INTRODUCTION
Diabetes mellitus (DM) is associated with the inability of the pancreas to secrete insulin or the body’s improper ability to react to insulin secretion. This condition causes hyperglycemia and disrupts carbohydrate, fat, and protein metabolisms. Physiologically, insulin increases glucose uptake into the body’s cells by activating the glucose transporter type 4 (GLUT-4) membrane transporter (DeFronzo et al., 2015). Glucose comes into the cell and is phosphorylated by hexokinase to become glucose-6-phosphate (G6Pase). Glucose incorporated in the cytoplasm undergoes metabolic processes in the liver and other target cells through glycogenesis and glycolysis pathways. In a DM condition, the metabolic balance, in which the liver has a significant role, is greatly affected. Enzyme activities are disrupted, e.g., decreased hexokinase activity and increased gluconeogenesis enzyme activity, worsening hyperglycemia (Petersen et al., 2017).
Chronic hyperglycemia can trigger body damage, including various tissue and organ failures. Currently, DM treatments include diet modifications, exercise, oral antidiabetics, and insulin therapy. Discoveries, developments, and research of novel antidiabetics that can prevent or treat DM and its complications are ongoing. Various studies are conducted to discover effective antidiabetics with minimum side effects (Zheng et al., 2018). The discovery and development of new drugs are conducted by mimicking actual physiological conditions, in this case, insulin’s role in controlling blood glucose. One of the mechanisms is regulating the activities of rate-limiting enzymes in glucose metabolism to achieve glucose homeostasis. Through this mechanism, glucose uptake increases by transferring GLUT-4 to the surface of liver cells, and blood glucose levels can be reduced.
Indonesia, a country with the third-largest natural resources globally, is encouraged to use native Indonesian plants to overcome health issues. Data has shown that traditional therapy is still an option for the Indonesian community; thus, further research on traditional antidiabetic plants that can be developed into oral antihyperglycemics is required (Arulselvan et al., 2014). Eventually, this can overcome the issues of high drug prices or lack of drug availabilities in some rural populations, particularly in developing countries (Soewondo et al., 2013).
One of the plants used by the community to treat DM is curry leaves or Murraya koenigii (MKE). This plant is in the Rutaceae family, commonly used by the community as a spice for cooking. Curry leaves have been shown to have antioxidant, anticancer, anti-inflammatory, and antimicrobial effects. Our previous study showed that curry leaf extract has an antihyperglycemic effect, and no study showed the effect of MKE on key enzymes of carbohydrate metabolism and GLUT-4 expression, so this study aims to investigate the ability of curry leaf MKE extract to overcome hyperglycemia based on its ability to regulate the activities of rate-limiting enzymes in glucose metabolism and GLUT-4 expression that serves to regulate the level of blood sugar in streptozotocin-nicotinamide (STZ-NA) -induced diabetic rats.
MATERIALS AND METHODS
Animals
This methodology, which used the liver and skeletal muscle of Sprague-Dawley rats, adult, male, 170–250 g, was approved by the Ethics Committee at the Faculty of Medicine, University of Indonesia (No. 643/UN2.F1/ETIK/2015). The study was carried out based on the Guide for the Care and Use of Laboratory Animals guidelines. STZ and NA were administered to the test animals to induce hyperglycemia. STZ was purchased from Sigma Chemical Co. and NA from Merck. This study used analytical-grade chemicals and reagents.
Extract preparation
Fresh and mature curry leaves were taken from a garden in Aceh, Indonesia. A taxonomist from the Indonesian Institute of Sciences, Indonesia, confirmed the identity of the leaves. 500 grams of curry leaves was desiccated for 2 weeks at ambient temperature. Next, the dry leaves were crushed by an electric grinder, resulting in a coarse powder. This was then soaked in 96% ethanol and intermittently shaken. A Whatman filter paper No. 1 was used to filter the extract before being turned into a dry mass using a rotary evaporator at 50°C. The product was kept at 4°C. Analysis of the extract’s secondary metabolite components was conducted using preparative liquid chromatography-mass spectrometry (LC-MS).
DM induction
Hyperglycemia was triggered by intraperitoneal intake of 120 mg/kg NA before an intraperitoneal injection of 55 mg/kg STZ. A 15-minute pause was given between the injections. The plasma glucose level was determined via the rat tail vein by a glucometer (Roche Diagnostics Ltd. Co., Germany). Rats with plasma glucose ≥250 mg/dl were classified as diabetic and used in the study. A previous study has discussed the result of this approach (Husna et al., 2018).
Experimental design
This study involved six groups of test animals (each consisting of five rats). All test animals were treated with a test or standard substance (30 days) following the procedure protocol.
Group 1: normal rats were given 0.5% CMC-NA solution (CON), Group 2: normal rats were given 400 mg/kg MKE (CON + MKE), Group 3: diabetic rats were given a 0.5% CMC-NA solution (DM), Group 4: diabetic rats were given 200 mg/kg MKE (DM + L), Group 5: diabetic rats were given 400 mg/kg MKE (DM + H), and Group 6: diabetic rats were given 1 mg/kg glibenclamide (DM + GLI).
MKE and glibenclamide suspensions, made by dispersion in a 0.8% CMC-NA solution, were given orally using a feeding cannula daily. The plasma glucose level of the rats was evaluated every week.
Tissue collection
Cervical decapitation was used to sacrifice the rats on day 30. The liver tissues were cut, soaked in ice-cold normal saline, and dried on clean filter paper before weighing. A part of the liver was put in a sterile and nuclease-free Eppendorf tube and stored at −80°C.
Determination of hexokinase and G6Pase dehydrogenase activities
The activities of rate-limiting enzymes, i.e., hexokinase and G6Pase dehydrogenase, in glucose metabolism were measured using a suitable colorimetric kit. The hexokinase activity in liver homogenate was determined using colorimetric kit ab136957 (Abcam®). The principle of hexokinase measurement is the conversion of glucose to G6Pase by hexokinase. The determination followed the instructions on the colorimetric kit using liver homogenate.
The hexokinase activity (nmol/min/ml or mU/ml) was calculated using the following formula:
ΔA= A2–A1.
B = amount of NADH based on the standard curve (nmol).
ΔT= reaction time T2–T1 (minutes).
V= volume of sample added in each well (ml).
One unit of hexokinase is equivalent to the amount of enzyme that can produce 1 μmol NADH per minutes at pH 8 and room temperature.
The determination of G6Pase dehydrogenase used colorimetric kit ab102529 (Abcam®). The determination followed the instructions on the colorimetric kit using liver homogenate.
The G6PDH activity (nmol/min/ml or mU/ml) was calculated using the following formula:
ΔA= A2–A1.
B = amount of NADH based on the standard curve (nmol).
ΔT= reaction time T2–T1 (minutes).
V= volume of sample added in each well (ml).
One unit of G6PDH is equivalent to the amount of enzyme that can produce 1 μmol NADH per min at pH 8 and 37°C.
Determination of mRNA G6P and phosphoenolpyruvate carboxykinase-1 (PCK)
The High Pure RNA Tissue Kit reagent (Roche, USA) was used to extract the total RNA from the liver tissues of each rat. The Transcriptor First Strand complementary DNA (cDNA) Synthesis Kit (Roche, USA) was used to synthesize the first cDNA strand using 1 μg total RNA. Quantitative real-time PCR analysis was conducted using FastStart Essential DNA Green Master (Roche, USA) in the LightCycler® Nano software system (Roche®) to determine the G6Pase and PCK gene expressions. The following gene-specific oligonucleotide primers were used:
Primer for rat G6Pase is 5?AACGTCTGTCTGTCCCGGATCTAC3? (forward) and 5?ACCTCTGGAGGCTGGCATTG3? (reverse). Primer for rat PCK-1 is (forward) and 5? GTTGCAGGCCCAGTTGTTGA3? (reverse). Primer for rat β-actin is 5? TACTGCCCTGGCTCCTA3? (forward) and 5?GGGCCGGACTCATCGTA3? (reverse).
The cycle circumstances were 42°C and 95°C (5 minutes each). Furthermore, it was set to 45 cycles of 95°C for 15 seconds, 60°C for 30 seconds, and 72°C for 30 seconds. Various parameters, such as the amount of endogenous control, β-actin, analyzed melting curves, and ΔΔCt method, were used to normalize the relative amount of the mRNAs (Life Technologies, 2014).
Determination of GLUT-4 mRNA
The TriPure RNA Kit Reagent (Roche, USA) was used to extract the total RNA from the skeletal muscle of each rat. Quantitative real-time PCR analysis was conducted using FastStart Essential DNA Green Master (Roche, USA) in the LightCycler® Nano software system (Roche®) to determine the GLUT-4 gene expression. The cycle circumstances were 42°C and 95°C (5 minutes each). Furthermore, it was set to 45 cycles of 95°C for 15 seconds, 60°C for 30 seconds, and 72°C for 30 seconds. Various parameters, such as the amount of endogenous control, β-actin, analyzed melting curves, and Livak-Schmittgen method (relative quantification), were used to normalize the relative amount of the mRNAs (Life Technologies, 2014).
Primer for rat GLUT-4 is 5?GATACTCATTCTCGGACGGTTC3? (forward) and 5?GGCGATTTCTCCCACATACA3? (reverse). Primer for rat β-actin is 5? TACTGCCCTGGCTCCTA3? (forward) and 5?GGGCCGGACTCATCGTA3? (reverse).
Statistical analysis
Data were displayed as mean ± standard error of the mean (SEM) and analyzed using one-way ANOVA and least significant difference post hoc via SPSS 20, with the significant level of 5% or p < 0.05.
RESULTS
The LC-MS analysis shows that MKE contains several compounds, i.e., mukoenine A, siamenol, girinimbine, murrayamine I, murraxonin, murrayaquinone B, 1,6,7-trihydroxy-3-methyl carbazole, 1?-O-acetyl-murrangatin, murralongin, murrayanine, koenimbine, and pyrayaquinone A (Fig. 1).
The effect of MKE on the activities of hexokinase and G6Pase dehydrogenase
The impact of MKE administration on hepatic hexokinase in diabetic-induced rats is presented in Figure 2A. The hexokinase activity of diabetic-induced rats declined. Administration of 200 and 400 mg/kg body weight of MKE improved hexokinase activity significantly compared to diabetic rats that were not given therapy (p = 0.00 and p = 0.003). The effect of MKE on the activity of hepatic G6PDH in diabetic rats is depicted in Figure 2B. A decline in G6PDH activity occurred in diabetic-induced rats. Administration of 200 and 400 mg/kg body weight of MKE improved G6PDH activity significantly for the treated diabetic rats (p = 0.008 each).
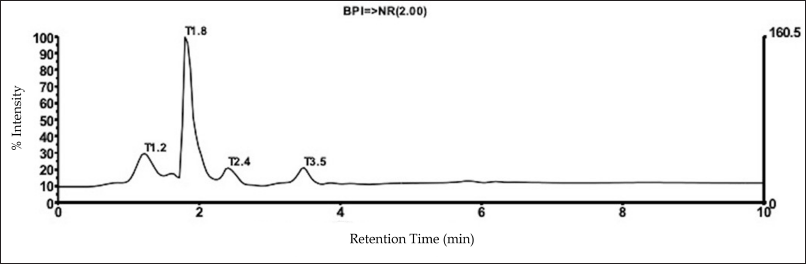 | Figure 1. LC-MS chromatogram of curry leaf (Murraya koenigii) extract. [Click here to view] |
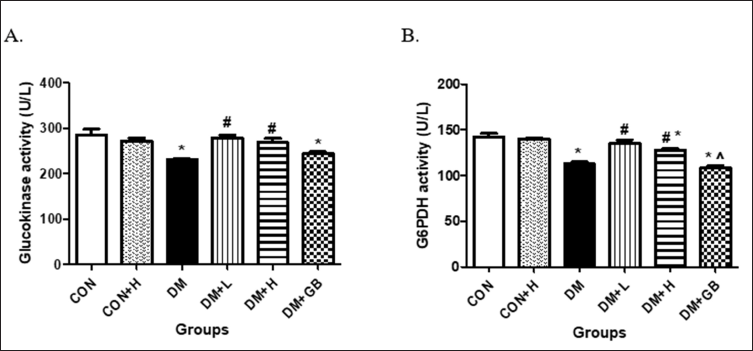 | Figure 2. The effect of MKE on hepatic hexokinase (A) and G6Pase dehydrogenase (B). Activities in STZ-NA-induced diabetic rats. Data presented as average, error bar shows ± SEM (n = five rats). *p < 0.05 versus CON group. #p < 0.05 versus DM group. $ p < 0.05 versus DM + L/H group. CON: control, DM: diabetes. DM+L: diabetes+ MKE 200 mg/kg b.wt, DM+H: diabetes+ MKE 400 mg/kg b.wt, DM+GB: diabetes + glibenclamide 1 mg/kg b.wt. [Click here to view] |
The effect of MKE on the relative expressions of glucose-6-phosphatase and phosphoenolpyruvate carboxykinase-1 mRNAs
The effect of MKE administration on the relative expression of hepatic G6Pase mRNA in diabetic rats is presented in Figure 3A. An increase in G6Pase mRNA expression occurred in diabetic-induced rats. Administration of 200 and 400 mg/kg body weight of MKE reduced the enzyme’s mRNA expression in the treated diabetic rats (p = 0.068 and p = 0.03).
The effect of MKE administration on the relative expression of hepatic PCK-1 mRNA in diabetic rats is shown in Figure 3B. The expression of PCK-1 mRNA in diabetic rats increased five times higher than in the standard rat group. Administration of 200 and 400 mg/kg body weight of MKE significantly reduced the expression of PCK-1 mRNA and was almost comparable to the condition of a healthy rat (p = 0.000 and p = 0.000).
The effect of MKE on the relative expression of GLUT-4
The effect of MKE administration on the relative expression of GLUT-4 mRNA in the skeletal muscle of diabetic rats is shown in Figure 4. DM induction decreased the relative expression of GLUT-4 mRNA significantly in the treated rats (p = 0.029). MKE administration in diabetic rats increased the expression of GLUT-4 mRNA; however, it was not significantly different compared to untreated diabetics rats (p = 0.057).
DISCUSSION
This study used rat liver experimentally induced by injecting STZ and NA to trigger DM. STZ is an alkylating agent that can cause DNA fragmentation and damage through the reactivation of methyl carbonium ion (CH3-). STZ is cytotoxic to β cells because it stimulates the production of poly (ADP-ribose) polymerase (PARP) that reduces the ratio of NAD+ in pancreatic β cells, hence triggering β cell necrosis (Eleazu et al., 2013). Meanwhile, the administration of NA protects the cell, hence minimizing pancreatic cell damage. NA is an NAD + precursor, PARP inhibitor, and niacin (vitamin B3) derivative; therefore, it plays a role as a free radical and NO scavenger and reduces DNA methylation, thus preventing damage due to STZ administration (Szkudelski, 2012).
This study used 55 mg/kg b.wt STZ and 120 mg/kg b.wt NA, which were injected 15 minutes before STZ injection. The STZ-NA model, a type 2 DM nongenetic and nonobese model, is often used in antihyperglycemic candidate screenings and antihyperglycemic effectiveness studies (Badole et al., 2013; Jangale et al., 2013). The test substance in this study is the ethanolic extract of MKE at 200 and 400 mg/kg body weight. As the standard drug for type 2 DM, glibenclamide was also used at a 1 mg/kg body weight dose. This section will discuss MKE and how it affects the livers of diabetic rats by comparatively analyzing the average activities of rate-limiting enzymes involved in carbohydrate metabolism and GLUT-4 mRNA expression.
Murraya koenigii is a plant with intraspecific variations which highly depends on the nature of its location. Therefore, the determination of chemical composition in plants is vital to obtaining the uniformity of phytochemical composition. The composition of active ingredients in plants is highly dependent on extrinsic and intrinsic factors (Rao et al., 2011). Based on the Ishikawa fishbone diagram, variations are caused by raw material, method, equipment, stability, and human (Balekundri and Mannur, 2020). Our previous study reported that MKE contains flavonoids, saponin, steroids, and essential oil. The essential oil component includes caryophyllene, benzo[a]naphthacene, hexadecen-1-ol, α-matrine, 2H-3,5A-epoxynaphth[2,1-B]oxepin, vitamin E, 12-epilicodolin, oxepin, bisnorlabdane, δ-sitosterol, noruns-12-ene, hexadecenoic acid, linoleic acid, and octanoic acid (Husna et al., 2020). Carbazole alkaloid is the active ingredient that plays a role in various effects of MKE extract. This study has identified secondary metabolites in MKE through preparative LC-MS analysis and discovered the presence of mukoenine A, siamenol, girinimbine, murrayamine I, murraxonin, murrayaquinone B, 1,6,7-trihydroxy-3-methyl carbazole, 1?-O-acetyl-murrangatin, murralongin, murrayanine, koenimbine, and pyrayaquinone A. The metabolites contained in MKE leaves are thought to be the compounds that mediate the numerous antihyperglycemic impacts of MKE on STZ-NA-induced diabetic rats.
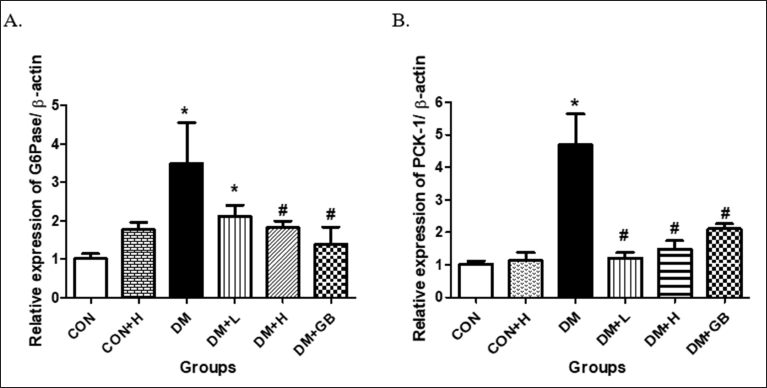 | Figure 3. The effect of MKE on the relative expression of hepatic G6Pase and phosphoenolpyruvate carboxykinase-1 in STZ-NA-induced diabetic rats. Data presented as average, error bar shows ± SEM (n = five rats). *p < 0.05 versus CON group. CON: control, DM: diabetes. DM+L: diabetes+ MKE 200 mg/kg b.wt, DM+H: diabetes+ MKE 400 mg/kg b.wt, DM+GB: diabetes + glibenclamide 1 mg/kg b.wt. [Click here to view] |
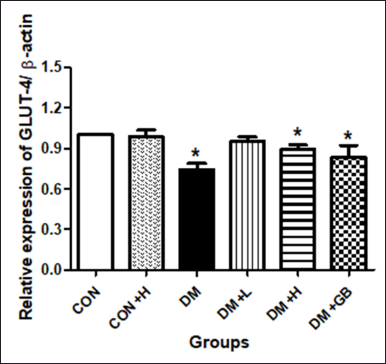 | Figure 4. The effect of MKE on the relative expression of GLUT-4 mRNA in the skeletal muscle of STZ-NA-induced diabetic rats. Data presented as average, error bar shows ± SEM (n = five rats). *p < 0.05 versus CON group. CON: control, DM: diabetes. DM+L: diabetes+ MKE 200 mg/kg b.wt, DM+H: diabetes+ MKE 400 mg/kg b.wt, DM+GB: diabetes + glibenclamide 1 mg/kg b.wt. [Click here to view] |
In a diabetic condition, the activities of key enzymes involved in glucose metabolism change drastically, thus causing hyperglycemia. Supplementation of a specific substance or compound that can improve enzyme activity can be a therapy option in managing DM (Kahn et al., 2014). The liver is involved in glucose metabolism, playing an essential role in maintaining glucose homeostasis because it is the primary organ that stores glucose as glycogen and produces endogenic glucose. At a normal condition, an increase in blood glucose level triggers blood glucose absorption to be stored in the liver and insulin secretion to suppress hepatic glucose production (Nelson and Cox, 2005). The liver can switch from storing hepatic glucose to a hepatic glucose producer (or vice versa) by regulating the activities of key enzymes in the glycolysis and glucogenesis pathways. The final amount of hepatic glucose relies on the activities of hexokinase and G6Pase (Sharabi et al., 2015).
Hexokinase is an insulin-dependent enzyme and is the earliest involved in glycolysis, glycogen synthesis, and the pentose phosphate pathway. The function of hepatic hexokinase is to lower blood glucose levels shortly after eating and convert it to G6Pase (Nelson and Cox, 2005). Hexokinase also acts as a glucose sensor in pancreatic β cells, thereby triggering insulin secretion. In a diabetic state, hepatic glucokinase activity decreases significantly. This situation can be caused by nonenzymatic glycation because the blood glucose increases, reducing the metabolized G6Pase (Jiang et al., 2020).
Another important enzyme is G6PDH, the first rate-limiting enzyme that catalyzes reactions in the pentose phosphate pathway to produce ribose-5-phosphate and nicotinamide adenine dinucleotide phosphate (NADPH). The pentose phosphate pathway is the main pathway that produces NADPH. A decline in the activity of rate-limiting enzymes in this process leads to a decrease in NADPH level, making cells more vulnerable to oxidative damage (Dasgupta and Wahed, 2014). Oxidative stress is highly related to diabetes (Lazarus et al., 2020). In a diabetic state, a decrease in G6PDH activity slows the pentose phosphate pathway and increases tissue susceptibility to oxidative stress (Jiang et al., 2020). Our study demonstrates that MKE administration increased G6PDH activity. This condition is strongly related to MKE’s ability to improve blood glucose level, which is associated with tissue oxidative processes.
Hexokinase and G6PDH are involved in hepatic glucose utilization and production that contributes significantly to blood glucose levels (Jiang et al., 2020; Petersen et al., 2017). This study demonstrates that the activities of hexokinase and G6PDH in diabetic rats were significantly lower than in normal rats. The result agrees with other studies examining the activities of rate-limiting enzymes in glucose metabolism using diabetic animals (Murali et al., 2013; Ramachandran and Saravanan, 2013; Sureka et al., 2021). The condition may be caused by further glycation of enzyme proteins involved in glucose metabolism, thereby reducing enzyme activities. MKE administration increased hepatic hexokinase and G6PDH activities in diabetic rats, possibly because MKE controls the blood glucose level; therefore, no further glycation of essential protein occurred, including this enzyme. MKE also showed a hepatoprotective effect that can protect the liver from hyperglycemia-induced damage. In addition, MKE’s antioxidant effect controls blood glucose levels in diabetic rats until it achieves a normal condition.
G6Pase is an enzyme that is the opposite of hexokinase. G6Pase is found in the endoplasmic reticulum and catalyzes the dephosphorylation of G6Pase to glucose via the glucogenesis pathway (Jiang et al., 2020). Glucogenesis is a glucose production pathway that uses a nonglucose substrate. This pathway maintains blood glucose levels when there is no carbohydrate intake and the hepatic glycogen reserve drops. G6Pase is only found in the liver and kidney (Sureka et al., 2021). In a diabetic condition, the G6Pase activity increases (Matschinsky, 1995). This study shows that MKE administration decreased the expression of G6Pase mRNA. This condition lowers endogenic glucose production via glucogenesis and, therefore, controls the blood glucose level.
Several other enzymes also control the substrate cycle between glucogenesis and glycolysis. Phosphoenolpyruvate carboxylase (PEPCK) is an essential glucogenesis enzyme (Jiang et al., 2020). The substrate cycle regulates the speed and direction of substrate flow that can be adjusted by changing the specific enzyme effector concentration, modifying enzyme covalent, and changing the gene expression of the enzyme (Prabhakar et al., 2014). The decrease in PEPCK activity in rats with MKE administration can control the substrate’s speed and flow direction, hence suppressing the glucogenesis pathway.
Apart from the liver, skeletal muscle is a primary tissue that plays a role in transporting glucose from the circulation, mediated by insulin. Insulin stimulates glucose uptake into the skeletal muscle via its role in the GLUT-4 vesicle trafficking to the plasma membrane (Bogan, 2012; Huang and Czech, 2007). When insulin binds to the α subunit of the insulin receptor, it activates intracellular signals. One of the signals is vesicle translocation that carries GLUT-4 to the cell membrane and facilitates glucose entry to target tissues (Kahn et al., 2014; Prabhakar et al., 2014). This study demonstrates that the relative expression of GLUT-4 mRNA in diabetic rats was lower than in their counterparts. This result is consistent with numerous studies showing decreases in GLUT-4 expression in skeletal muscle and adipose tissue of diabetic rats (Lazarus et al., 2020; Yousefi et al., 2017). In this study, the increase of GLUT-4 expression in the diabetic group with MKE administration shows that MKE can potentially improve glucose uptake or utilization in the skeletal muscle by raising GLUT-4 movement to the plasma membrane, therefore improving the resistance state.
CONCLUSION
This study clearly indicates that the antihyperglycemic effect of MKE extract resulting from attenuation of the activity of rate-limiting enzymes of glucose metabolism on liver hyperglycemic rats increases glucose uptake into the skeletal muscle through the increased GLUT-4 translocation in the skeletal muscle.
ACKNOWLEDGMENTS
The authors would like to thank the Directorate Research and Community Services, Universitas Syiah Kuala, Indonesia.
AUTHOR’S CONTRIBUTIONS
FH conducted the research, collected, organized, analyzed, and interpreted data, and drafted the article. WA and FDS designed the study, provided logistic support, and revised the draft. EHP designed the study and revised the draft. MH identified the extract. LC-MS, RR, and CWA analyzed and interpreted data. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
FUNDING
This research was funded by Lembaga Penelitian Dan Pengabdian Masyarakat Universitas Syiah Kuala, Indonesia (Grant No. 178/UN11.2.1/PT.01.03/PNPB/2021).
ETHICAL APPROVAL
This experiment was conducted after being approved by the Ethics Committee of the Faculty of Medicine, University of Indonesia (No. 643/UN2.F1/ETIK/2015).
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Arulselvan P, Ghofar HAA, Karthivashan G, Halim MFA, Ghafar MSA, Fakurazi S. Antidiabetic therapeutics from natural source: a systematic review. Biomed Prev Nutr, 2014; 4(4):607–17. CrossRef
Badole SL, Mahamuni SP, Bagul PP, Khose RD, Joshi AC, Ghule AE, Bodhankar SL, Raut CG, Khedkar VM, Coutinho EC, Wagh NK. Cycloart-23-ene-3β, 25-diol stimulates GLP-1 (7-36) amide secretion in streptozotocin-nicotinamide induced diabetic Sprague-Dawley rats: a mechanistic approach. Eur J Pharmacol, 2013; 698(1–3):470–9. CrossRef
Balekundri A, Mannur V. Quality control of the traditional herbs and herbal products: a review. Futur J Pharm Sci, 2020; 6(1):1–9. CrossRef
Bogan JS. Regulation of glucose transporter translocation in health and diabetes. Annu Rev Biochem, 2012; 81(1):507–32. CrossRef
Dasgupta A, Wahed A. Carbohydrate metabolism, diabetes, and hypoglycemia. In: Dasgupta A, Wahed A (eds.). Clinical chemistry, immunology and laboratory quality control. Elsevier, pp 107–26, 2014. CrossRef
DeFronzo RA, Ferrannini E, Groop L, Henry RR, Herman WH, Holst JJ, Hu FB, Kahn CR, Raz I, Shulman GI, Simonson DC, Testa MA, Weiss R. Type 2 diabetes mellitus. Nat Rev Dis Prim, 2015; 1(1):15019; doi:10.1038/nrdp.2015.19 CrossRef
Eleazu CO, Eleazu KC, Chukwuma S, Essien UN. Review of the mechanism of cell death resulting from streptozotocin challenge in experimental animals, its practical use and potential risk to humans. J Diabetes Metab Disord, 2013; 12(1):60. CrossRef
Huang S, Czech MP. The GLUT4 glucose transporter. Cell Metab, 2007; 5(4):237–52. CrossRef
Husna F, Suyatna FD, Arozal W, Poerwaningsih EH. Anti-diabetic potential of Murraya Koenigii (L) and its antioxidant capacity in nicotinamide-streptozotocin induced diabetic rats. Drug Res (Stuttg), 2018; 68(11):631–6. CrossRef
Husna F, Suyatna FD, Arozal W, Purwaningsih EH, Sani Y. Restoration of pro-inflammatory cytokines and histopathological changes in pancreas and liver of hyperglycemic rats by Murraya koenigii leaves extract. J Appl Pharm Sci, 2020; 10(1):8–15. CrossRef
Jangale NM, Devarshi PP, Dubal AA, Ghule AE, Koppikar SJ, Bodhankar SL, Chougale AD, Kulkarni MJ, Harsulkar AM. Dietary flaxseed oil and fish oil modulates expression of antioxidant and inflammatory genes with alleviation of protein glycation status and inflammation in liver of streptozotocin–nicotinamide induced diabetic rats. Food Chem, 2013; 141(1):187–95. CrossRef
Jiang S, Young JL, Wang K, Qian Y, Cai L. Diabetic-induced alterations in hepatic glucose and lipid metabolism: the role of type 1 and type 2 diabetes mellitus (Review). Mol Med Rep, 2020; 22(2):603–11. CrossRef
Kahn SE, Cooper ME, Del Prato S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet, 2014; 383(9922):1068–83. CrossRef
Lazarus G, Alexander S, Kusuma GO, Wijaya K, Soetikno V. Antioxidative activities of alpha-mangostin in high-fat/high-glucose diet and streptozotocin-induced insulin-resistant rodents. J Appl Pharm Sci, 2020; 10(11):35–9.
Life Technologies. Realtime PCR handbook. Applied Biosystems. Life Technologies, Carlsbad, CA, pp 1–70, 2014.
Matschinsky FM. A lesson in metabolic regulation inspired by the glucokinase glucose sensor paradigm. Diabetes, 1995; 45(2):223–41. CrossRef
Murali R, Srinivasan S, Ashokkumar N. Antihyperglycemic effect of fraxetin on hepatic key enzymes of carbohydrate metabolism in streptozotocin-induced diabetic rats. Biochimie, 2013; 95(10):1848–54. CrossRef
Nelson DL, Cox MM. Lehninger Principles of Biochemistry. 4th edition, WH Freeman, Madison, WI, pp 521–55, 2005.
Petersen MC, Vatner DF, Shulman GI. Regulation of hepatic glucose metabolism in health and disease. Nat Rev Endocrinol, 2017; 13(10):572–87; doi:10.1038/nrendo.2017.80 CrossRef
Prabhakar PK, Kumar A, Doble M. Combination therapy: a new strategy to manage diabetes and its complications. Phytomedicine, 2014; 21(2):123–30. CrossRef
Ramachandran V, Saravanan R. Efficacy of asiatic acid, a pentacyclic triterpene on attenuating the key enzymes activities of carbohydrate metabolism in streptozotocin–induced diabetic rats. Phytomedicine, 2013; 20(3–4):230–6. CrossRef
Rao BRR, Rajput DK, Mallavarapu GR. Chemical diversity in curry leaf (Murraya koenigii) essential oils. Food Chem, 2011; 126(3):989–94. CrossRef
Sharabi K, Tavares CDJ, Rines AK, Puigserver P. Molecular pathophysiology of hepatic glucose production. Mol Aspects Med, 2015; 46:21–33. CrossRef
Soewondo P, Ferrario A, Tahapary DL. Challenges in diabetes management in Indonesia: a literature review. Global Health, 2013; 9(1):63–80. CrossRef
Sureka C, Elango V, Al-Ghamdi S, Aldossari KK, Alsaidan M, Geddawy A, Abdelaziz MA, Mohideen AP, Ramesh T. Ameliorative property of Sesbania grandiflora on carbohydrate metabolic enzymes in the liver and kidney of streptozotocin-induced diabetic rats. Saudi J Biol Sci, 2021; 28(7):3669–77. CrossRef
Szkudelski T. Streptozotocin-nicotinamide-induced diabetes in the rat. Characteristics of the experimental model. Exp Biol Med, 2012; 237(5):481–90. CrossRef
Yousefi MR, Bakhtiyari S, Valizadeh A. Reviewing and comparing the impact of aerobic exercise (3 and 5 times per week) on insulin receptors, glucose transporter protein (GLUT4), and skeletal muscle insulin sensitivity in diabetic rats. J Appl Pharm Sci, 2017; 7(2):132–6.
Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol, 2018; 14(2):88–98; doi:10.1038/nrendo.2017.151. CrossRef