR) and is associated with increased long-term morbidity and mortality. Thirty percent of NS deaths are due to AMR (Hayes et al., 2021; Shelar, 2019).
AMR is a global problem, which is particularly challenging in developing countries like India (Murthy et al., 2019; Singh et al., 2022). Among GNBs, cephalosporin resistance rates ranged from 26% to 84%, and carbapenem resistance rates ranged from 0% to 81%. The percentage of GPBs resistant to glycopeptides ranged from 0% to 45% (Li et al., 2020).
Due to increasing healthcare expenses globally, pharmacoeconomic considerations, including the cost of illness and the cost-effectiveness of health interventions, have become crucial for developing and implementing health policies (Fenny et al., 2020; Gastmeier et al., 2004). In India, neonatal intensive care is among the most expensive components of pediatric care. The per-capita income of an Indian parent is only $1,920, making NS a catastrophic financial burden (Prinja et al., 2013; The World Bank, 2021). Among the very few publications on the costs of NS in India, a systematic review estimated the cost of treatment for NS per patient in India and the United States as $55 and $129,632, respectively (Salman et al., 2020). The paucity of investigations on AMR patterns, mortality, and the cost of NS in India creates an urgent need to increase epidemiological studies for implementing targeted interventions. This study aims to assess the antibiogram and evaluate factors associated with the length of NICU stay, mortality, and the costs incurred due to NS.
MATERIALS AND METHOD
Our study included case records of neonates clinically diagnosed with NS between January 2017 and September 2019. Only neonates with culture-positive bacteria were included. Culture sensitivity reports were retrospectively collected from the Laboratory Information Service software maintained by the hospital. Fungal or viral sepsis and incomplete records were excluded. Demographic details of neonates [gender, GA (gestational age), birth weight, and morbidities] were collected from case records. The study also classified EOS and LOS based on the National Institute for Health and Care Excellence guidelines (Cortese et al., 2016).
Statistical analysis
Patient demography, susceptibility patterns of bacterial isolates, and cost are indicated in percentages, mean (±SD), and median (±IQR) values. The skewed data sets were transformed to log data for statistical analysis. To study the impact of neonatal and maternal variables on the length of NICU stay and cost, we performed a multiple linear regression analysis using the backward selection method (to avoid multicollinearity between the variables) with p < 0.05 considered statistically significant. Multicollinearity between independent variables was determined by Tolerance (T) values (significant if T <0.1). Variance inflation factor above five was considered significant. We tested the correlation between cost and clinically significant neonatal variables using ‘Pearson’s correlation (two-tailed) (Ananya19b, 2021). We also performed binomial logistic regression using the backward stepwise (likelihood ratio) method to determine the association between risk factors, mortality, and LOS/EOS (Schober et al., 2018). All statistical analyses were carried out using the SPSS 28.0 package (IBM Corp. IBM SPSS Statistics for Windows, Armonk, NY).
RESULTS
Between 2017–18 and 2018–19, the number of neonates admitted to the NICU was 3,818 and 3,856, respectively. The overall incidence of sepsis was between 9% and 10%, of which bacterial sepsis occurred in only 2.4% of cases annually. Of the 186 cases of NS, 57% were males. Cesarean section (C-section) and vaginal birth constituted 68% and 32%, respectively. The neonatal mortality rate (NMR) was 44%. The demographic details are presented in Table 1.
Culture sensitivity tests
Culture sensitivity tests showed that 78% of neonates were infected with GNBs, while 22% were infected GPBs. EOS was associated with 43% and 15% GNBs and GPBs, respectively. Thirty-four percent of GNBs and 8% of GPBs were associated with LOS. Klebsiella pneumoniae 55% (n = 129) was the most abundant, followed by E. coli 17% (n = 40), Acinetobacter spp. 14% (n = 34) and Enterobacter cloacae 9% (n = 21). Among different strains, extensively drug-resistant/Pandrug-resistant (XDR/PDR) strains of Klebsiella pneumonia (56%) were higher followed by E. coli (18%) and Acinetobacter spp. (15%). However, multidrug-resistant (MDR) strains of E. coli (80%) were higher followed by Enterococcus spp. (75%). Enterobacter cloacae (47%) and K. pneumoniae (33%) (Fig. 1).
Empirical antibiotics were administered to 31% (n = 58) of patients. Commonly used antibiotics were ampicillin (20%), followed by amikacin (19%), piperacillin-tazobactam (15%), etc. Sixty-nine percent (n = 128) of the patients received definitive therapy (either continuing the empirical treatment or changing antibiotics based on the culture sensitivity test). The most frequently prescribed antibiotics in the definitive therapy category were amikacin (91%), followed by piperacillin-tazobactam (69%) (Table 2). Results of the antimicrobial susceptibility testing are depicted in the supplementary file (Supplementary Figs. S1–S5). The risk factors associated with EOS is mentioned in Table 3.
Mortality
Eighty-one (44%) neonates died, of whom 57% had EOS. Binomial logistic regression using a backward stepwise method (likelihood ratio) showed that septic shock (p < 0.027) and platelet count (p < 0.001) were significantly associated with mortality (Table 5). Mechanical ventilation (MV) was associated with 75% of neonatal mortality. Other risk factors associated with mortality are shown in Table 4.
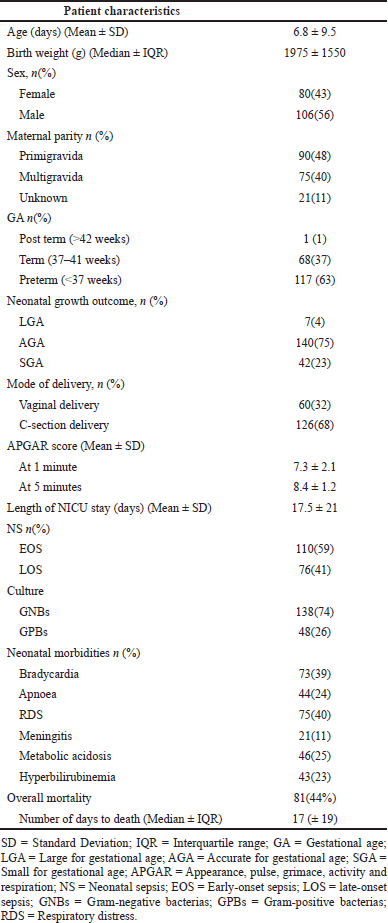 | Table 1. Demographic features of the sample population (Total N = 186). [Click here to view] |
Length of NICU stay
Small gestational age (SGA) (p < 0.02), LBW (p < 0.036), intrauterine growth restriction (IUGR) (p < 0.013), heart disease (p < 0.049), vasopressor (p < 0.001), surfactants (p < 0.006), EOS (p < 0.001), LOS (p < 0.014), persistent pulmonary hypertension of the neonates (PPHN) (p < 0.001), and the number of definitive antibiotics administered per patient (p < 0.001) were significantly associated with the length of NICU stay (Table 6). A residual scatter plot indicates that the linear model for length of stay fits the data well (Supplementary Fig. S6).
Cost of NS
The median antibiotic cost and the median length of NICU stay cost for LOS were twice and thrice that of the EOS, respectively. The median cost for LOS is double the total cost of EOS (Table 7). The antibiotic cost constitutes 6% and 2% of the total drug and total costs, respectively. Antibiotic costs and the overall treatment cost for extremely low birth weight (ELBW) were higher and were closely followed by VLBW and LBW.
Multivariate linear regression analysis shows that overall treatment cost increased significantly with an increase in the NICU stay (p < 0.001). Co-morbidities further increased the length of NICU stay. Heart disease (p < 0.001), seizures (p < 0.001), respiratory syncytial virus (RSV) infection (p < 0.004), anemia (p < 0.051), and peritonitis (p < 0.046) were significantly associated with the total cost (Table 8). A residual scatter plot showed that the linear model for the cost of the length of NICU stay fits the data well (Fig. 2).
DISCUSSION
NS remains a serious clinical concern in neonatology, with high morbidity and mortality rate, particularly in LMICs such as India, especially with the rapid emergence of AMR. This study shows that the incidence of bacterial sepsis in 2.4% of live births for two consecutive years. The Burden of Antibiotic Resistance in Neonates from Developing Societies study has shown the incidence of bacterial sepsis as 0.04% (Milton et al., 2022). Two different studies from the USA reported the incidence of bacterial sepsis from the Indian sub-continent as 40.1 per 1,000 live births and 6.7 per 1,000 live births respectively (Panigrahi et al., 2017; Sundaram et al., 2009). The extremely low rate of NS in our hospital’s NICU demonstrates the effectiveness of our infection control policies and the maintenance of an aseptic atmosphere while performing any invasive procedures.
This study assessed the demographics and antibiogram of all the neonates. The mean neonatal age was 6.8 ± 9.5 days, predominantly males (n = 106; 56%). GNBs (74%) outnumbered GPBs (26%). Klebsiella pneumoniae 55% (n = 129) was the most abundant, followed by E. coli 17% (n = 40), Acinetobacter spp. 14% (n = 34) and E. cloacae 9% (n = 21). Klebsiella pneumoniae constituted a high proportion of XDR/PDR (56%) and MDR (33%) strains highly resistant to ampicillin (99%), cefotaxime (84%), cefuroxime (84%), cefepime (82%) and moderately resistant toward meropenem (74%), piperacillin-tazobactam (73%), and cefoperazone (72%). A study from Pakistan reported a mean neonatal age of 11.41 ± 6.76 days, predominantly affecting females (57.6%), with culture sensitivity reports of K. pneumoniae (34.1%) followed by Staphylococcus aureus (31.7%), E. coli (19.5%), and coagulase-negative Staphylococci (12.2%) (Liyakat et al., 2021). Klebsiella pneumoniae is the most frequent cause of outbreaks in NICU leading to significant morbidity and mortality. In our study, MDR [Beta (β) = 1.882, 95% CI 2.708–15.923, p < 0.0001 and XDR/PDR] (β = 1.101, 95% CI 1.354–6.684, p < 0.007) strains of K. pneumoniae was highly associated with LOS unadjusted with all other study variables. Similarly susceptible (β = 1.236, 95% CI 0.089-0.953, p < 0.041) and MDR (β = 1.905, 95% CI 0.024–0.928, p < 0.041) strains of Acinetobacter spp. and, MDR (β = 2.085, 95% CI 0.049–0.953, p < 0.0001) and XDR/PDR (β = 1.340, 95% CI 0.119–0.953, p < 0.003) strains of K. pneumoniae were highly associated with EOS unadjusted with all other variables respectively. According to the Lancet report, K. pneumoniae was responsible for the majority of NS and mortality (124,000 deaths in 2019) (Dall, 2022). MDR strains frequently emerge in intensive care unit (ICU) settings as a result of prolonged and excessive usage of multiple antibiotics. The lack of specific signs and symptoms makes it extremely difficult to diagnose and treat sepsis. Lengthy turnaround times for laboratory diagnoses force continued empirical antibiotics until suspected sepsis is ruled out. The rise of MDR and XDR bacteria restricts available treatment alternatives, hindering effective therapy. Ampicillin combined with amikacin is the first-line empirical treatment in our NICU. A third-generation cephalosporin and piperacillin-tazobactam is the second line of treatment if there is no improvement. Until blood culture results are received, carbapenems are used as a last resort (Hassuna et al., 2020). In our study, a combination of ampicillin (20%) and amikacin (19%) was preferred as the first-line agent for NS. However, most isolates were resistant to ampicillin, shifting definitive therapy choices to piperacillin-tazobactam (69%) and amikacin (91%). Third-generation cephalosporins such as cefoperazone-sulbactam and fluoroquinolones such as ciprofloxacin were infrequently used. When the isolates (e.g., XDR/PDR strains of K. pneumoniae) were found resistant to most of the antibiotics, colistin and carbapenems were prescribed as a last resort. Our hospital consistently prescribed doses of antibiotics recommended by Micromedex NeoFax Essentials guidelines (Micormedex NeoFax Essentials, 2014).
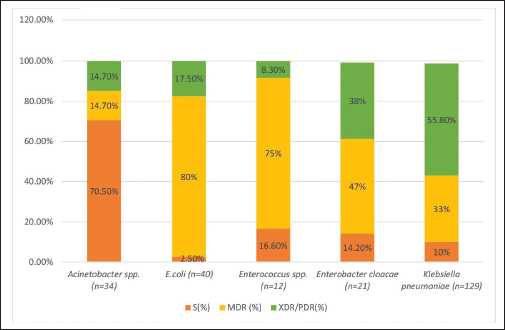 | Figure 1. MDR, PDR, and sensitive strains of different GNBs (%), and GPBs (%) found in the study. [Click here to view] |
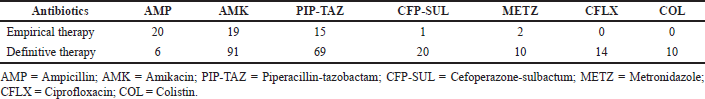 | Table 2. Antibiotics used in neonates [values in percentages (%)]. [Click here to view] |
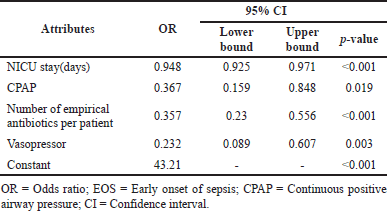 | Table 3. Risk factors associated with EOS. [Click here to view] |
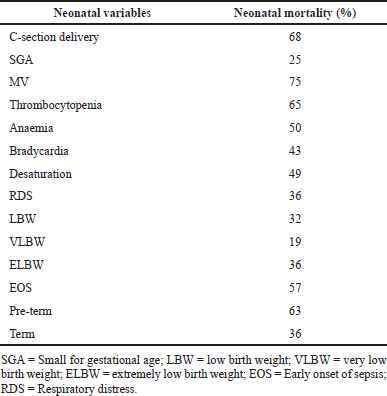 | Table 4. Risk factors associated with mortality. [Click here to view] |
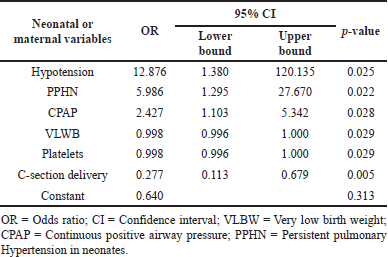 | Table 5. Association between neonatal variables and mortality. [Click here to view] |
Understanding resistance patterns require continuous surveillance of antimicrobial susceptibility. India is at the epicenter of this expanding public health hazard, with various levels of government and healthcare organizations undertaking AMR action plans. Kerala (2018) was one of the first Indian states to establish policies and prioritize activities to address AMR hazards, followed by Madhya Pradesh and New Delhi (2020). However, ensuring the state-wide execution of an AMR action plan needs collaborative and committed efforts from healthcare professionals from both private and public sectors (Chandra et al., 2022; Singh et al., 2021). Neonatologists must be familiar with the bacteriological profile and the local geographic susceptibility patterns to reduce the morbidity and mortality associated with NS.
NS predisposes neonates to an increased risk of mortality or morbidity, especially by MDR organisms. The incidence (59%) and mortality (57%) were higher for EOS than LOS, similar to the results of a systematic review from Germany (Fleischmann et al., 2021). Preterm babies are more susceptible to infection in early life. However, appropriate care and rational antibiotic prescriptions could prevent LOS or mortality. While we found that LOS accounted for 43% of the total cases (n = 76), Taiwan reported 23% in a similar study (Tsai et al., 2014).
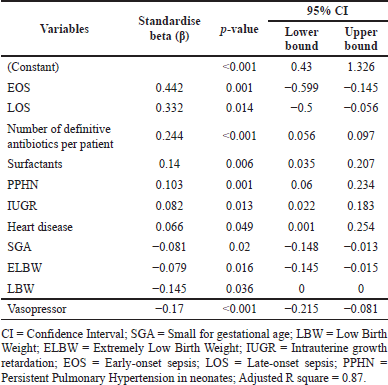 | Table 6. Association between neonatal variables and NICU length of stay. [Click here to view] |
This study showed that mortality was significantly associated with hypotension, presence of PPHN, C-section delivery, and neonates receiving continuous positive airway pressure (CPAP). CPAP has been shown to reduce fatality risk by 48%. Costly surfactants and MV reduce fatality risk by 50%, and fewer days of NICU stay. However, prolonged use of CPAP has been reported to cause sepsis (possibly from inappropriate handling or lack of knowledge on CPAP administration, especially in LMICs) which is an independent factor for neonatal mortality (Dewez et al., 2018; Duke, 2014; Thukral et al., 2016).
We also checked the association between bacterial isolates and mortality. However, with or without adjustment for age, preterm, term, LBW, VLBW, and ELBW, there was no significant association between bacterial isolates and mortality.
C-section deliveries increased by more than twice as much as vaginal deliveries between 2017 and 2019 in our hospital. The C-section delivery had higher mortality than vaginal delivery and was significantly associated [OR = 0.277 (95% CI 0.113 to 0.679), p < 0.005] with mortality. A study from India reported C-section delivery was positively associated with neonatal mortality [OR 1.19 (95% CI 1.02 to 1.39), p < 0.001] (Gondwe et al., 2020). In our study, neonates born via C-section were more likely to require NICU admission, oxygen supplementation, ventilatory support for respiratory distress syndrome (RDS), desaturation, and PPHN than those born via vaginal delivery, which may have progressed to severe respiratory failure and resulted in death. For newborns delivered via C-section without experiencing labor, respiratory morbidity caused by failure to remove fetal lung fluid is common and can be challenging. Numerous studies have shown that respiratory morbidity such as RDS, transient tachypnea, and PPHN has led to the need for supplemental oxygen (extracorporeal membrane oxygenation) or death (Kamath et al., 2009). The C-section rate in India, which may influence neonatal outcomes, appears to be influenced by a combination of socioeconomic, medical, and maternal factors. Worldwide, the rate of C-section deliveries has been rising rapidly, accounting for more than half of births today. Data from more than 150 countries suggest that 5% to 10% of C-section delivery rates accounted for the lowest neonatal and maternal mortality rates. When cesarean delivery rates exceeded 10%, mortality rates seemed to stabilize, suggesting that more cesarean deliveries do not reduce mortality. WHO advises that the national cesarean delivery rate should not exceed 15% (Boerma et al., 2018; Ye et al., 2016). India’s National Family Health Survey for 2015–2016, reported that cesarean deliveries are rising and account for 17.2% of all births, with a massive gap between rural (12.9%) and urban (28.3% ) households (Gondwe et al., 2020).
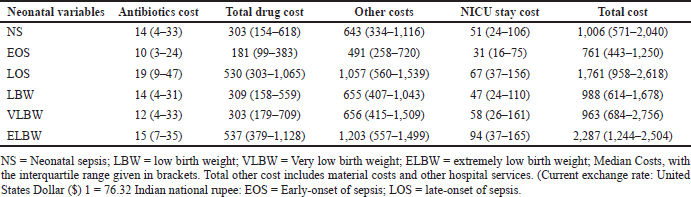 | Table 7. Median costs incurred (in $) due to NS. [Click here to view] |
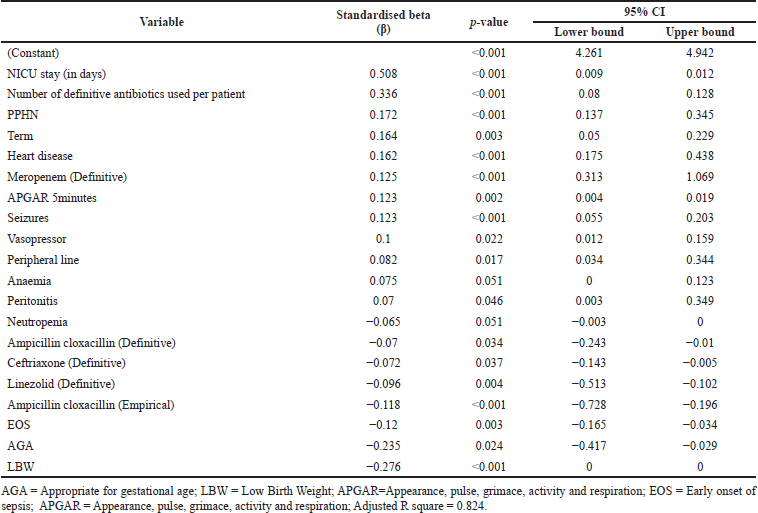 | Table 8. Association between neonatal variables and total cost. [Click here to view] |
Decreased platelet count (thrombocytopenia) has been associated with neonatal mortality. Thrombocytopenia in NS increases mortality risk nearly four-fold. The bacterial infection triggers thrombocytopenia, severe sepsis, and disseminated intravascular coagulation, leading to death. Both thrombocytopenia and bacterial infections are independent risk factors for mortality (Ree et al., 2017). The global NMR (within the first 28 days of life) in 2020 was 17 deaths per 1,000 live births (UNICEF, 2021). NMR in India, per 1,000 live births, fell drastically from 84.32 in 1970 to 16 (2017) 23 (2018), 21 (2019), and 20 (2020). India plans to reduce this to 12 per 1,000 live births by 2030 (Bora and Saikia, 2018). NMR in Karnataka in 2018 was 16 per 1000 live births which is much higher than other South Indian states such as Kerala (2020; 3.4 per live births) and Tamil N?du (2019); 11 per 1000 live births (Ahuja, 2021; Bhaskar, 2021; Bora and Saikia, 2018). A study from Haryana, India, reported that district-to-district variation for life expectancy at birth was highest for the Udupi district in Karnataka and lowest for Kargil in Jammu and Kashmir (Kesarvani, 2015).
 | Figure 2. Scatterplot overall cost and neonatal variable-predicted value (X-axis) versus residual value (Y-axis). [Click here to view] |
The major causes of neonatal mortality in India are prematurity, birth asphyxia or injury, and infections. Efforts toward decreasing mortality from birth asphyxia, preterm births, and LBW have been met with varied success. The incidence of NS and mortality in our hospital (the only tertiary care hospital within the Udupi district) is very low, suggesting that the standard of care given by the NICU, effectiveness of home-based neonatal care, better socioeconomic circumstances, and new governmental health schemes such as National Rural Health Mission have enhanced the health of rural and underserved communities (Bang et al., 1999). A study from Gadchiroli, India, showed that the decline in the incidence of NS and associated mortality results from the effective implementation of government-run programs and the standard of care given by both private and public hospitals (Bang et al., 2021). Madhya Pradesh, an Indian state with one of the highest NMR, also improved significantly (59 in 2,000 vs. 35 in 2018) because of substantial progress in maternal and child health interventions at the government level (Maternal and Neonatal Health in Madhya Pradesh, 2022.).
This study has also demonstrated a significant positive correlation between length of NICU stay and neonatal clinical variables such as EOS (p < 0.001) and LOS (p < 0.114), followed by the number of definitive antibiotics per patient (p < 0.001), PPHN (0.001) and IUGR (p < 0.013) and heart disease (p < 0.049). A study from Eritrea showed that GA, birth weight <2,000 g, SGA, and pneumonia were associated with an increase in the length of NICU stay (Shah et al., 2012). Multiple reports suggest that morbidities such as heart disease, IUGR, PPHN, EOS, and LOS are associated with a prolonged NICU stay, in agreement with our results, suggesting the need for extensive clinical monitoring while recovering from NS (Liyakat et al., 2021; Niknajad et al., 2012; Pokhrel et al., 2018; Shrestha et al., 2007). Anticipating the length of NICU stay or time to discharge would help resource allocation, planning, treatment quality, research, and medical practice. LBW, followed by SGA and ELBW, are negatively correlated with the length of NICU stay, possibly because early deaths reduced the median length of NICU stay (Niknajad et al., 2012).
We have shown that the median overall cost of treating NS was $1,006 (571–2040), higher than other LMICs. Antibiotic cost constitutes 6% and 2% of the total drug and overall treatment costs, respectively. The overall treatment cost for managing NS was higher in ELBW ($2,287) group and was least for VLBW ($963) group. A study from the USA reported the overall treatment cost for the management of LOS in ELBW is more than $20,000 compared to VLBW ($2,994) (Johnson et al., 2013). Prolonged antibiotic administration in the 1st week of life or suspected NS increases morbidity and mortality, necrotizing enterocolitis (NEC), compromises gut microbiota, escalates AMR, separates the mother and child, and increases healthcare costs (Boverman et al., 2022). A retrospective study from the US showed that every additional day on antibiotics increases the risk of developing NS, NEC, or fatality by 24%, increasing the healthcare cost (Cantey et al., 2018). AMR also increases healthcare costs disastrously. According to the CDC in 2013, AMR alone may increase hospital bills for treating bacterial infections by approximately $1,400 in the US, possibly raising annual healthcare expenditures by more than $2 billion. Many estimates suggest that by 2050, the global cost of AMR might be between $300 billion and more than $1 trillion yearly. AMR’s immediate financial impact on healthcare includes high costs of expensive and extended treatments and increased resource usage (Dadgostar, 2019).
This study showed that overall treatment costs were significantly associated with anemia (p < 0.051), presence of peripheral line (p < 0.017), seizures (p < 0.001), and heart disease (p < 0.001). Overall, treatment cost was lower in EOS because cost decreases when appropriate treatment is received early. A study from Nepal showed that the median cost for NS was $111.7 (69.8–155.5) (Shrestha et al., 2007). A multicentric study from Mozambique and South Africa showed that the median overall cost of treatment was $ 75.12 [IQR, 149.43–386.12] and $653.62 (543.33–827.53), respectively (Aerts et al., 2022). Our study is the first to evaluate the factors responsible for the higher cost in India. Based on a few LMICs studies, the median hospitalization costs in our study appear higher than in previous reports. The cost of treatment depends on inter-country variations in resource unit costs, length of NICU stay, availability of standard care within the hospital, and willingness to pay. LMICs such as India, with a high burden of infection and low resources settings, the cost of treatment is generally above the affordable range. The average Indian per capita income stands at $1,947 (2020–21), making insurance a prominent social need. India’s healthcare expenditure is below $15, with the bulk of the cost of therapy met by out-of-pocket payments (IANS, 2021).
This study has a few limitations: The results are based on a single center. The small sample size may limit the extrapolation of results to the general population. We could not estimate the indirect costs. Thirdly, we did not collect data on family income, working parents’/caregivers’ salaries, time away from work, travel expenses, or caregivers’ living costs, which limited the estimated expenditures and out-of-pocket expenses, with long-term economic consequences for poorer households seeking care in private hospitals.
CONCLUSION
Sepsis in neonates is often fatal. Klebsiella pneumoniae was the predominant organism isolated from samples, with a high incidence of XDR/PDR isolates. EOS was associated with higher mortality rates than LOS. NICU length of stay increases with morbidities in neonates. Septic shock and platelet count were significantly associated with mortality. The median total cost for LOS is twice that of EOS. The total cost of treatment was significantly associated with the onset of EOS, length of NICU stay, presence of peripheral line, and heart disease in neonates. Clinical data being scarce in India, such studies could help frame informed policy decisions toward developing and implementing targeted interventions to reduce the burden of NS within and outside India.
ACKNOWLEDGMENT
The trial is registered at the Clinical Trial Registry of India CTRI identifier (CTRI/2020/06/025920).
AUTHOR CONTRIBUTIONS
Sl. No Author Contribution
1 P C Involved in the conception and designing of the study. Also involved in the statistical analysis and interpretation and drafting of the article.
2 F I Involved in the conception and designing of the study. Also involved in the statistical analysis and interpretation and drafting of the article.
3 U MK Involved in drafting and revising the manuscript for its intellectual content.
4 J P Involved in revising the manuscript for its intellectual content.
5 PA S Involved in revising the manuscript for its intellectual content.
6 E AS Involved in the statistical analysis and interpretation.
7 R V Involved in revising the manuscript for its intellectual content.
8 SR M Involved in revising the manuscript for its intellectual content.
9 L E SL Involved in conception and designing, revising the manuscript for its intellectual content, and the final approval of the version to be published.
FINANCIAL SUPPORT
There is no financial assistance from any of the organizations in conducting the study.
CONFLICT OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study was approved by the institutional ethics committee (IEC: 81/2020) and registered at the Clinical Trial Registry India (CTRI identifier: CTRI/2020/06/025920).
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Aerts C, Leahy S, Mucasse H, Lala S, Bramugy J, Tann CJ, Madhi SA, Bardají A, Bassat Q, Dangor Z, Lawn JE, Jit M, Procter SR. Quantifying the acute care costs of neonatal bacterial sepsis and meningitis in Mozambique and South Africa. Clin Infect Dis Off Publ Infect Dis Soc Am, 2022; 74:S64–9; https://doi.org/10.1093/cid/ciab815
Ahuja A. Despite improved infant and child mortality rates and feeding practices, malnutrition has increased in Kerala: NFHS-5. NDTV, 2021. Available via https://swachhindia.ndtv.com/despite-improved-infant-and-child-mortality-rates-and-feeding-practices-malnutrition-has-increased-in-kerala-nfhs-5-55455/ (Accessed 15 March 2022).
Ananya19b. Multicollinearity: problem, detection and solution. Analytics Vidhya. 2021. Available via https://www.analyticsvidhya.com/blog/2021/02/multicollinearity-problem-detection-and-solution/ (Accessed 29 April 2022)
Bang AT, Bang RA, Baitule SB, Reddy MH, Deshmukh MD. Effect of home-based neonatal care and management of sepsis on neonatal mortality: field trial in rural India. Lancet Lond Engl, 1999; 354:1955–61; https://doi.org/10.1016/S0140-6736(99)03046-9
Bang A, Deshmukh M, Baitule S, Duby J. Decline in the incidence of neonatal sepsis in rural Gadchiroli, India during the twenty-one years (1998-2019) following the home-based neonatal care field-trial. Pediatr Infect Dis J, 2021; 40:1029–33; https://doi.org/10.1097/INF.0000000000003248
Bhaskar S. 2021. Despite improved infant and child mortality rates and feeding practices, malnutrition has increased in Kerala: NFHS-5. NDTV-Dettol Banega Swasth Swachh India. Available via https://swachhindia.ndtv.com/despite-improved-infant-and-child-mortality-rates-and-feeding-practices-malnutrition-has-increased-in-kerala-nfhs-5-55455/ (Accessed 4 April 2022).
Boerma T, Ronsmans C, Melesse DY, Barros AJ, Barros FC, Juan L, Moller AB, Say L, Hosseinpoor AR, Yi M, Neto DDLR, Temmerman M. Global epidemiology of use of and disparities in caesarean sections. Lancet, 2018; 392(10155):1341–8.
Bora JK, Saikia N. Neonatal and under-five mortality rate in Indian districts with reference to sustainable development goal 3: an analysis of the national family health survey of India (NFHS), 2015–2016. Plos One, 2018; 13:e0201125; https://doi.org/10.1371/journal.pone.0201125
Boverman G, Perez C, Vij S, Tgavalekos K, Ravindranath S, Antonescu C, Chambers-Hawk B. Neonatal ICU antibiotic use trends within an integrated delivery network. Antimicrob Resist Infect Control, 2022; 11:21; https://doi.org/10.1186/s13756-022-01057-3
Cantey JB, Pyle AK, Wozniak PS, Hynan LS, Sánchez PJ. Early antibiotic exposure and adverse outcomes in preterm, very low birth weight infants. J Pediatr, 2018; 203:62–7; https://doi.org/10.1016/j.jpeds.2018.07.036
Chandra P, Rajesh V, Surulivelrajan M, Shastry CS, Unnikrishnan MK. Multidrug-resistant Acinetobacter baumannii infections: looming threat in the Indian clinical setting. Expert Rev Anti Infect Ther, 2022; 20:721–32; https://doi.org/10.1080/14787210.2022.2016393
Cortese F, Scicchitano P, Gesualdo M, Filaninno A, De Giorgi E, Schettini F, Laforgia N, Ciccone MM. Early and late infections in newborns: where do we stand? A review. Pediatr Neonatol, 2016; 57:265–73; https://doi.org/10.1016/j.pedneo.2015.09.007
Dadgostar P. Antimicrobial resistance: implications and costs. Infect Drug Resist, 2019; 12:3903–10; https://doi.org/10.2147/IDR.S234610
Dall. Report highlights the deadly impact of bacterial infections. CIDRAP, 2022. Available via https://www.cidrap.umn.edu/antimicrobial-stewardship/report-highlights-deadly-impact-bacterial-infections (Accessed 24 November 2022).
Dewez JE, Chellani H, Nangia S, Metsis K, Smith H, Mathai M, van den Broek N. Healthcare workers’ views on the use of continuous positive airway pressure (CPAP) in neonates: a qualitative study in Andhra Pradesh, India. BMC Pediatr, 2018; 18:347; https://doi.org/10.1186/s12887-018-1311-8
Duke T. CPAP: a guide for clinicians in developing countries. Paediatr Int Child Health, 2014; 34:3–11; https://doi.org/10.1179/2046905513Y.0000000102
Fenny AP, Otieku E, Labi KAK, Asante FA, Enemark U. Costs and extra length of stay because of neonatal bloodstream infection at a teaching hospital in Ghana. PharmacoEcon Open, 2020; 5:111–20; https://doi.org/10.1007/s41669-020-00230-x
Fleischmann C, Reichert F, Cassini A, Horner R, Harder T, Markwart R, Tröndle M, Savova Y, Kissoon N, Schlattmann P, Reinhart K, Allegranzi B, Eckmanns T. Global incidence and mortality of neonatal sepsis: a systematic review and meta-analysis. Arch Dis Child, 2021; 106:745–52; https://doi.org/10.1136/archdischild-2020-320217
Gastmeier P, Geffers C, Schwab F, Fitzner J, Obladen M, Rüden H. Development of a surveillance system for nosocomial infections: the component for neonatal intensive care units in Germany. J Hosp Infect, 2004; 57:126–31; https://doi.org/10.1016/j.jhin.2003.12.038
Gondwe T, Betha K, Kusneniwar GN, Bunker CH, Tang G, Simhan H, Haggerty CL. Adverse infant outcomes associated with caesarean section delivery in India. Int Health, 2020; 12(5):411–6.
The World Bank. GDP per capita (current US$). 2021. Available via https://data.worldbank.org/indicator/NY.GDP.PCAP.CD (Accessed 30 March 2022).
Hassuna NA, AbdelAziz RA, Zakaria A, Abdelhakeem M. Extensively-drug resistant Klebsiella pneumoniae recovered from neonatal sepsis cases from a major NICU in Egypt. Front Microbiol, 2020; 11:1375.
Hayes R, Hartnett J, Semova G, Murray C, Murphy K, Carroll L, Plapp H, Hession L, O’Toole J, McCollum D, Roche E, Jenkins E, Mockler D, Hurley T, McGovern M, Allen J, Meehan J, Plötz FB, Strunk T, de Boode WP, Polin R, Wynn JL, Degtyareva M, Küster H, Janota J, Giannoni E, Schlapbach LJ, Keij FM, Reiss IKM, Bliss J, Koenig JM, Turner MA, Gale C, Molloy EJ, Infection, Inflammation, Immunology and Immunization (I4) section of the European Society for Paediatric Research (ESPR). Neonatal sepsis definitions from randomized clinical trials. Pediatr Res, 2021; https://doi.org/10.1038/s41390-021-01749-3
Hornik CP, Fort P, Clark RH, Watt K, Benjamin DK, Smith PB, Manzoni P, Jacqz-Aigrain E, Kaguelidou F, Cohen-Wolkowiez M. Early and late onset sepsis in very-low-birth-weight infants from a large group of neonatal intensive care units. Early Hum Dev, 2012; 88(Suppl 2):S69–74; https://doi.org/10.1016/S0378-3782(12)70019-1
Johnson TJ, Patel AL, Jegier BJ, Engstrom JL, Meier PP. Cost of morbidities in very low birth weight infants. J Pediatr, 2013; 162:243–9; https://doi.org/10.1016/j.jpeds.2012.07.013
Kamath BD, Todd JK, Glazner JE, Lezotte D, Lynch AM. Neonatal outcomes after elective cesarean delivery. Obst Gynecol, 2009; 113(6):1231.
Kesarwani R. Estimation of life expectancy from infant mortality rate at districts level. Int Res J Soc Sci, 2015; 4:52–63.
Leal YA, Álvarez-Nemegyei J, Velázquez JR, Rosado-Quiab U, Diego-Rodríguez N, Paz-Baeza E, Dávila-Velázquez J. Risk factors and prognosis for neonatal sepsis in southeastern Mexico: analysis of a four-year historic cohort follow-up. BMC Pregnancy Childbirth, 2012; 12:48; https://doi.org/10.1186/1471-2393-12-48
Li G, Bielicki JA, Ahmed ASMNU, Islam MS, Berezin EN, Gallacci CB, Guinsburg R, da Silva Figueiredo CE, Santarone Vieira R, Silva AR, Teixeira C, Turner P, Nhan L, Orrego J, Pérez PM, Qi L, Papaevangelou V, Triantafyllidou P, Iosifidis E, Roilides E, Sarafidis K, Jinka DR, Nayakanti RR, Kumar P, Gautam V, Prakash V, Seeralar A, Murki S, Kandraju H, Singh S, Kumar A, Lewis L, Pukayastha J, Nangia S, KNY, Chaurasia S, Chellani H, Obaro S, Dramowski A, Bekker A, Whitelaw A, Thomas R, Velaphi SC, Ballot DE, Nana T, Reubenson G, Fredericks J, Anugulruengkitt S, Sirisub A, Wong P, Lochindarat S, Boonkasidecha S, Preedisripipat K, Cressey TR, Paopongsawan P, Lumbiganon P, Pongpanut D, Sukrakanchana PO, Musoke P, Olson L, Larsson M, Heath PT, Sharland M. Towards understanding global patterns of antimicrobial use and resistance in neonatal sepsis: insights from the NeoAMR network. Arch Dis Child, 2020; 105:26–31; https://doi.org/10.1136/archdischild-2019-316816
Liyakat H, Khan M, Tahirkheli N, Bader -u-Nisa, Ashfaq M. Bacteriological profile and antibiogram of neonatal sepsis. J Dow Univ Health Sci JDUHS, 2021; 15:130–5; https://doi.org/10.36570/jduhs.2021.3.1240
Maternal and Neonatal Health in Madhya Pradesh: Trends, Insights and Scope. (2022). India Health Action Trust (IHAT). Available via https://www.ihat.in/resources/mnh-in-madhya-pradesh-trends-insights-and-scope/ (Accessed 29 April 2022)
McGovern M, Giannoni E, Kuester H, Turner MA, van den Hoogen A, Bliss JM, Koenig JM, Keij FM, Mazela J, Finnegan R, Degtyareva M, Simons SHP, de Boode WP, Strunk T, Reiss IKM, Wynn JL, Molloy EJ, Infection, Inflammation, Immunology and Immunization (I4) section of the ESPR. Challenges in developing a consensus definition of neonatal sepsis. Pediatr Res, 2020; 88:14–26; https://doi.org/10.1038/s41390-020-0785-x
Micormedex NeoFax Essentials 2014 (1).pdf. (2014). Available via http://ypeda.com/attachments/fil/Micormedex%20NeoFax%20Essentials%202014%20(1).pdf (Accessed 04 April 2022).
Milton R, Gillespie D, Dyer C, Taiyari K, Carvalho MJ, Thomson K, Sands K, Portal EAR, Hood K, Ferreira A, Hender T, Kirby N, Mathias J, Nieto M, Watkins WJ, Bekele D, Abayneh M, Solomon S, Basu S, Nandy RK, Saha B, Iregbu K, Modibbo FZ, Uwaezuoke S, Zahra R, Shirazi H, Najeeb SU, Mazarati JB, Rucogoza A, Gaju L, Mehtar S, Bulabula ANH, Whitelaw AC, Walsh TR, Chan GJ, BARNARDS Group. Neonatal sepsis and mortality in low-income and middle-income countries from a facility-based birth cohort: an international multisite prospective observational study. Lancet Glob Health, 2022; 10(5):e661–72.
Murthy S, Godinho MA, Guddattu V, Lewis LES, Nair NS. Risk factors of neonatal sepsis in India: a systematic review and meta-analysis. Plos One, 2019; 14:e0215683; https://doi.org/10.1371/journal.pone.0215683
Niknajad A, Ghojazadeh M, Sattarzadeh N, Bashar Hashemi F, Dezham Khoy Shahgholi F. Factors affecting the neonatal intensive care unit stay duration in very low birth weight premature infants. J Caring Sci, 2012; 1:85–92; https://doi.org/10.5681/jcs.2012.013
Panigrahi P, Chandel DS, Hansen NI, Sharma N, Kandefer S, Parida S, Satpathy R, Pradhan L, Mohapatra A, Mohapatra SS, Misra PR, Banaji N, Johnson JA, Morris JG, Gewolb IH, Chaudhry R. Neonatal sepsis in rural India: timing, microbiology and antibiotic resistance in a population-based prospective study in the community setting. J Perinatol Off J Calif Perinat Assoc, 2017; 37:911–21; https://doi.org/10.1038/jp.2017.67
Pokhrel B, Koirala T, Shah G, Joshi S, Baral P. Bacteriological profile and antibiotic susceptibility of neonatal sepsis in neonatal intensive care unit of a tertiary hospital in Nepal. BMC Pediatr, 2018; 18:208; https://doi.org/10.1186/s12887-018-1176-x
Prinja S, Manchanda N, Mohan P, Gupta G, Sethy G, Sen A, van den Hombergh H, Kumar R. Cost of neonatal intensive care delivered through district level public hospitals in India. Indian Pediatr, 2013; 50:839–46; https://doi.org/10.1007/s13312-013-0234-6
Ree IMC, Fustolo-Gunnink SF, Bekker V, Fijnvandraat KJ, Steggerda SJ, Lopriore E. Thrombocytopenia in neonatal sepsis: incidence, severity and risk factors. Plos One, 2017; 12:e0185581; https://doi.org/10.1371/journal.pone.0185581
Salman O, Procter SR, McGregor C, Paul P, Hutubessy R, Lawn JE, Jit M. Systematic review on the acute cost-of-illness of sepsis and meningitis in neonates and infants. Pediatr Infect Dis J, 2020; 39:35–40; https://doi.org/10.1097/INF.0000000000002500
Schober P, Boer C, Schwarte LA. Correlation coefficients: appropriate use and interpretation. Anesth Analg, 2018; 126:1763–8; https://doi.org/10.1213/ANE.0000000000002864
Shah S, Zemichael O, Meng HD. Factors associated with mortality and length of stay in hospitalized neonates in Eritrea, Africa: a cross-sectional study. BMJ Open, 2012; 2:e000792; https://doi.org/10.1136/bmjopen-2011-000792
Shelar J. Indian neonates highly resistant to first-line antibiotics. The Hindu. 2019 (Accessed 18 November 2022)
Shrestha P, Das BK, Bhatta NK, Jha DK, Das B, Setia A, Tiwari A. Clinical and bacteriological profiles of blood culture positive sepsis in newborns. J Nepal Paediatr Soc, 2007; 27:64–7; https://doi.org/10.3126/jnps.v27i2.1411
Singh S, Charani E, Devi S, Sharma A, Edathadathil F, Kumar A, Warrier A, Shareek PS, Jaykrishnan AV, Ellangovan K. A road-map for addressing antimicrobial resistance in low- and middle-income countries: lessons learnt from the public private participation and co-designed antimicrobial stewardship programme in the State of Kerala, India. Antimicrob Resist Infect Control, 2021; 10:32; https://doi.org/10.1186/s13756-020-00873-9
Singh M, Alsaleem M, Gray CP. Neonatal sepsis. In: StatPearls. StatPearls Publishing, Treasure Island, FL, 2022.
Sundaram V, Kumar P, Dutta S, Mukhopadhyay K, Ray P, Gautam V, Narang A. Blood culture confirmed bacterial sepsis in neonates in a North Indian tertiary care center: changes over the last decade. Jpn J Infect Dis, 2009; 62:46–50.
Thomson KM, Dyer C, Liu F, Sands K, Portal E, Carvalho MJ, Barrell M, Boostrom I, Dunachie S, Farzana R, Ferreira A, Frayne F, Hassan B, Jones E, Jones L, Mathias J, Milton R, Rees J, Chan GJ, Bekele D, Mahlet A, Basu S, Nandy RK, Saha B, Iregbu K, Modibbo F, Uwaezuoke S, Zahra R, Shirazi H, Syed NU, Mazarati JB, Rucogoza A, Gaju L, Mehtar S, Bulabula ANH, Whitelaw A, van Hasselt JGC, Walsh TR, BARNARDS Group. Effects of antibiotic resistance, drug target attainment, bacterial pathogenicity and virulence, and antibiotic access and affordability on outcomes in neonatal sepsis: an international microbiology and drug evaluation prospective substudy (BARNARDS). Lancet Infect Dis, 2021; 21:1677–88; https://doi.org/10.1016/S1473-3099(21)00050-5
Thukral A, Sankar MJ, Chandrasekaran A, Agarwal R, Paul VK. Efficacy and safety of CPAP in low- and middle-income countries. J Perinatol, 2016; 36:S21–8; https://doi.org/10.1038/jp.2016.29
Tsai MH, Hsu JF, Chu SM, Lien R, Huang HR, Chiang MC, Fu RH, Lee CW, Huang YC. Incidence, clinical characteristics and risk factors for adverse outcome in neonates with late-onset sepsis. Pediatr Infect Dis J, 2014; 33:e7–13; https://doi.org/10.1097/INF.0b013e3182a72ee0
UNICEF. Neonatal mortality. UNICEF DATA. 2021. Available via https://data.unicef.org/topic/child-survival/neonatal-mortality/ (Accessed 29 April 2022)
WHO. Newborns: improving survival and well-being. Available via https://www.who.int/news-room/fact-sheets/detail/newborns-reducing-mortality (Accessed 15 May 2022)
Ye J, Zhang J, Mikolajczyk R, Torloni MR, Gülmezoglu AM, Betran AP. Association between rates of caesarean section and maternal and neonatal mortality in the 21st century: a worldwide population-based ecological study with longitudinal data. BJOG Int J Obst Gynaecol, 2016; 123(5):745–53.
SUPPLEMENTARY MATERIAL
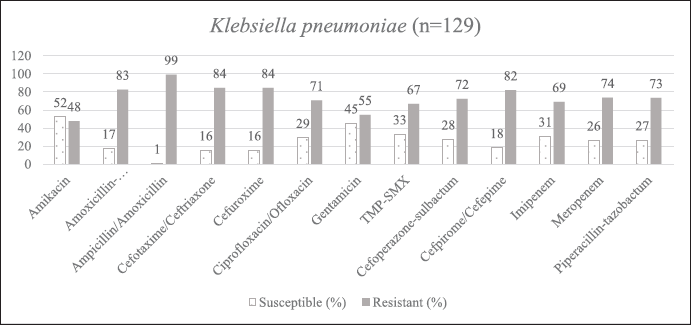 | Supplementary Figure S1. AST of K. pneumoniae. [Click here to view] |
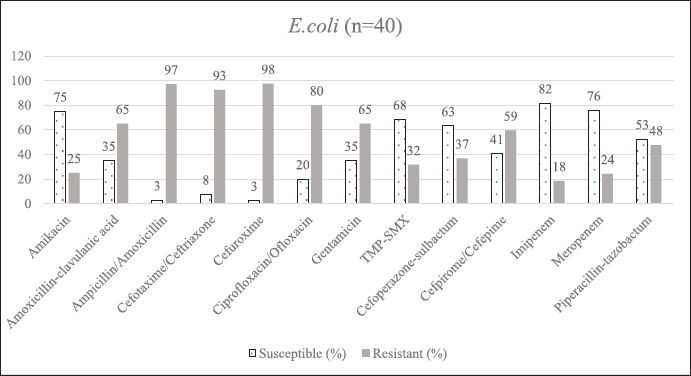 | Supplementary Figure S2. AST of E.coli. [Click here to view] |
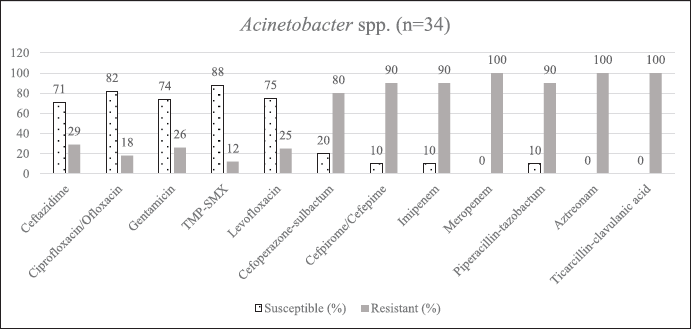 | Supplementary Figure S3. AST of Acinetobacter spp. [Click here to view] |
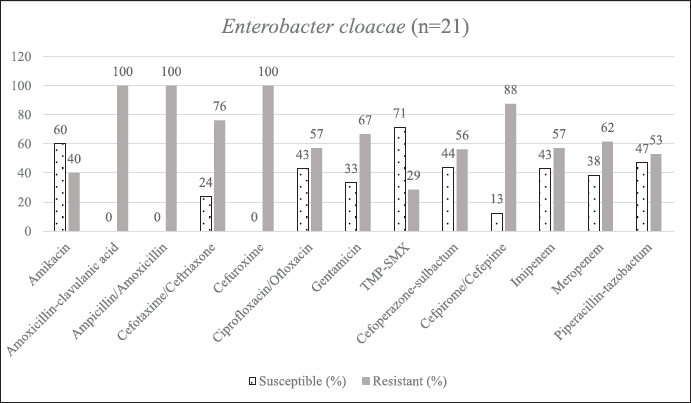 | Supplementary Figure S4. AST of E. cloacae. [Click here to view] |
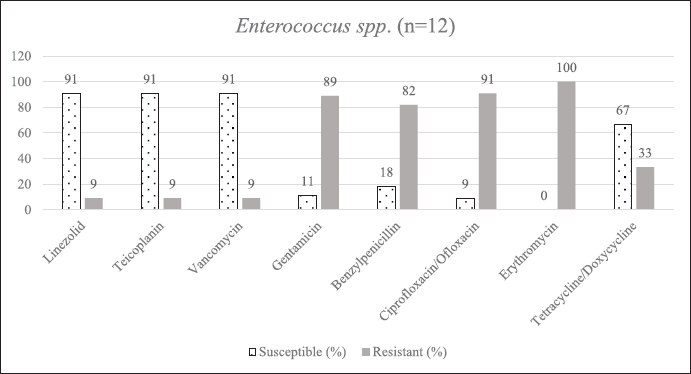 | Supplementary Figure S5. AST of Enterococcus spp. [Click here to view] |
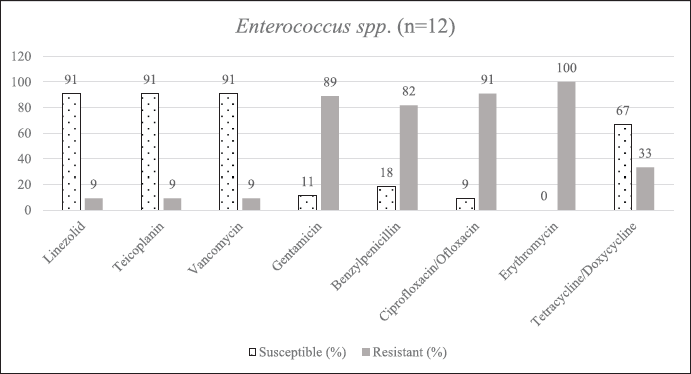 | Supplementary Figure S5. AST of Enterococcus spp. [Click here to view] |
 | Supplementary Figure S6. Scatterplot NICU stay and neonatal attributes -predicted value (X-axis) versus residual value (Y-axis). [Click here to view] |