INTRODUCTION
Curculigo latifolia (family: Hypoxidaceae), locally known as “Marasi,” is an annual plant commonly found in Southeast Asia, including Indonesia. Some tribes in Indonesia, the Batak Karo (Sumatra), use this species as an ornamental garden plant, traditional healthcare, and food additive (Silalahi and Nisyawati, 2018). Recent studies have shown that C. latifolia has high phenolic content and significantly exhibits antioxidant and α-glucosidase inhibitor activities (Umar et al., 2021a). The main components that had pharmacological activities included 1,1-bis-(3,4-dihydroxyphenyl)-1-(2-furan)-methane, (1S,2R)-O-methylnyasicoside, 2,4-dichloro-5-methoxy-3-methylphenol, curculigoside B and curculigosaponin G, H, and I, orchioside B, and orcinol glucoside (Umar et al., 2021a). The other groups of compounds identified in Curculigo spp. were phenolic glucoside, norlignans, and terpenoids (Wang et al., 2021), with various bioactivities, such as anti-inflammatory (Zhu et al., 2015a), neuroprotection (Zhao et al., 2020), antidepression (Zhang et al., 2017), antiosteoporosis and anti-rheumatoid arthritis (Han et al., 2020), and sweet-tasting and taste-modifying (Okubo et al., 2021). Callus extract of C. latifolia also has antioxidant and antibacterial effects (Farzinebrahimi et al., 2016).
The culture technology of cell/callus, tissue, and plant organs has proven to be an efficient method for producing secondary metabolites from a plant (Li et al., 2021). The metabolites can be produced in a sustainable system without environmental constraints due to biotic and abiotic conditions. Temperature, light exposure, nutrient availability, and pH can be easily controlled under in vitro system (Cabañas-García et al., 2021). Therefore, in vitro culture is a potential tool to obtain compounds with selected biological activities, particularly from uncultivated plants like C. latifolia in Indonesia.
Compounds in in vitro propagules can be identified quickly using a metabolomic approach (Kim et al., 2021). This approach provides an overview of all the metabolites present in plant tissues. Complex metabolomic data need to be supported by chemometric techniques, that is, principal component analysis (PCA) and partial least squares (PLS), to help discriminate, group, and identify the essential compounds as well as the marker metabolites of the analyzed samples (Bertol et al., 2021). Phenolic content, antioxidant activity, and phenolics profiling in callus, plantlet leaves, and organs of C. latifolia have never been investigated. The phenolics profile of the in vitro propagules and the mother plant organs in this study was determined by ultrahigh-performance liquid chromatography-quadrupole-orbital ion trap analyzer-high-resolution mass spectrometry (UHPLC-Q-Orbitrap HRMS). It helped group samples based on similarities and differences in compounds and identified any marker metabolites distinguishing the two types of samples. It is necessary to distinguish the essential metabolites in the mother plant organs and in vitro propagules, mainly when the latter are to be developed as an alternative plant source (Kim et al., 2021). We give more profound knowledge of the phytochemicals contained in the in vitro propagules and in the mother plant organs of this species, along with their potential as antioxidants.
MATERIAL AND METHODS
Chemicals and reagents
Gallic acid, 1,1-diphenyl-2-picrylhydrazyl (DPPH), 1,3,5-tri(2-pyridyl)-2,4,6-triazine, and 6-hydroxy-2,5,7,8-tetramethyl-chroman-2-carboxylic acid (Trolox) were purchased from Sigma Aldrich (St Louis). Water [liquid chromatography-mass spectrometry (LC-MS grade)], acetonitrile (LC-MS grade), methanol (LC-MS grade), formic acid, aluminum chloride, Folin Ciacolteu reagent, sodium carbonate, ferric chloride, ethanol, and sodium tetraborate were from Merck (Darmstadt, Germany).
Plant material
The plant materials of C. latifolia were obtained from Puncak District, Sinjai Regency (South Sulawesi), Indonesia (5°13′25″S, 120º02′46″E, ± 959 m.asl), in February 2019 (rainy season). The samples were identified based on the published flora and voucher specimens deposited at the Herbarium Bogoriense, Research Center for Biology, the Indonesian Institute of Sciences, Indonesia, under voucher number 184. The plants collected from the field were planted in pots under greenhouse conditions in Bogor (West Java), from which explants for callus culture were taken. The media for callus initiation, proliferation, and regeneration were MS (Murashige and Skoog, 1962) with 3 mgl–1 6-benzylaminopurine (BAP) and 5 mgl–1 indole-3-butyric acid (IBA). The detailed tissue culture method for callus initiation and plantlet regeneration has been described in Umar et al. (2021b). The callus and plantlet leaves (Fig. 1) were then used for this study.
Sample preparation
The samples from the callus of C. latifolia (CCL), plantlet leaf of C. latifolia (PLL), and from the rhizome, petiole, and leaf of C. latifolia originating from Sinjai-Puncak Sinjai-Puncak (RLSK, PLSK, and LLSK, respectively) were prepared as previously described by Umar et al. (2021a). Sinjai-Puncak is situated in South Sulawesi.
Determination of total phenolics
Total phenolic content (TPC) was determined using the method described by Umar et al. (2021a). Phenolic content was expressed as gallic acid equivalent (mg GAE g–1). For each sample, this assay was replicated three times.
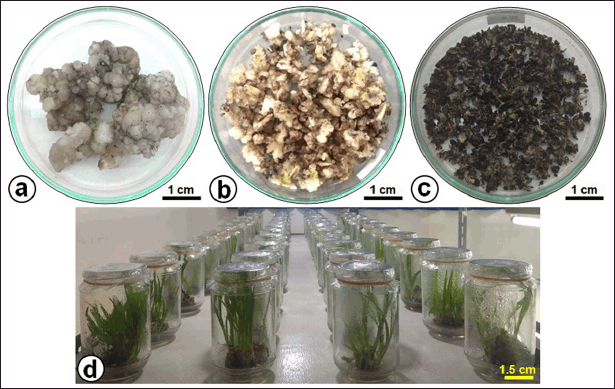 | Figure 1. The yield of in vitro culture of C. latifolia. (a) Freshly harvested callus, (b) chopped callus, (c) dried callus used for extraction, and (d) plantlets grown on MS culture medium with a BAP-IBA combination. [Click here to view] |
Determination of antioxidant activities
DPPH free radical scavenging activity
The DPPH free radical scavenging activity of sample extracts was measured in 96-well plates, according to the procedure described in Umar et al. (2021a) with slight modifications. An aliquot of 10 μl of appropriately diluted sample or Trolox solution (31.25–1000 μM) was added to 190 μl of DPPH solution (in ethanol). The mixture was incubated at 37°C for 30 minutes, and the absorbance was measured at 517 nm. All samples and the control were tested in triplicate. The ability to scavenge the DPPH radical was calculated as a percentage as follows:
DPPH (scavenging effect)% = [(ADPPH – AS)/ADPPH] × 100,
where ADPPH is the absorbance of the control, and AS is the absorbance of the sample. The IC50 value was determined to be the effective concentration at which DPPH radicals were inhibited by 50%. In addition, to determine the IC50 of samples on DPPH, a series of six different concentrations were used. The ethanol instead of the sample was made as a blank control, while Trolox was used as the standard.
2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) free radical scavenging activity
We used the procedure of Zhu et al. (2015b) to determine the antioxidant activity by the ABTS assay. About 10 μl of the sample was diluted appropriately. Then, 190 μl of ABTS+ solution was added to a 96-well plate. This assay was replicated three times. Trolox was used as the standard. The equation used to determine the antioxidant activity followed the DPPH scavenging method described above. The scavenging activities of different concentrations of samples against ABTS+ would also result in the IC50.
Ferric reducing/antioxidant power (FRAP) assay
As described previously by Zhu et al. (2015b), a FRAP assay assay was performed in this study. Approximately 10 μl of properly diluted samples and 30 μl of distilled water were added to 260 μl of freshly prepared FRAP reagent in a 96-well plate. The mixture was incubated at 37°C for 10 minutes. The absorbance of the reaction mixture was measured at 593 nm by a microplate reader. It was carried out in triplicate. The calibration curve was plotted on a FeSO4·7H2O standard at a concentrations range of 0.125 to 2 mM, and FRAP activity was expressed as mmol Fe2+ equivalent per g sample (mmol Fe2+ equivalent g–1). A high FRAP value indicates a greater antioxidant capacity.
UHPLC-Q-Orbitrap HRMS analysis
The extraction and analysis procedure followed exactly the method described by Umar et al. (2021a). Metabolite profiling was performed in an Orbitrap High-Resolution Mass Spectrometer (Vanquish Flex UHPLC-Q Exactive Plus) using Accucore™ Phenyl Hexyl (100 × 2.1 mm, 2.6 μm) as the separation column and UV detector at 254 nm (Thermo Scientific™, Waltham, MA). Rhizome, petiole, and leaves collected from the field were dried for 3 days in a drying cabinet at 40°C. About 250 mg of fresh callus and plantlet leaves were dried and extracted in the same way. All samples were analyzed in three replications.
Data analysis
Data of total phenolics and the determination of antioxidant activity (DPPH, ABTS, and FRAP) were expressed as means ± SD of three replicates for each sample. The data were statistically analyzed using R ver. I386 3.6.0 (R Core Team, 2018). Data from UHPLC-Q-Orbitrap HRMS analysis were proceeded to the Compound Discoverer™ Software (Thermo Scientific™, Waltham, MA), MS-DIAL ver. 4.70, and MS-FINDER ver. 3.52 (Tsugawa et al., 2015). The compounds identification used an in-house database and MSP File (in MS/MS positive and negative mode). Clustering analysis was performed using PCA and hierarchical clustering analysis (HCA). A supervised PLS discrimination analysis (PLS-DA) helped to evaluate the differences in metabolite levels. An orthogonal partial least square discriminant analysis (OPLS-DA) assisted in identifying the marker compounds in in vitro propagules (CCL and PLL) and mother plant organs (RLSK, PLSK, and PLSK). The results were subjected to several validation tools such as R2 and Q2 (permutation value) and variables of importance in projection (VIP) to confirm the reliability of the PLS-DA and OPLS-DA model. Finally, analysis of variances was performed to determine significant differences, if any, with a 95% confidence level by MetaboAnalyst 5.0 (https://www.metaboanalyst.ca) (Chong et al., 2019).
RESULTS
TPC
The TPC of the dried extracts of callus, plantlet leaves, rhizomes, leaves, and petiole is listed in Table 1. The rhizome’s TPC was the highest (457.80 ± 0.51 mg GAE g–1), followed by plantlet leaves, callus, petiole, and leaves.
Antioxidant activity
In this work, we used three different methods, DPPH, ABTS, and FRAP assays, to assess and compare the antioxidant potential of in vitro propagules and plant organs. The results are shown in Table 1. Antioxidant activity of the rhizome was significantly the highest, followed by plantlet leaves, callus, petiole, and leaves, reflected by the DPPH and ABTS radical scavenging activities. The antioxidant activities corroborated with the TPC in the tissues. The antioxidant activity increased as the TPC increased in each organ. However, FRAP gave the highest value to leaves.
Metabolites in in vitro propagules and plant organs
Representative chromatogram from UHPLC-Q-Orbitrap HRMS for callus (CCL), plantlet leaf (PLL), and rhizome organ (RLSK), petiole (PLSK), and leaf (LLSK) of C. latifolia with MS full scan type (100–1,500), relative abundance (0–100), and retention time (0–32 minutes) showed differences in peaks, height, and peak area at the retention times of 1.3–3.7, 4.5–8.5, 9.0–11, 12.0–18.0, and 22.0–26.0 (Fig. S1).
The heatmap analysis with the sample group model (Fig. 2) represents the phenolic compounds identified in each sample group. In general, the distribution of compounds identified in in vitro propagules (callus and plantlet leaves) was in positive (Fig. 2a) and negative (Fig. 2b) modes, and they were more abundant (on the right-hand side) than those in the mother plant organ samples.
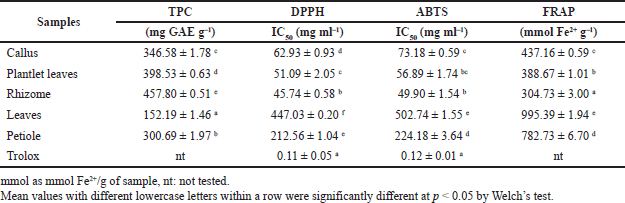 | Table 1. Total phenolics content and antioxidant activity of in vitro propagules and plant organs of C. latifolia. [Click here to view] |
The phenolic compounds identified in the samples are shown in Table 2, in [M + H]+ and [M – H]– modes, but the majority were in negative than positive mode. Phenolic groups dominated the compounds in all samples. Alkaloids, saponins, steroids, and terpenoids were also found. All tentative compounds are summarized in Table S1, including the retention time, type of adduction, molecular formula, experimental m/z, MS and MS/MS fragments, accuracy (ppm), and classes of metabolites.
Multivariate analysis
Multivariate statistical analysis was used to classify the differences between callus, plantlet leaves, and mother plant organs (rhizome, petiole, and leaves) of C. latifolia, and the results are presented in the PCA (Fig. 3a and b) and HCA (Fig. 3c and d). Unsupervised PCA revealed that the total PC value of the two main components was 100% (PC-1 94.1% and PC-2 5.9%) (Fig. 3a). The HCA showed four clusters, that is, a, b, c, and d (Fig. 3c). Cluster c demonstrated the similarity between callus, leaves, rhizome, and petiole. In the negative mode (Fig. 3b), the total PC value of the two main components was 99.1% (PC-1 92.3% and PC-2 6.8%). The cluster analysis also resulted in four clusters (Fig. 3d). Samples belonging to the same cluster indicated the presence of similar compounds. HCA was made using a dendrogram model with Euclidean distance parameters, a complete clustering algorithm, and auto scale standardization between groups, with 3.0e+08 and 1e+08 similarity for positive and negative modes, respectively.
A supervised PLS-DA was applied to see the patterns of discrimination using a score plot (Fig. S2a and b) and the essential features (Fig. 4a and b). The score plots in positive and negative modes revealed total component values of 99.9% and 99.1%, respectively. In contrast, metabolites with VIP values > 1 were orcinol glucoside in positive mode, and nyasicoside and orcinol glucoside were in negative mode.
The OPLS-DA loadings S-plot (the essential features) (Fig. 5a and b) demonstrated that orcinol glucoside and vanillin were the marker compounds, separating the in vitro propagules (IVP = CCL and PLL) and the mother plant organs (OPO = RLSK, LLSK, and PLSK) in the positive mode and nyasicoside and orcinol glucoside in the negative mode. The OPLS-DA score plot produced a total T score of 72.6% in positive mode and 59.1% in negative mode (Fig. S3a and b). The OPLS-DA model showed a good level of goodness-of-fit (R2 = 0.814) and had a high predictability level (Q2 = 0.693) in positive mode and R2 = 0.864 and Q2 = 0.781 in negative mode (Fig. S4a and b).
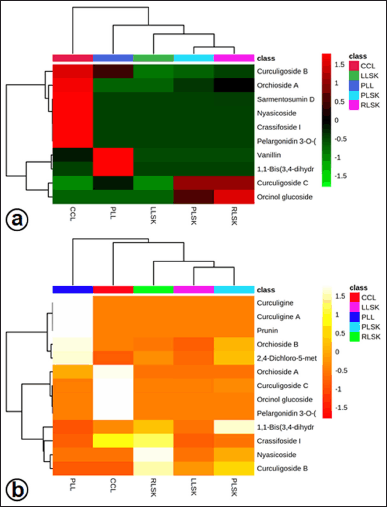 | Figure 2. Heatmap analysis representing phenolic compounds identified in callus (CCL), plantlet leaf (PLL), rhizome (RLSK), petiole (PLSK), and leaf (LLSK) organ of C. latifolia. The color scale shows the relative abundance of each compound. Each row represents a compound, and each column represents a sample group. (a) Positive and (b) negative mode. [Click here to view] |
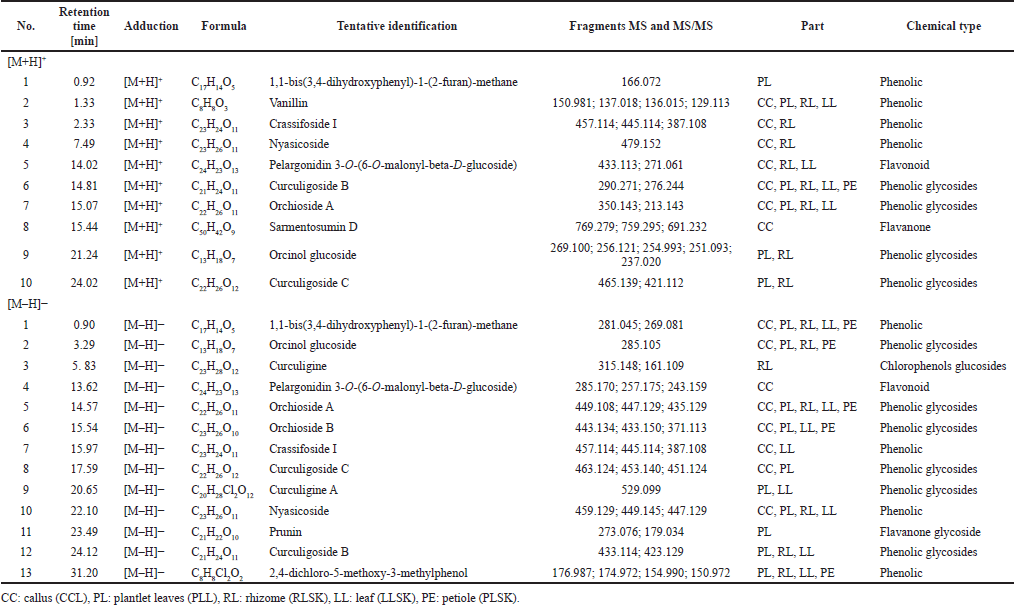 | Table 2. Phenolic compounds identified in 70% ethanol extract of callus, plantlet leaves, rhizome, leaf, and petiole of C. latifolia in positive [M + H]+ and negative [M – H]– modes. [Click here to view] |
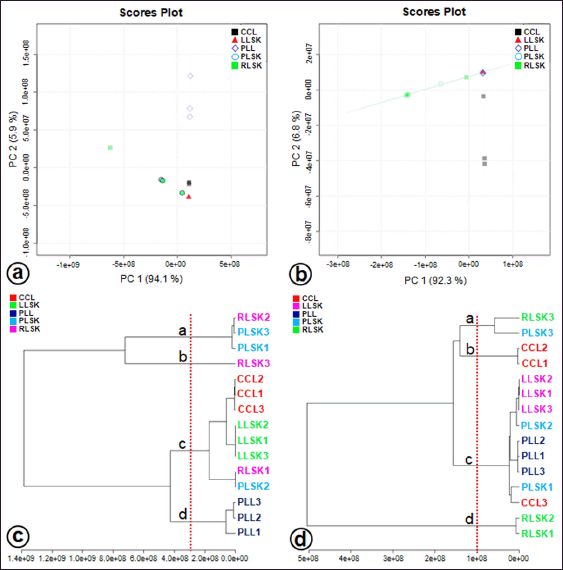 | Figure 3. Factorial distribution of principal components 1 (PC1) and 2 (PC2) in (a) positive and (b) negative modes generated from UHPLC-Q-Orbitrap-HRMS data of ethanol extracts of in vitro propagules and mother plant organs and hierarchical cluster analysis (HCA) plot, showing four clusters at positive mode (c) and negative mode (d) (n = 3 replicates). Colors indicate the types of samples. [Click here to view] |
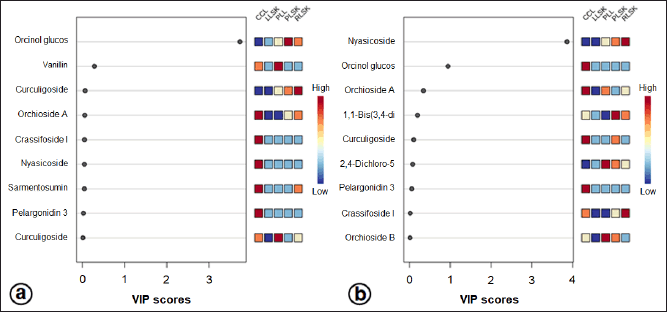 | Figure 4. Importance in projection (VIP) scores of bioactive metabolites in PLS-DA at positive (a) and negative (b) modes. In vitro propagules and mother plant organs represented the peak area. The colored boxes (on the right) indicate the relative concentration of the corresponding metabolites. The red color indicates a high level, and the blue indicates a low level. [Click here to view] |
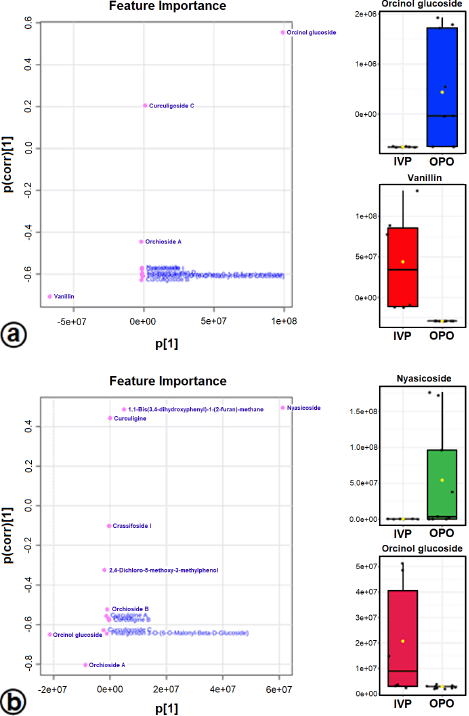 | Figure 5. The OPLS-DA loadings S-plot and the compounds distinguishing the IVP from the OPO in positive (a) and negative (b) modes. Orcinol glucoside, vanillin, and nyasicoside are the marker compounds in in vitro propagules and in mother plant organs. [Click here to view] |
DISCUSSION
Phenolic compounds have widely attracted attention due to their potential as antioxidants and their ability to reduce the generation and scavenge free radicals. In this study, the highest phenolic content was found in the rhizome (Table 1). This high level of total phenolics is supported by the results of the metabolite profiling that we have carried out (Table 2). The high level of phenolics is most probably influenced by the soil pH and micronutrient content. In the rainy season, the increase of micronutrient solubility results in the decrease of the soil pH, which can be attributed to lower mineralization processes at lower pH. Micronutrients in the soil directly affect the biosynthesis and concentration of secondary metabolites in plants, augmenting the phenolics content (Kumar et al., 2022). The accumulation of secondary metabolites was reported to have an essential role in plant tolerance to biotic and abiotic stresses. The plants adapt to fluctuating environmental conditions, among others, due to their high phenolic content and their antioxidant capacity (Hashim et al., 2020). Callus and plantlet leave also accumulated a relatively high level of phenols. It is generally accepted that phenolic compounds act synergistically with auxin in the developing callus and organs. A high level of phenolics protects auxin from oxidation, making it more effective (Jagie??o-Kubiec et al., 2021). The environmental condition and plant growth stage influence the secondary metabolite content and composition (Yeshi et al., 2022).
The antioxidant activity of plant extracts cannot be evaluated by a single method because of the complex nature of phytochemicals, and the determination of antioxidant activity is highly dependent on the reaction mechanism (Yaermaimaiti et al., 2021). Several chemical or biological assays have been developed to evaluate their antioxidant activity and explain the action mechanism of an antioxidant in plant extracts. The DPPH, ABTS, and reducing power tests are the most commonly used (Lee and Cho, 2021; Yaermaimaiti et al., 2021). Therefore, in this study, we also employed different methods to compare the antioxidant potential of in vitro propagules and the field-raised mother plant organs of C. latifolia. Many antioxidants react with DPPH by the hydrogen-atom transfer mechanism or the single electron transfer mechanism (SET), depending on the antioxidants, free radicals, and the reaction environment. ABTS has the exact mechanism of action as the DPPH, as these two radicals (DPPH and ABTS) are soluble in water and in organic solvents, but SET seems to be the primary mechanism occurring in the DPPH assay (Baschieri and Amorati, 2021). IC50 is a parameter widely used in measuring antioxidant activity, including DPPH and ABTS. A lower IC50 value indicates higher antioxidant activity (Trinh et al., 2022). The FRAP assay helps in establishing the reductive activity by reducing Fe (III) to Fe (II) under acidic conditions, presenting the ability of the compound to reduce free radicals via electron transfer (Butkeviciute et al., 2021). Differences in the antioxidant activity values are due to the different mechanisms of each method taken to measure antioxidant activities (Xu et al., 2019). In this study, the rhizome extract exhibited the best antioxidant activity (DPPH and ABTS), probably due to the high level of total phenolics, while with the FRAP method, the highest antioxidant activity was obtained from the leaf extract, although its phenolic content was moderate. This fact drove us to assume that phenolics do not exert this property in leaves. It might probably be undertaken by norlignan, sitosterol, or cycloartane, also identified in this organ (Table S1). As reported by Li et al. (2012) by the DPPH method, norlignan of in vitro Curculigo sinensis showed strong radical scavenging activities. By the DPPH, ABTS, and FRAP methods, sitosterol from Phlomis thapsoides had antioxidant ability (Sobeh et al., 2016), and cycloartane of Astragalus plumosus demonstrated the potential for antioxidant activity (Denizli et al., 2014).
The antioxidant capacities from the DPPH, ABTS, and FRAP methods on the in vitro propagules and organs of C. latifolia gave different results. Callus and plantlet leaves had better antioxidant activity than the leaves and petiole of the mother plant. By DPPH and superoxide dismutase assays, Farzinebrahimi et al. (2016) also reported that the callus of this species has potential components as an antioxidant. Additionally, they noticed that callus generated from rhizomal explant and rhizome collected from the field performed superior antioxidant activities than those from leaf-derived callus and the original leaves. A similar result was also obtained by Hejazi et al. (2018) and Kushalan et al. (2022), who reported that the rhizome of Curculigo orchioides had the highest activity as an antioxidant. Based on the collected information and our results, it is suggested that the rhizome of Curculigo spp. is the main site of secondary metabolites accumulation, at least those exerting as antioxidants. In this study, the leaves and plantlet leaves of C. latifolia have lower antioxidant activity than the rhizomes. Our previous study (Umar et al., 2021a) revealed that the leaves of C. latifolia and C. orchioides had a high level of total flavonoids compared to other organs. Flavonoids that are also included in the phenolic group have synergistic or opposite effects as antioxidants.
UHPLC-Q-Orbitrap HRMS has been widely used to characterize metabolites in a complex mixture, particularly in in vitro propagules (López-Ramírez et al., 2021; Ramabulana et al., 2021). We identified 27 tentative compounds in the callus and plantlet leaves (Table S1). In general, the compounds were dominated by phenolics, and the other significant groups were alkaloids, steroids, and terpenoids. A study by Umar et al. (2021a) found that the compounds having antioxidant and α-glucosidase inhibition effects in C. latifolia were 1,1-bis-(3,4-dihydroxyphenyl)-1-(2-furan)-methane, (1S,2R)-O-methylnyasicoside, 2,4-dichloro-5-methoxy-3-methylphenol, curculigoside B, curculigosaponin G, H, and I, orchioside B, and orcinol glucoside. They were identified in the mother plant organs as well as in the callus and plantlet leaves. Similar compound types in plant organs and in vitro propagules might originate from similar biosynthetic pathways.
Figure 2 shows that several compounds identified in plantlet leaves had higher concentrations (peak area) than those in the callus and mother plant organs. The plantlets were cultured on MS basal medium with plant growth regulators. Plant callus as an alternative source of raw material can be induced to increase its metabolites content through several interventions, for example, by adding biotic or abiotic elicitors (Ferdausi et al., 2021), manipulating biosynthetic pathways (Inyai et al., 2021), or genetic engineering (Shi et al., 2021).
Differences were detected in the chromatogram pattern and tentative analysis of compounds in the callus, plantlet leaves, and plant organs. Concentration differences in various in vitro propagules had been reported in Bidens pilosa (Ramabulana et al., 2021), Cleome dendroides (de Castro et al., 2021), and Nelumbo nucifera (Deng et al., 2020). The gap in quinine concentration between Cinchona ledgeriana in vitro cells and plant organs (Ratnadewi et al., 2021) and variable substance composition in in vitro propagules and mother plant organs of Saraca asoca (Vignesh et al., 2022) have been stated. Those differences may be due to the absence of intercellular translocation in the cultured cells (Ratnadewi et al., 2021), the types of plant growth regulators used versus the natural hormones (Babich et al., 2021), and the employment of elicitors (Rady et al., 2021).
Untargeted metabolomic analysis is a comprehensive assessment to get an overall picture of metabolites and to systematically identify and quantify any essential metabolites from a biological sample (Zhang et al., 2011). Compounds found in several other plant species were also identified in the CCL, including pelargonidin 3-O-(6-O-malonyl-beta-D-glucoside), which belongs to anthocyanin of Kadsura coccinea (Huang et al., 2021); sarmentosumin D in Piper sarmentosum, used to treat vascular endothelial dysfunction (Md. Salleh et al., 2021); ecdysterone, a steroid found in Achyranthes bidentata, used to treat various degenerative diseases (Dinan et al., 2021); salidroside in Rhodiola rosea, used as a neuroprotective (Zhu et al., 2021). In C. latifolia plantlet leaves, we found some prunins, while prunin from Prunus persica was used as an antiobesity agent (Li et al., 2021). In the oriental regions where herbal medication is commonly practiced, like in Indonesia, research on the composition and quantity of any suspected bioactive is encouraged to support local hereditary wisdom.
Due to the extensive information and data complexity, approaches with unsupervised methods, such as PCA and HCA, were used to identify the differences among samples (Ramabulana et al., 2021). PCA, with principal components 1 (PC1) and 2 (PC2), collectively accounted for 100% and 99.1% at the total variance’s positive and negative modes. This value showed excellent accuracy and differentiation among the samples (Lella et al., 2021), in this case among the callus, plantlet leaves, and the original organs (rhizome, petiole, and leaves). In this study, the HCA supports the clustering of the sample in the PCA.
A supervised PLS-DA evaluated differences in metabolite levels in in vitro propagules and mother plant organs. The important variables in interpreting the PLS-DA data are VIP. The basis for ranking the metabolites was their VIP scores; only top-ranking metabolites with the highest VIP scores were considered (Wang et al., 2021). Metabolites with VIP values > 1 are suspected of playing an essential role in differentiating the sample (Mashiane et al., 2021). The OPLS-DA model was used to determine the marker compounds among the samples studied (Mashiane et al., 2021). The marker compounds, orcinol glucoside, nyasicoside, and vanillin, were detected in this study; they have been reported to have an antioxidant capacity (Bai et al., 2021; Hoque et al., 2021; Wang et al., 2021). These compounds could be developed as a reliable chemical marker to distinguish the in vitro propagules from the mother plant organs of C. latifolia. Complex chemical components in plants are very difficult to evaluate; thereby, specificity is needed using one or several chemical markers (chemical reference substance). In addition, chemical markers are also used for quality control of traditional medicinal raw materials (Wang et al., 2022).
CONCLUSION
Phenolic groups generally dominated the secondary metabolites in C. latifolia. Rhizome had the highest TPC and antioxidant activity (DPPH and ABTS), followed by the plantlet leaves and callus. However, FRAP analysis indicated that there were substances other than phenolics that function as antioxidants too. In vitro propagules have great potential as sources of antioxidants. This information would be valuable for herbalists or herbal medicine producers in using the plant organs as well as the in vitro callus. Orcinol glucoside, nyasicoside, and vanillin are reliable marker compounds in discriminating the in vitro propagules and the mother plant organs of C. latifolia.
AUTHOR CONTRIBUTIONS
Abdul Halim Umar, Diah Ratnadewi, and Mohamad Rafi: Conceptualization, Methodology; Abdul Halim Umar: Collect plant samples; Abdul Halim Umar, Diah Ratnadewi, and Mohamad Rafi: Software, Validation, Data curation, Formal analysis; Abdul Halim Umar: Writing-original draft preparation; Abdul Halim Umar, Diah Ratnadewi, Mohamad Rafi, Yohana Caecilia Sulistyaningsih, and Hamim Hamim: Writing-Reviewing and Editing. All authors have read and agreed to the published manuscript.
FINANCIAL SUPPORT
The authors gratefully acknowledged the Directorate of Higher Education, the Ministry of Research and Higher Education the Republic of Indonesia for funding this research, which is part of the scholarship (BPP-DN Scheme 2017–2020) granted to Abdul Halim Umar.
CONFLICT OF INTEREST
No potential conflicts of interest were reported by the authors.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Babich O, Sukhikh S, Pungin A, Astahova L, Chupakhin E, Belova D, Prosekov A, Ivanova S. Evaluation of the conditions for the cultivation of callus cultures of Hyssopus officinalis regarding the yield of polyphenolic compounds. Plants, 2021; 10:915. CrossRef
Bai Y, Jian J, Liu D, Zhao X. Synthesis, characterization and application of a new biomass-based antioxidant derived from vanillin and methyl ethyl ketone. J Clean Prod, 2021; 316:128315. CrossRef
Baschieri A, Amorati R. Methods to determine chain-breaking antioxidant activity of nanomaterials beyond DPPH: a review. Antioxidants, 2021; 10:1551. CrossRef
Bertol G, Cobre AF, Pontarolo R. Differentiation of Mikania glomerata and Mikania laevigata species through mid-infrared spectroscopy and chemometrics guided by HPLC-DAD analyses. Rev Bras Farmacogn, 2021; 31:442–52. CrossRef
Butkeviciute A, Petrikaite V, Jurgaityte V, Liaudanskas M, Janulis V. Antioxidant, anti-inflammatory, and cytotoxic activity of extracts from some commercial apple cultivars in two colorectal and glioblastoma human cell lines. Antioxidants, 2021; 10:1098. CrossRef
Cabañas-García E, Areche C, Gómez-Aguirre YA, Borquez J, Muñoz R, Cruz-Sosa F, Balch EPM. Biomass production and secondary metabolite identification in callus cultures of Coryphantha macromeris (Engelm.) Britton & Rose (Cactaceae), a traditional medicinal plant. S Afr J Bot, 2021; 137:1–9; doi:10.1016/j.sajb.2020.10.002 CrossRef
Chong J, Wishart DS, Xia J. Using MetaboAnalyst 4.0 for comprehensive and integrative metabolomics data analysis. Curr Protoc Bioinformatics, 2019; 68:e86. CrossRef
de Castro TC, Simões-Gurgel C, Gayer CR, Coelho MGP, Albarello N. Micropropagation of Cleome dendroides (Cleomaceae), an endemic Brazilian species, as a source of glucosinolates. Plant Biosyst, 2021; 155:281–90. CrossRef
Deng X, Xiong Y, Li J, Yang D, Liu J, Sun H, Song H, Wang Y, Ma J, Liu Y, Yang M. The establishment of an efficient callus induction system for lotus (Nelumbo nucifera). Plants, 2020; 9:1436. CrossRef
Denizli N, Horo I, Gülcemal D, Masullo M, Festa M, Capasso A, Koz Ö, Piacente S, Alanku?-Çal??kan Ö. Cycloartane glycosides from Astragalus plumosus var. krugianus and evaluation of their antioxidant potential. Fitoterapia, 2014; 92:211–8. CrossRef
Dinan L, Dioh W, Veillet S, Lafont R. 20-Hydroxyecdysone, from plant extracts to clinical use: therapeutic potential for the treatment of neuromuscular, cardio-metabolic and respiratory diseases. Biomedicines, 2021; 9:492. CrossRef
Farzinebrahimi R, Mat Taha R, Rashid KA, Ali Ahmed B, Danaee M, Rozali SE. Preliminary screening of antioxidant and antibacterial activities and establishment of an efficient callus induction in Curculigo latifolia Dryand (Lemba). Evid Based Complement Alternat Med, 2016; 2016:e6429652. CrossRef
Ferdausi A, Chang X, Jones M. Enhancement of galanthamine production through elicitation and NMR-based metabolite profiling in Narcissus pseudonarcissus cv. Carlton in vitro callus cultures. In Vitro Cell Dev Biol-Plant, 2021; 57:435–46. CrossRef
Han J, Wan M, Ma Z, Hu C, Yi H. Prediction of targets of Curculigoside A in osteoporosis and rheumatoid arthritis using network pharmacology and experimental verification. Drug Des Devel Ther, 2020; 14:5235–50. CrossRef
Hashim AM, Alharbi BM, Abdulmajeed AM, Elkelish A, Hozzein WN, Hassan HM. Oxidative stress responses of some endemic plants to high altitudes by intensifying antioxidants and secondary metabolites content. Plants, 2020; 9:869. CrossRef
Hejazi II, Khanam R, Mehdi SH, Bhat AR, Rizvi MMA, Thakur SC, Athar F. Antioxidative and anti-proliferative potential of Curculigo orchioides Gaertn in oxidative stress induced cytotoxicity: in vivo, ex vivo and in silico studies. Food Chem Toxicol, 2018; 115:244–59. CrossRef
Hoque MdA, Ahmad S, Chakrabarty N, Khan MF, Hafez Kabir MS, Brishti A, Raihan MdO, Alam AHMK, Haque Md. Anwarul, Nasrin MstS, Haque Md. Areeful, Reza ASMA. Antioxidative role of palm grass rhizome ameliorates anxiety and depression in experimental rodents and computer-aided model. Heliyo, 2021; 7:e08199. CrossRef
Huang D, Ming R, Yao S, Li L, Huang R, Tan Y. Identification of anthocyanins in the fruits of Kadsura coccinea using UPLC-MS/MS-based metabolomics. Biochem Syst Ecol, 2021; 98:104324. CrossRef
Inyai C, Yusakul G, Komaikul J, Kitisripanya T, Likhitwitayawuid K, Sritularak B, Putalun W. Improvement of stilbene production by mulberry Morus alba root culture via precursor feeding and co-elicitation. Bioprocess Biosyst Eng, 2021; 44:653–60. CrossRef
Jagie??o-Kubiec K, Nowakowska K, ?ukaszewska AJ, Pacholczak A. Morpho-anatomical and biochemical changes associated with rooting of micropropagated ninebark cuttings. Plant Cell Tiss Organ Cult, 2021; 147:229–37. CrossRef
Kim WS, Seo JH, Lee JI, Ko ES, Cho SM, Kang JR, Jeong JH, Jeong YJ, Kim CY, Cha JD, Ryu YB. The metabolite profile in culture supernatant of Aster yomena callus and its anti-photoaging effect in skin cells exposed to UVB. Plants, 2021; 10:659. CrossRef
Kumar D, Punetha A, Verma PPS, Padalia RC. Micronutrient based approach to increase yield and quality of essential oil in aromatic crops. J Appl Res Med Aromat Plants, 2022; 26:100361. CrossRef
Kushalan S, Yathisha UG, Khyahrii SA, Hegde S. Phytochemical and anti-oxidant evaluation of in vitro and in vivo propagated plants of Curculigo orchioides. In Vitro Cell Dev Biol-Plant, 2022; 58:382–91. CrossRef
Lee JH, Cho YS. Assessment of phenolic profiles from various organs in different species of perilla plant (Perilla frutescens (L.) Britt.) and their antioxidant and enzyme inhibitory potential. Ind Crops Prod, 2021; 171:113914. CrossRef
Lella SD, Porta NL, Tognetti R, Lombardi F, Nardin T, Larcher R. White rot fungal impact on the evolution of simple phenols during decay of silver fir wood by UHPLC-HQOMS. Phytochem Anal, 2021; 33:170–83. CrossRef
Li L, Li S, Cui Z, Wang Y, Li Y, Kong L, Luo J. Improving sesquiterpenoids production of Sarcandra glabra callus culture. Ind Crops Prod, 2021; 169:113636. CrossRef
Li N, Li SP, Wang KJ, Yan GQ, Zhu YY. Novel norlignan glucosides from rhizomes of Curculigo sinensis. Carbohydr Res, 2012; 351:64–67. CrossRef
López-Ramírez Y, Cabañas-García E, Areche C, Trejo-Tapia G, Pérez-Molphe-Balch E, Gómez-Aguirre YA. Callus induction and phytochemical profiling of Yucca carnerosana (Trel.) McKelvey obtained from in vitro cultures. Rev Mex Ing Quim, 2021; 20:823–37. CrossRef
Mashiane P, Manhivi VE, Shoko T, Slabbert RM, Sultanbawa Y, Sivakumar D. Cooking african pumpkin leaves (Momordica balsamina L.) by stir-frying improved bioactivity and bioaccessibility of metabolites-metabolomic and chemometric approaches. Foods, 2021; 10:2890. CrossRef
Md. Salleh MFRR, Aminuddin A, Hamid AA, Salamt N, Japar Sidik FZ, Ugusman A. Piper sarmentosum Roxb. attenuates vascular endothelial dysfunction in nicotine-induced rats. Front Pharmacol, 2021; 12:667102. CrossRef
Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant, 1962; 15:473–97. CrossRef
Okubo S, Terauchi K, Okada S, Saito Y, Yamaura T, Misaka T, Nakajima K, Abe K, Asakura T. De novo transcriptome analysis and comparative expression profiling of genes associated with the taste-modifying protein neoculin in Curculigo latifolia and Curculigo capitulata fruits. BMC Genom, 2021; 22:347. CrossRef
R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, 2018.
Rady MR, Gierczik K, Ibrahem MM, Matter MA, Galiba G. Anticancer compounds production in Catharanthus roseus by methyl jasmonate and UV-B elicitation. S Afr J Bot, 2021; 142:34–41. CrossRef
Ramabulana AT, Steenkamp PA, Madala NE, Dubery IA. Application of plant growth regulators modulates the profile of chlorogenic acids in cultured Bidens pilosa cells. Plants, 2021; 10:437. CrossRef
Ratnadewi D, Fendiyanto MH, Satrio RD, Miftahudin M, Laily AN. Strictosidine synthase coding gene expression towards quinine biosynthesis and accumulation: inconsistency in cultured cells and fresh tissues of Cinchona ledgeriana. Int J Agric Biol, 2021; 26:131–8. CrossRef
Shi M, Liao P, Nile SH, Georgiev MI, Kai G. Biotechnological exploration of transformed root culture for value-added products. Trends Biotechnol, 2021; 39:137–49. CrossRef
Silalahi M, Nisyawati N. The ethnobotanical study of edible and medicinal plants in the home garden of Batak Karo sub-ethnic in North Sumatra, Indonesia. Biodiversitas, 2018; 19:229–38. CrossRef
Sobeh M, Mamadalieva NZ, Mohamed T, Krstin S, Youssef FS, Ashour ML, Azimova SS, Wink M. Chemical profiling of Phlomis thapsoides (Lamiaceae) and in vitro testing of its biological activities. Med Chem Res, 2016; 25:2304–15. CrossRef
Trinh NTN, Tuan NN, Thang TD, Kuo PC, Thanh NB, Tam LN, Tuoi LH, Nguyen THD, Vu DC, Ho TL, Anh LN, Thuy NTT. Chemical composition analysis and antioxidant activity of Coffea robusta monofloral honeys from Vietnam. Foods, 2022; 11:388. CrossRef
Tsugawa H, Cajka T, Kind T, Ma Y, Higgins B, Ikeda K, Kanazawa M, Vander Gheynst J, Fiehn O, Arita M. MS-DIAL: data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat Methods, 2015; 12:523–6. CrossRef
Umar AH, Ratnadewi D, Rafi M, Sulistyaningsih YC. Untargeted metabolomics analysis using FTIR and UHPLC-Q-Orbitrap HRMS of two Curculigo species and evaluation of their antioxidant and α-glucosidase inhibitory activities. Metabolites, 2021a; 11:42. CrossRef
Umar AH, Ratnadewi D, Rafi M, Sulistyaningsih YC, Hamim H. Callus of Curculigo latifolia Dryand. ex W.T.Aiton: initiation, regeneration, secretory structure and histochemistry. IOP Conf Ser Earth Environ Sci, 2021b; 948:012051. CrossRef
Vignesh A, Selvakumar S, Vasanth K. Comparative LC-MS analysis of bioactive compounds, antioxidants and antibacterial activity from leaf and callus extracts of Saraca asoca. Phytomed Plus, 2022; 2:100167. CrossRef
Wang R, Qin Y, Zhou J, Wang J, Shu H, Zhou S, Peng X. Comprehensive evaluation of Bletilla striata and its substitutes by combining phenotypic characteristic, chemical composition, and anti-melanogenic activity. Phytochemistry, 2022; 195:113059. CrossRef
Wang Y, Li J, Li N. Phytochemistry and pharmacological activity of plants of genus Curculigo: an updated review since 2013. Molecules, 2021; 26:3396. CrossRef
Xu YB, Chen GL, Guo MQ. Antioxidant and anti-inflammatory activities of the crude extracts of Moringa oleifera from Kenya and their correlations with flavonoids. Antioxidants, 2019; 8:296. CrossRef
Yaermaimaiti S, Wu T, Aisa HA. Bioassay-guided isolation of antioxidant, antimicrobial, and antiviral constituents of Cordia dichotoma fruits. Ind Crops Prod, 2021; 172:113977. CrossRef
Yeshi K, Crayn D, Ritmejeryt? E, Wangchuk P. Plant secondary metabolites produced in response to abiotic stresses has potential application in pharmaceutical product development. Molecules, 2022; 27:313.
Zhang A, Sun H, Wang P, Han Y, Wang X. Modern analytical techniques in metabolomics analysis. Analyst, 2011; 137:293–300. CrossRef
Zhang Y, Ge JF, Wang FF, Liu F, Shi C, Li N. Crassifoside H improve the depressive-like behavior of rats under chronic unpredictable mild stress: possible involved mechanisms. Brain Res Bull, 2017; 135:77–84. CrossRef
Zhao Y, Guo Y, Chen Y, Liu S, Wu N, Jia D. Curculigoside attenuates myocardial ischemia?reperfusion injury by inhibiting the opening of the mitochondrial permeability transition pore. Int J Mol Med, 2020; 45:1514–24. CrossRef
Zhu L, Liu Z, Ren Y, Wu X, Liu Y, Wang T, Li Y, Cong Y, Guo Y. Neuroprotective effects of salidroside on ageing hippocampal neurons and naturally ageing mice via the PI3K/Akt/TERT pathway. Phytother Res, 2021; 35:5767–80. CrossRef
Zhu FB, Wang JY, Zhang YL, Quan RF, Yue ZS, Zeng LR, Zheng WJ, Hou Q, Yan SG, Hu YG. Curculigoside regulates proliferation, differentiation, and pro-inflammatory cytokines levels in dexamethasone-induced rat calvarial osteoblasts. Int J Clin Exp Med, 2015a; 8:12337–46.
Zhu MZ, Wu W, Jiao LL, Yang PF, Guo MQ. Analysis of flavonoids in lotus (Nelumbo nucifera) leaves and their antioxidant activity using macroporous resin chromatography coupled with LC-MS/MS and antioxidant biochemical assays. Molecules, 2015b; 20:10553–65. CrossRef
SUPPLEMENTARY MATERIAL
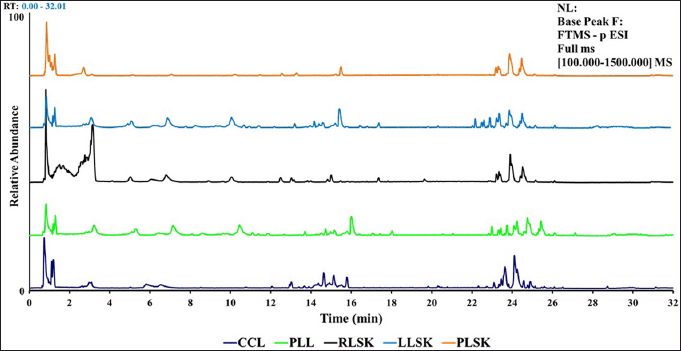 | Figure S1. UHPLC-Q-Orbitrap HRMS chromatogram of 70% ethanol extract from CCL, PLL, RLSK, LLSK, and PLSK of C. latifolia. [Click here to view] |
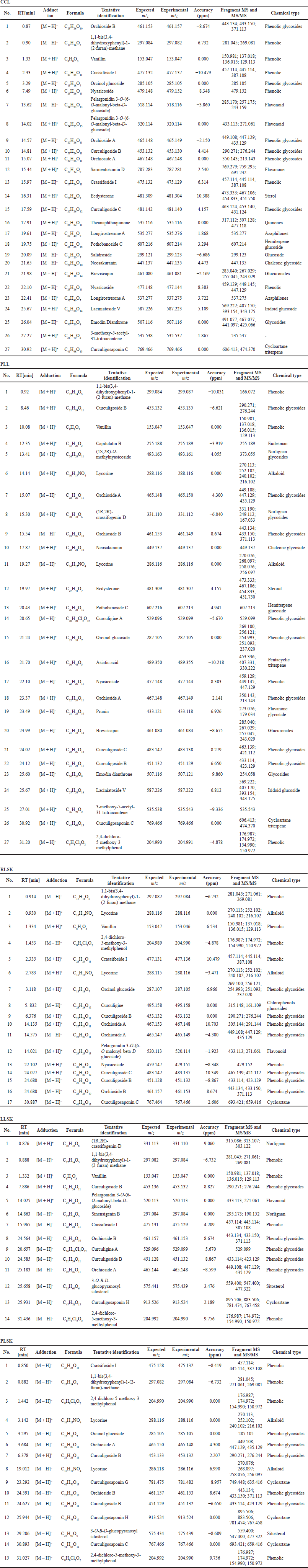 | Table S1. Compounds identified in 70% ethanol extract from CCL, PLL, RLSK, LLSK, and PLSK of C. latifolia. [Click here to view] |
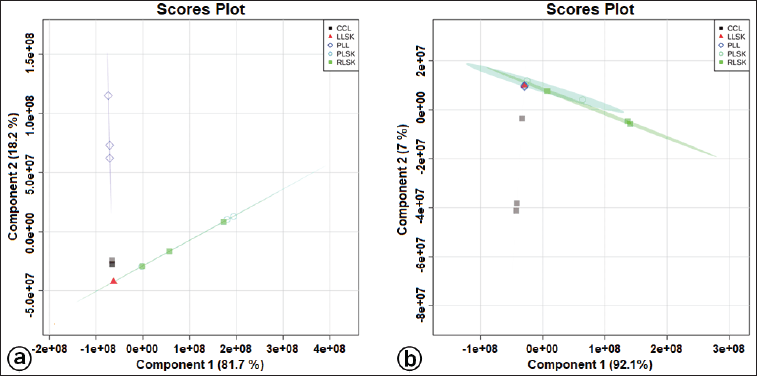 | Figure S2. Supervised PLS-DA score plot showing separation of clusters for in vitro propagules and mother plant organs based on bioactive metabolites at positive (a) and negative (b) modes generated by the UHPLC-Q-Orbitrap HRMS analysis. [Click here to view] |
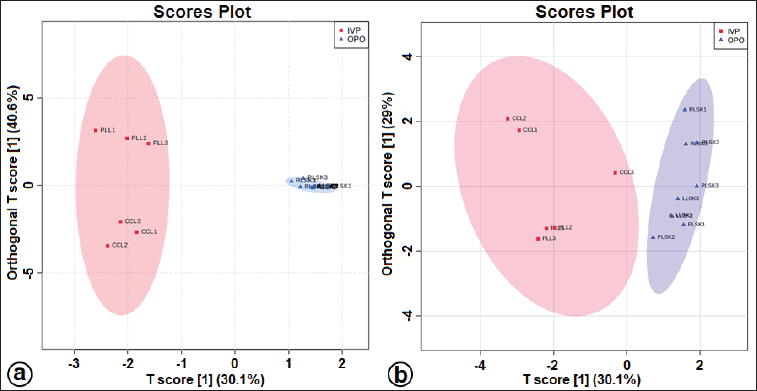 | Figure S3. The OPLS-DA score plot at positive (a) and negative (b) modes for separating in vitro propagules and mother plant organs of C. latifolia. [Click here to view] |
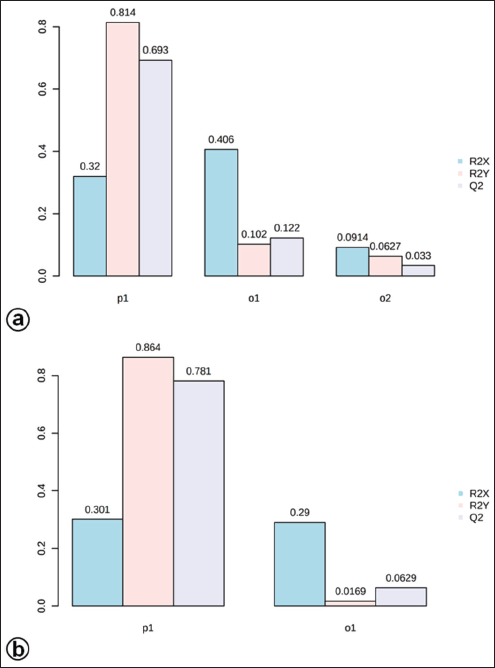 | Figure S4. The OPLS-DA model shows the level of goodness-of-fit (R2) and predictability level (Q2) at positive (a) and negative (b) modes. [Click here to view] |