INTRODUCTION
Gastric carcinoma is an aggressive malignancy, the most common cancer worldwide and the leading cause of cancer mortality (Olnes and Martinson, 2021; Rawla and Barsouk, 2019). Gastric cancer is also known as stomach cancer. The mucous membrane lining the stomach is made up of columnar epithelial cells and glands. These cells are prone to gastritis, an inflammation that can progress to peptic ulcers and eventually stomach cancer (Rawla and Barsouk, 2019). Various risk factors which contribute to gastric cancer include Helicobacter pylori infection (Ahmed, 2005; Biagioni et al., 2019), genetics (Boland and Yurgelun, 2017), intake of salty food (Biagioni et al., 2019; Tsugane and Sasazuki, 2007), alcohol consumption (Biagioni et al., 2019; Ma et al., 2017), tobacco smoking (Sadjadi et al., 2014), gastroesophagal reflux disease (Wu et al., 2003; Ye et al., 2001), chemical exposure (Chang et al., 2018), radiation (Ali et al., 2018), pernicious anemia (Kuipers, 2015), gastric surgery (Ahn et al., 2008; Komatsu et al., 2012), obesity, and other environmental factors (Lin et al., 2014). However, H. pylori infection is more closely associated with gastric cancer and related host gene polymorphism (He et al., 2013; Herrera and Personnet, 2009).
Classification of gastric cancer
Gastric cancer is a complicated, complex, and heterogeneous disease with various characteristics. The accessible diversity of histological classification systems is due to this variability. The Laurén, WHO, and Goseki classification systems are the most generally used. According to the Laurén classification, there are four forms of gastric adenocarcinomas as diffuse, intestinal, indeterminate, and mixed (Laurén, 1965). The WHO classification is based on the appearance of histomorphology. Gastric cancer is classified as tubular, papillary, mucinous, weakly cohesive (including signet ring cell carcinoma), or mixed carcinomas by the World Health Organization (Berlth et al., 2014; Nagtegaal et al., 2020). The Goseki classification divides gastric cancer into four types based on intracellular mucin production and tubular differentiation. Tubules that are well differentiated but lacking in intracellular mucin are classified as Group I; tubules that are well differentiated but rich in intracellular mucin are classified as Group II; tubules that are poorly differentiated but lacking in intracellular mucin are classified as Group III; tubules that are poorly differentiated but rich in intracellular mucin are classified as Group IV (Fontana et al., 2003; Goseki et al., 1992; Martin et al., 1994; Nagtegaal et al., 2020).
Gastric cancer most likely begins with chronic active gastritis, which progresses through atrophic gastritis, intestinal metaplasia, and dysplasia, ultimately causing stomach cancer. Gastric cancer is attributed to H. pylori infection and host gene polymorphism in addition to environmental, dietary, and genetic variables (Fig. 1). Infection with H. pylori has been associated with the development of chronic active gastritis, leading to adenocarcinoma (Xiao et al., 2017).
Therapeutic aspects of gastric cancer
Gastric cancer treatment requires a multidisciplinary approach for optimal therapy (Fabian and Michael, 2019). Common treatment approaches include surgery, radiation, targeted therapies, immunotherapy, chemotherapy, etc. (Jun et al., 2021; Robert et al., 2018). Surgical resection is one of the curative modalities for localized gastric cancer. However, survival is poor with surgery alone, perioperative chemotherapy or postoperative chemoradiotherapy is required to improve the outcomes of surgical treatment (Charalampakis et al., 2018). Radiation therapy utilized high-intensity rays for killing the cancerous cell in a specific part of the body (Baskar et al, 2012). In cases of unresectable stomach cancer with anemia and/or pyloric blockage, radiotherapy is indicated. Although the effect is often brief (3–6 months), it is a simple therapeutic approach (Asakura et al., 2011; Tey et al., 2007). Multimodal therapy for gastric cancer also includes targeted therapy in the form of small molecule inhibitors and monoclonal antibodies (Fabian and Michael, 2019). It includes drugs that target human epidermal growth factor receptor, e.g., trastuzumab (Gravalos and Jimeno, 2008), drugs that target vascular endothelial growth factor receptor, e.g., ramucirumab (Fuchs et al., 2014). Immunotherapy involves the utilization of drugs for helping a person’s immune system to locate and inhibit cancerous cells significantly (Fabian and Michael, 2019; Jun et al., 2021; Kang et al., 2017; Mellman et al, 2011). Currently, chemotherapy is the most common type of cancer treatment. Chemotherapy uses anticancer drugs that are injected into a vein or given by mouth as pills (Nygren et al, 2001). High doses of chemotherapy drugs, on the other hand, have some side effects because of their rapid release into the gastrointestinal tract. Oral anticancer drugs are associated with a variety of side effects, including systemic side effects, diarrhea, and gastrointestinal disturbances, such as nausea and vomiting (GIT). Therefore, a gastroretentive dosage form with a proper release profile can be considered a fantastic and impressive technology to overcome various side effects associated with conventional formulations (Ehsan et al., 2013).
Anticancer drugs for gastric cancer
For the treatment of gastric cancer, a variety of anticancer drugs are utilized, including docetaxel, doxorubicin hydrochloride, 5-fluorouracil, mitomycin, capecitabine, carboplatin, cisplatin, paclitaxel, irinotecan, oxaliplatin, etc. (Sastre et al, 2006). Chemotherapy is still the most effective treatment option for stomach cancer. However, traditional oral chemotherapy for gastric cancer suffers from significant limitations such as inadequate absorption, multiple doses, systemic toxicity, etc. (Fig. 2). All of which contribute to poor treatment outcomes. Also, oral chemotherapy may involve low or variable bioavailability, associated with premature drug release, limited drug solubility, and less retention period as well (Narvekar et al., 2014). As a result, novel drug delivery platforms must be designed for managing drug release while simultaneously extending their gastroretentive duration. Gastroretentive drug delivery system (GRDDS) has been successfully designed to retain the dosage form localized precisely in the stomach and extend the dosing interval for stomach cancer (Nana et al., 2019). The gastroretentive floating drug delivery system is particularly well suited to overcome the pharmacokinetic challenges of oral chemotherapy (Mandal et al., 2016). Fabrication of a gastroretentive floating system is also needed to address several issues associated with conventional dosage forms and to maximize oral absorption of various therapeutic molecules with prolonged stomach residence duration and regulated drug delivery (Kotreka and Moji, 2011). Owing to its prolonged gastric residence time (GRT) and controlled drug release input, gastroretentive devices aid in attaining tailored drug release (Verma et al., 2016). The above-described systems can also be utilized to improve the efficacy of anticancer drugs like 5-fluorouracil, with a narrow absorption window (Malik et al., 2015).
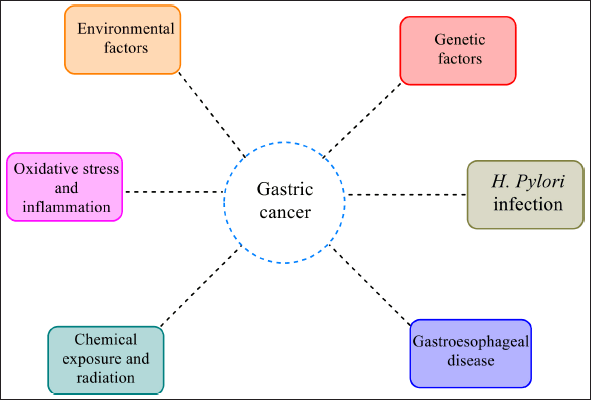 | Figure 1. Factors influencing gastric cancer. [Click here to view] |
Gastroretentive drug delivery system
The oral route is the most preferred route of administration due to its various important advantages such as administration convenience, high patient compliance, cost-effectiveness, noninvasiveness, etc. (Lee and Mukherjee, 2002; Lopes et al., 2016; Narayana et al., 2010; Rai et al., 2018; Shahiwala, 2011). Nevertheless, owing to the heterogeneity of the gastrointestinal system, the oral drug delivery system faces various limitations such as low bioavailability, gastric retention time variabilities, etc. (Lopes et al., 2016). Various modern technological developments have also resulted in many novel pharmaceutical aspects, especially controlled release systems for solving these constraints (El Nabarawi et al., 2017). Oral controlled release dosage forms have been broadly used for enhancing bioavailability. However, oral controlled systems may also not be able for retaining dosage forms in the stomach for a prolonged time period along with less localization in the desired region of the gastrointestinal tract (Patil et al., 2006). Therefore, to keep the dosage form in the stomach for the required time, GRDDS are being proposed by scientists in recent years (Awasthi et al., 2014; Das et al., 2020). GRDDS is a novel and advanced approach that not only displays the spatial placement of dosage form retained in the stomach but also offers temporal placement by releasing the drug in a controlled and prolonged manner (Mirani et al., 2016). These systems provide several impressive and pivotal advantages, including extended GRT of dosage forms in the stomach for several hours, improved therapeutic effects of drugs through enhanced drug absorption, and suitable for specific delivery in the gastric region. Furthermore, GRDDS can improve the controlled drug delivery by constantly releasing the drug at the desired rate and at a specific absorption site for an extended period of time until the drug has been released entirely from the dosage form (Pawar et al., 2011; Prajapati et al., 2013; Rouge et al., 1996). Prolonged residence time is highly suitable for drugs that have a narrow window of absorption in the GIT or which are unstable in the colonic and intestine environment or have low solubility in the alkaline pH (Arora et al., 2005; Pahwa et al., 2012b; Shah et al., 2017). A gastroretentive floating system provides feasibility for site-specific delivery; also offers a reduction in a number of doses in the regimen, along with enhanced bioavailability as compared to conventional dosage forms (Chen et al., 2018). Various commercialized formulations based on gastroretentive technology are depicted in Table 1 (Mandal et al., 2016; Pawar et al., 2012).
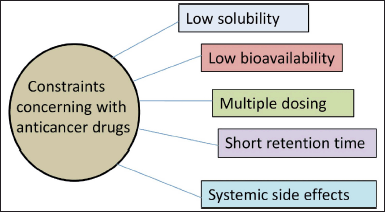 | Figure 2. Numerous constraints concerning with anticancer drugs. [Click here to view] |
Salient advantages
Following advantages of GRDDS are mentioned in the subsequent portion (Krishana et al., 2021; Kumar and Philip, 2007; Pahwa et al., 2011; Pahwa et al., 2012a, 2013; Pahwa et al., 2021). These are evidently portrayed in Figure 3.
- Increased bioavailability of drugs with a narrow window of absorption in the upper gastrointestinal tract.
- Increased patient comfort and compliance as a result of low dose frequency.
- Increased dosage retention time in the stomach.
- Gastric discomfort can be avoided by using a sustained release profile.
- Drug release is consistent, with no possibility of dumping of dose.
- Less inter and intra-subject variability.
- Site specificity.
- Maximum utilization of drugs with minimum side effects.
Drawbacks aspects
However, few limitations associated with the gastroretentive system include drugs with limited solubility and stability in stomach/acidic medium that are not suitable for gastroretentive system. Also, drugs that causes irritation in the stomach cannot be developed into gastroretentive formulations (Adibkia et al., 2011; Kumar and Kaushik, 2018).
The main approaches for gastric retention that have been examined include the floating system (Chen et al., 2018; Mohapatra et al., 2020; Vasvari et al., 2019), expandable system (Zhao et al., 2014), bioadhesive system (Ishak, 2015; Pawar et al., 2012; Raviteja et al., 2014; Simons and Wagner, 2019), high-density system (Du et al., 2019; Prajapati et al., 2011) superporous hydrogels (Bardonnet et al., 2006; Mirani et al., 2016), magnetic system (Bardonnet et al., 2006; Melocchi et al., 2019), dual functioning system (Singh and Kim et al., 2000; Tripathi et al., 2019), etc. A floating drug delivery system helps to maintain the prolonged duration of action of drug by enhancing buoyancy over gastrointestinal contents. It aids in reducing the dosage frequency and ultimately improves patient compliance (Arora et al., 2005; Mohapatra et al., 2020). Also, expandable dosage forms withstand stomach transit because of their bigger size than the pyloric spincture diameter (Neumann et al., 2021). Additionally, bioadhesive systems are employed to localize and adhere the delivery device within the lumen to improve absorption of the drug in site-specific manner and extend GRT (Ishak, 2015). Furthermore, high-density system utilizes their weight for gastric retention mechanism. Superporous hydrogel systems with an average pore size > 100 μm, swell to a large size and withstand the pressure of gastric contraction resulting in gastric retention (Hejaji and Amiji, 2002). In a magnetic system, gastrointestinal transit of dosage form can be controlled by using magnet (Melocchi et al., 2019). Dual functioning system based on the combination of bioadhesion and floating principle provide enhanced bioavailability profile (Singh and Kim et al., 2000). Mechanistic insight for floating system as low-density dosage form which remain buoyant in the gastric fluid for enhanced time period includes effervescent and non-effervescent approaches. Effervescent system employs matrices developed using swellable polymeric materials such as polysaccharides, methocel, etc. along with effervescent components. The system is designed in such a manner that, when it comes into contact with gastric acid, carbon dioxide is generated as a result of an effervescent reaction between organic acid and carbonate-bicarbonate salt. The buoyancy of the dosage form is ultimately maintained by the gas which entrapped inside the gellified hydrocolloids. Non-effervescent systems, comprises gel-forming, swellable, cellulosic hydrocolloids, matrix forming polymers or polysaccharides. Alginate beads, porous systems, hollow microspheres, and hydrodynamically balanced systems are examples of non-effervescent systems that can be developed using gel forming swellable polymers (Pahwa et al., 2012b, 2013). Various research findings on anticancer drug based gastroretentive dosage forms have been reported. Table 2 summarizes various recent investigational studies of anticancer gastroretentive dosage forms.
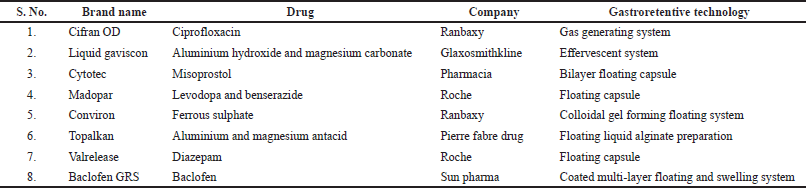 | Table 1. List of commercialized gastroretentive formulations. [Click here to view] |
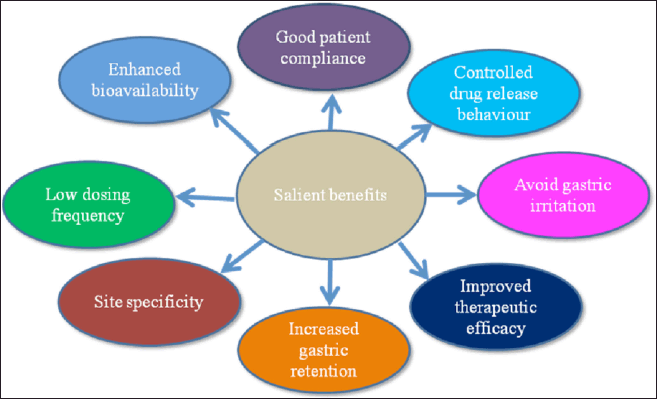 | Figure 3. Salient benefits of GRDDS. [Click here to view] |
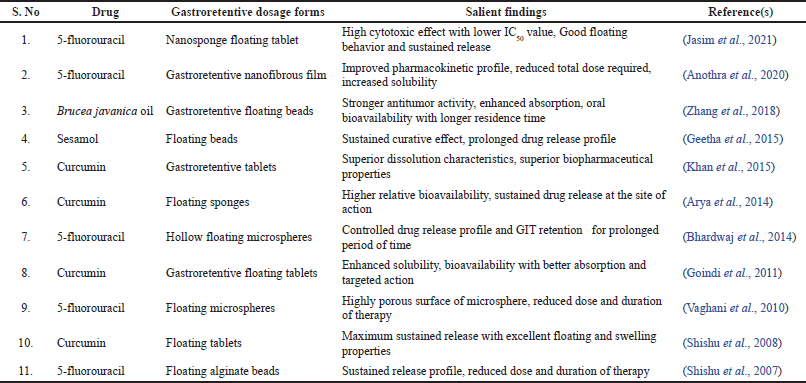 | Table 2. Gastroretentive dosage forms in the management of gastric cancer. [Click here to view] |
 | Table 3. Some recent patents based on gastroretentive technology. [Click here to view] |
Therefore, substantial and continual research efforts have been done globally toward designing innovative gastroretentive delivery system of anticancer drugs. Targeting gastric cancer using gastroretentive dosage forms appears to represent a promising option with site-specificity and unique drug release kinetics for achieving newer insights as per therapeutic needs. Several recent patents on gastroretentive technology are presented in Table 3.
Future perspectives
Various research investigational studies on gastroretentive technologies have been conducted so far using single technological approaches like floating, expandable, mucoadhesive systems, etc. However, utilizing sophisticated combinatorial strategies along with versatile modifications in polymeric materials may be effective in reducing gastric retention time variability. Currently, there is an urgent need to design an array of advanced gastroretentive dosage forms of anticancer drugs having a narrow absorption window in the GIT according to the clinical requirements. Significant research advancements are needed to achieve expected gastric retention with anticancer drugs. Future work needs to focus on gastroretentive dosage form of anticancer drugs to be retained specifically in the GIT for prolonged period of time. Moreover, using a quality by design (QbD) can also be helpful in determining the impact of formulation as well as processing factors on quality characteristics of effective gastroretentive systems. Use of QbD techniques in pharmaceutical field may be helpful in understanding and control of the manufacturing process, lowering the possibility of product failure significantly. Future directions in gastroretentive technology of anticancer drugs may need to focus on increased and controlled swelling qualities of therapeutically accessible dosage forms and optimizing buoyant behavior. Additionally, plethora of research and more intensive investigation should be developed for effective recognition of gastroretentive technology of anticancer drugs in the successful management of gastric cancer and further avoiding any side effects.
CONCLUSION
Gastric cancer is a malignancy that has a poor prognosis. Classification and factors affecting this multifactorial disease have been elucidated in the present paper. Eradication of H. pylori, adopting a healthy lifestyle along with precise therapeutic interventions utilizing anticancer gastroretentive dosage forms play crucial role in the management of gastric cancer with promising outcomes. Gastroretentive dosage form is a significant strategy for retaining dosage in the GIT for prolonged time period and also recognized as impressive and rational technological consideration. Substantial advancements in retention of the dosage form in the gastrointestinal region with this targeted drug delivery highlight the immense relevance of gastroretentive systems of anticancer drugs. The present article provides an overview of gastric cancer and several unique therapeutic approaches employing gastroretentive delivery. Enormous potential of encouraging gastroretentive technologies for the management of gastric cancer and for obtaining maximum therapeutic benefits from gastroretentive dosage forms of anticancer drugs are also highlighted in this paper.
CONFLICT OF INTEREST
The authors have no financial or other conflicts of interest to disclose.
FUNDING
There is no funding to report.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Adibkia K, Hamedeyazadan S, Javadzadeh Y. Drug release kinetics and physicochemical characteristics of floating drug delivery system. Expert Opin Drug Deliv, 2011; 8(7):891–903.
Ahmed N. A 23 years of the discovery of Helicobacter pylori: is the debate over? Ann Clin Microbiol Antimicrob, 2005; 17:1–3.
Ahn HS, Kim JW, Yoo MW, Park DJ, Lee HJ, Lee KU, Yang HK. Clinicopathological features and surgical outcomes of patients with remnant gastric cancer after a distal gastrectomy. Ann Surg Oncol, 2008; 15(6):1632–9.
Ali RY, Kamran BL, Peivand B, Maryam R Zahra K. Risk factors for gastric cancer: a systemic review. Asian Pac J Cancer Prev, 2018; 19(3):591–603.
Anothra P, Pradhan D, Naik PK, Ghosh G, Rath G. Development and characterization of 5-fluorouracil nanofibrous film for the treatment of stomach cancer. J Drug Deliv Sci Technol, 2020; 61:1–26.
Arora S, Ali J, Ahuja A, Khar Roop K, Baboota S. Floating drug delivery systems: a review. AAPS Pharm Sci Tech, 2005; 6(3):372–90.
Arya P, Pathak K. Assessing the viability of microsponges as gastro retentive drug delivery system of curcumin optimization and pharmacokinetics. Int J Pharm, 2014; 460:1–2.
Asakura H, Hashimoto T, Harada H, Mizumoto M, Furutani K, Hasuike N, Matsuoka M, Ono H, Boku N, Nishimura T. Palliative radiotherapy for bleeding from advanced gastric cancer: is a schedule of 30 Gy in 10 fractions adequate? J Cancer Res Clin Oncol, 2011; 137(1):125–30.
Awasthi R, Kulkarni GT. Decades of research in drug targeting to the upper gastrointestinal tract using gastroretention technologies: where do we stand. Drug Deliv, 2014; 23(2):1–17.
Bardonnet PL, Faivre V, Pugh WJ, Piffaretti JC, Falson F. Gastroretentive dosage forms: overview and special case of Helicobacter pylori. J Control Release, 2006;111(1–2):1–18.
Baskar R, Lee KA, Yeo R, Yeoh KW. Cancer and radiation therapy: current advances and future directions. Int J Med Sci, 2012; 9(3):193–9.
Berlth F, Bollschweiler E, Drebber U, Hoelscher AH, Moenig S. Pathohistological classification systems in gastric cancer: diagnostic relevance and prognostic value. World J Gastroenterol, 2014; 20(19):5679–84.
Bhardwaj P, Chaurasia D, Singh R, Swarup A. Development and characterization of novel site specific hollow floating microspheres bearing 5-Fu for stomach targeting. Sci World J, 2014:705259–70.
Biagioni A, Skalamera I, Peril S, Schiavone N, Cianchi F, Giommoni E, Magnelli L, Papucci L. Update on gastric cancer treatments and gene therapies. Cancer Metastasis Rev, 2019:537–48.
Boland CR, Yurgelun MB. Historical perspective on familial gastric cancer. Cell Mol Gastroenterol and Hepatol, 2017; 3(2):192–200
Chang CJ, Tu YK, Chen PC, Yang HY. Talc exposure and risk of stomach cancer: systematic review and meta-analysis of occupational cohort studies. J Formos Med Assoc, 2018; 119(4):781–92.
Charalampakis N, Economopoulou P, Kotsantis I, Tolia M, Schizas D, Liakakos T, Elimova E, Ajani JA, Psyrri A. Medical management of gastric cancer a 2017 update. Cancer Med, 2018; 7(1):123–33.
Chen R, Guo X, Liu X, Cui H, Wang R, Han J. Formulation and statistical optimization of gastric floating alginate/oil/chitosan capsules loading procyanidins: in vitro and in vivo evaluations. Int J Bio Macromol, 2018; 108:1082–91.
Das S, Kaur S, Rai KV. Gastroprotective drug delivery system: a recent update on clinical pertinence and drug delivery. Drug Deliv Transl Res, 2020, 1–28.
Du F, Wu Y, Du F, Zhang L, Feng W, Zhao L. Construction of catechol-grafted chitosan alginate/barium sulfate microcapsules for computed tomography real-time imaging and gastroretentive drug delivery. Int J Nanomedicine, 2019; 14:6001–18.
Ehsan TD, Mohamed IN, Ali K, Behnam K, Abdoreza SF, Hamid AJ. Preparation and characterization of a gastric floating dosage form of capecitabine. BioMed Res Int, 2013:1–8.
El Nabarawi MA, Teaima MH, EL-Monem RAA, El Nabarawi NA, Gaber DA. Formulation release characteristics and bioavailability study of gastroretentive floating matrix tablet and floating raft system of meheverine HCl. Drug Des Dev Ther, 2017; 11:1080–93.
Fabian MJ, Michael B. Updates on management of gastric cancer. Curr Oncol Rep, 2019; 21(8):1–9.
Fontana MG, La Pinta M, Moneghini D, Villanacci V, Donato F, Rindi G, Paparini S, Baronvhelli C, Bertoli G, Alquati P. Prognostic value of Goseki histological classification in adenocarcinoma of the cardia. Br J Cancer, 2003; 88(3):401–5.
Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. The Lancet, 2014; 383(9911):31–9.
Geetha T, Deol PK, Kaur IP. Role of sesamol-loaded floating beads in gastric cancers a pharmacokinetic and biochemical evidence. J Microencapsul, 2015; 32(5):478–87.
Goindi S., Mann K., Aggarwal N. Gastro-retentive floating beads of curcumin β cyclodextrin complex to treat stomach tumors. Altern Med Stud, 2011; 1(1):49–53.
Goseki N, Takizawa T, Koike M. Differences in the mode of the extension of gastric cancer classified by histological type: new histological classification of gastric carcinoma. Gut, 1992; 33(5):606–12.
Gravalos C, Jimeno A. HER2 in gastric cancer: a new prognostic factor and a novel therapeutic target. Ann Oncol, 2008; 19(9):1523–9.
He C, Tu H, Sun L, Xu Q, Li P, Gong Y, Dong N Yuan Y. Helicobacter pylori-related host gene polymorphisms associated with susceptibility of gastric carcinogenesis: a two-stage case?control study in Chinese. Carcinogenesis, 2013; 34(7):1450–7.
Hejaji R, Amiji M. Stomach specific and anti H.pylori therapy: Preparation and characterization of tetracycline of a floating multiple unit capsule a high density loaded chitosan microcapsules. Int J Pharm, 2002; 235:87–94.
Herrera V Parsonnet J. Helicobacter pylori and gastric adenocarcinoma. Clin Microbiol and Infect, 2009; 15(11):971–6.
Ishak RAH. Buoyancy-generating agents for stomach-specific drug delivery: an overview with special emphasis on floating behavior. J Pharm Pharm Sci, 2015; 18(1):77–100.
Jasim IK, Alaa AA, Shaimaa N, Abd A. Nanosponge based gastroretentive drug delivery system of 5-fluorouracil for gastric cancer targeting. Int J Drug Deliv Technol, 2021; 11(3):959–63.
Jun X, Liling F, Li J. Immunotherapy of gastric cancer: past, future perspective and challenges. Pathol Res and Pract, 2021; 218:1–11.
Kang Y-K, Boku N, Satoh T, Ryu MH, Chao Y, Kato K. Nivolumab in patients with advanced gastric or gastro esophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet, 2017; 390(10111):2461–71.
Khan MA, Akhtar N, Sharma V, Pathak K. Product development studies on sonocrystallized curcumin for the treatment of gastric cancer. Pharmaceutics, 2015; 7(2):43–63.
Komatsu S, Ichikawa D, Okamoto K, Ikoma D, Tsujiura M, Nishimura Y, Murayama Y, Shiozaki A, Ikoma H, Kuriu Y, Nakanishi M, Fujiwara H, Ochiai T, Kokuba Y, Otsuji E. Progression of remnant gastric cancer is associated with duration of follow-up following distal gastrectomy. World J Gastroenterol, 2012; 18(22):2832–6.
Kotreka U, Moji CA. Gastroretentive floating drug-delivery systems: a critical review. Crit Rev Ther Drug Carr Syst, 2011; 28(1):47–99.
Krishana, Kumar A, Shrivastav R. In vitro in vivo studies on floating microspheres for gastroretentive drug delivery system: a review. Asian J Pharm Clin Res, 2021; 14(1):13–26.
Kuipers EJ. Pernicious anemia, atrophic gastritis, and the risk of gastric cancer. Clin Gastroenterol Hepatol, 2015; 13(13):2290–2.
Kumar M, Kaushik D, An overview on various approaches and recent patents on gastroretentive drug delivery systems, Recent Pat Drug Deliv Formul, 2018; 12:84–92.
Kumar R, Philip A. Gastroretentive dosage forms for prolonging gastric residence time. Int J Pharm Med, 2007; 21:157–71.
Laurén P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma: an attempt at a histo-clinical classification. Acta Pathol Microbiol Scand, 1965; 64(1):31–49.
Lee VHL, Mukherjee SK. Drug delivery: oral colon specific. In: Swarbrick J (ed.). Encyclopedia of pharmaceutical technology, Informa Healthcare, London, UK, pp 1228–41, 2002.
Lin XJ, Wang CP, Liu XD, Yan KK, Li S, Bao H., Zhao LY, Liu X. Body mass index and risk of gastric cancer: a meta-analysis. Jpn J Clin Oncol, 2014; 44(9):783–91.
Lopes CM, Bettencourt C, Rossi A, Buttini F, Barata P. Overview on gastroretentive drug delivery systems for improving drug bioavailability. Int J Pharm, 2016; 510(1):144–58.
Ma K, Baloch Z, He TT, Xia X. Alcohol consumption and gastric cancer risk: a meta-analysis. Med Sci Monit, 2017; 23:238–46.
Malik R, Garg T, Goyal AK, Rath G. Polymeric nanofibers: targeted gastro-retentive drug delivery systems. J Drug Target, 2015;23(2):109–24.
Mandal UK, Chatterjee B, Senjoti FG. Gastro-retentive drug delivery systems and their in vivo success: a recent update. Asian J Pharm Sci, 2016; 11(5):575–84.
Martin IG, Dixon MF, Sue-Ling H, Axon AT, Johnston D. Goseki histological grading of gastric cancer is an important predictor of outcome. Gut, 1994; 35(6):758–63.
Meghpara K, Vaghashiya J, Shah NH, Desai D, Phuapradit W, Sandhu HK, Vaka SRK, Shelke NB, Chatterji A, Gastroretentive dosage form for sustained drug delivery, US 2021/0236421 A1, 2021.
Mellman I, George C, Glenn D. Cancer immunotherapy comes of age. Nature, 2011:480–9.
Melocchi A, Uboldi M, Inverardi N, Briatico Vangosa F, Baldi F, Pandini S. Expandable drug delivery system for gastric retention based on shape memory polymers: development via 4D printing and extrusion. Int J Pharm, 2019; 570:1–36.
Menachem AB, Zalit I, Expandable gastroretentive dosage form, US 2022/0160630 A1, 2022.
Mirani AG, Patankar SP, Kadam VJ. Risk based approach for systemic development of gastroretentive drug delivery system. Drug Deliv Trans Res, 2016; 6(5):579–96.
Mohapatra PR, Satyavani CH, Sahoo S. Design and development of carvedilol gastroretentive floating drug delivery systems using hydrophilic polymers and in vitro characterization. Int J Pharm Pharm Sci, 2020;12(7):66–73.
Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington MK, Carneiro F, Cree IA. The 2019 WHO classification of tumors of the digestive system. Histopathol, 2020;76(2):182–8.
Nana C, Jing’e N, Qian L, Jianyin L, Xinping C, Yuan R, Guotai W, Yingqian L, Yanbin S. Development and evaluation of a new gastroretentive drug delivery system: nanomicelles-loaded floating mucoadhesive beads. J Drug Deliv Sci Technol, 2019; 51:485–92.
Narayana CR, Basavaraj BV, Madhavan V. Microballoons of famotidine: a non-effervescent gastroretentive controlled drug delivery system using Eudragit L-100. Int J Pharm Sci Rev Res, 2010; 5(10):135–9.
Narvekar M, Xue HY, Eoh JY, Wong LH. Nanocarrier for poorly water-soluble anticancer drugs-barriers of translation and solutions. AAPS Pharm Sci Tech, 2014; 15(4):822–33.
Neumann M, Heimhardt C, Seidlitz K, Koziolek M, Schneider F, Schiller C, Weitschies W. Development of a furosemide-containing expandable system for gastric retention. J Control Release, 2021; 338:105–18.
Nygren P. What is cancer chemotherapy? Acta Oncologica, 2001; 40:166–74.
Olnes MJ, Martinson HA. Recent advances in immune therapies for gastric cancer. Cancer Gene Ther, 2021:924–34.
Pahwa R, Bhardwaj BY, Sharma A, Piplani M, Kumar M. Gastroretentive floating technology for eradication of Helicobactor pylori: an insight view. Int J Appl Pharm, 2021;13(3):1–10.
Pahwa R, Bisht S, Kumar V, Kohli K. Recent advances in gastric floating drug delivery technology: a review. Curr Drug Deliv, 2013; 10(3):286–98.
Pahwa R, Jindal S, Chhabra L, Dutt H Rao R. Development and in vitro characterization of effervescent floating drug delivery system of famotidine. Int J Pharm Sci Res, 2011; 3(1):241–6.
Pahwa R, Saini N, Kumar V, Kohli K. Chitosan-based gastroretentive floating drug delivery technology: an updated review. Expert Opin Drug Deliv, 2012a; 9(5):525–39.
Pahwa R, Singh M, Kumar V, Kohli K. Recent advances and patent perspectives in gastroretentive technology. Recent Pat Drug Deliv and Formul, 2012b; 6(3):278–90.
Patil JM, hirlekar RS, Gide PS and Kadam VJ. Trends in floating drug delivery system. J Sci Ind Res, 2006; 65:11–21.
Pawar VK, Kansal S, Asthana S, Chourasia MK. Industrial perspective of gastroretentive drug delivery systems: physicochemical, biopharmaceutical, technological and regulatory consideration. Expert Opin Drug Deliv, 2012; 9(5):551–65.
Pawar VK., Kansal S, Garg Garima, Awasthi R, Singodia D, Kulkarni GT. Gastroretentive dosage forms: a review with special emphasis on floating drug delivery systems. Drug Deliv, 2011; 18(2):97–110.
Prajapati ST, Patel LD, Patel CN. Polymers for floating drug delivery system. Syst Rev Pharm, 2011; 2(1):1–7.
Prajapati VD, Jani GK, Khutliwala TA, Zala BS. Raft forming system-An upcoming approach of gastroretentive drug delivery system. J Control Release, 2013; (2):151–65.
Rai VK, Mishra N, Yadav KS, Yadav NP. Nanoemulsion as pharmaceutical carrier for dermal and transdermal drug delivery: formulation development, stability issues, basic considerations and applications. J Control Release, 2018; 270:203–25.
Raviteja G, Narayana RKVV, Baskaran M, Meghana G, Ganesh G. A mucoadhesive gastroretentive dosage form for valacyclovir. Int J Pharm Pharm Sci, 2014; 6(9):422–7.
Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Gastroenterol Rev, 2019; 14(1):26–38.
Robert S, Ma?gorzata S, Jerzy M, G Johan A Offerhaus, Ryszard M, Wojciech PP. Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res, 2018; 10:239–48.
Rouge N, Buri P, Doelker E. Drug absorption sites in the gastrointestinal tract and dosage forms for site-specific delivery. Int J Pharm, 1996; 136(1–2):117–39.
Sadjadi A, Derakhshan MH, Yazdanbod A, Boreiri M, Parsaeian MBM, Alimohammadian M, Samadi F, Etemadi A, Pourfarzi F, Ahmadi E, Delavari A, Islami F, Farzadfar F, Sotoudeh M, Nikmanesh A, Alizadeh BZ, De BGH. Malekzadeh R. Neglected role of hookah and opium in gastric carcinogenesis: a cohort study on risk factors and attributable fractions. Int J Cancer, 2014; 134(1):181–8.
Sastre J, Jose AS, Eduardo DR. Chemotherapy for gastric cancer. World J Gastroenterol, 2006; 12(2):204–13.
Sethuraman V, Hedden DB, Leskow KM, Gastro – retentive sustained - release oral dosage form of a bile acid sequestrant, US 2022/0110876 A1, 2022.
Shah H, Prajapati S, Patel C. Gastroretentive drug delivery systems: from conception to commercial success. J Crit Rev, 2017; 4(2):10–21.
Shah HN, Desai D, Phuapradit W, Vaghashiya J, Meghpara K, Self regulating osmotic gastroretentive drug delivery system, US 2022/0031604 A1, 2022.
Shahiwala A. Formulation approaches in enhancement of patient compliance to oral drug therapy. Expert Opin Drug Deliv, 2011; 8(11):1521–9.
Shishu, Gupta N, Aggarwal N. Bioavailability enhancement and targeting of stomach tumors using gastro-retentive floating drug delivery system of curcumin technical note. AAPS PharmSciTech, 2008; 9(3):810–3.
Shishu, Gupta N, Aggarwal N. Stomach-specific drug delivery of 5-fluorouracil using floating alginate beads. AAPS PharmSciTech, 2007; 8(2):143–9.
Simons FJ, Wagner KG. Modeling, design and manufacture of innovative floating gastroretentive drug delivery systems based on hot-melt extruded tubes. Eur J Pharm Biopharm, 2019; 137:196–208.
Singh BN, Kim KH. Floating drug delivery systems: an approach to oral controlled drug delivery via gastric retention. J Control Release, 2000; 63(3):235–59.
Tey J, Back MF, Shakespeare TP, Mukherjee RK, Lu JJ, Lee KM, Wong LC, Leong CN, Zhu M. The role of palliative radiation therapy in symptomatic locally advanced gastric cancer. Int J Radiat Oncol Biol Phys, 2007; 67(2):385–88.
Tripathi J, Thapa P, Maharjan R, Jeong SH. Current state and future perspectives on gastroretentive drug delivery systems. Pharmaceutics, 2019; 11(4):1–22.
Tsugane S, Sasazuki S. Diet and the risk of gastric cancer: review of epidemiological evidence. Gastric Cancer, 2007; 10:75–83.
Vaghani S, Vasanti S, Chaturvedi K, Satish CS, Jivani NP. Stomach-specific drug delivery of 5-fluorouracil using ethyl cellulose floating microspheres. Pharm Dev Technol, 2010;15(2):154–61.
Vasvari G, Haimhoffer A, Horvath L, Budai I, Trencsenyi G, Beresova M. Development and characterization of gastroretentive solid dosage form based on melt foaming. AAPS PharmSciTech, 2019; 20:1–11.
Verma A, Dubey J, Hegde RR, Rastogi V, Pandit JK. Helicobacter pylori: past, current and future treatment strategies with gastroretentive drug delivery systems. J Drug Target, 2016; 24(10):897–915.
Wu AH, Tseng CC, Bernstein L. Hiatal hernia. Reflux symptoms, body size, and risk of esophageal and gastric adenocarcinoma. Cancer, 2003; 98(5):940–8.
Xiao YZ, Pei YZ and Mouard AM, Aboul S. From inflammation to gastric cancer: role of Helicobacter pylori. Oncol Lett, 2017; 13(2):543–8.
Ye W, Chow WH, Lagergren J. Risk of adenocarcinomas of the esophagus and gastric cardia in patients with gastroesophageal reflux diseases and after antireflux surgery. Gastroenterology, 2001; 121(6):1286-93.
Zalit I, Menachem AB, Gastroretentive devices, US 2022/0008334 A1, 2022.
Zhang Y, Zhang L, Zhang Q, Zhang X, Zhang T, Wang B. Enhanced gastric therapeutic effects of Brucea javanica oil and its gastroretentive drug delivery system compared to commercial products in pharmacokinetics study. Drug Des Deliv Ther, 2018; 12:53544.
Zhao S, Lv Y, Zhang J Bin, Wang B, Lv GJ, Ma XJ. Gastroretentive drug delivery systems for the treatment of Helicobacter pylori. World J Gastroenterol, 2014; 20(8):9321–9.