INTRODUCTION
Obesity is a pathological condition in which excess body fat is stored to such an extent that it may be harmful to an individual’s health, resulting in a shorter life span and/or more health issues (Chong and Lee, 2018). Obesity, oxidative stress, and the inflammatory process are believed to have a pathological interaction, according to a recent research (Dludla et al., 2019). Over the prior few decades, the massive increase in people who are obese and overweight has become a global problem.
Obesity from excessive calorie intake results in an overexpenditure of energy, in general, but heredity, health problems, and lifestyle problems are also aspects to consider (Skolnik and Ryan, 2014). Obesity can exacerbate insulin resistance (IR), diabetes mellitus type 2 (DM2), dyslipidemia, and visceral fat deposition. When these variables are combined, a metabolic syndrome is formed, which is associated with nonalcoholic fatty liver disease (Caixàs et al., 2014).
Obesity oxidative stress is a condition characterized by an imbalance of free radicles and antioxidants, as well as the malfunction of organs to preserve and eliminate free radicals, which can have severe consequences (Souza Cruz et al., 2020). This condition is accompanied by the presence of advanced free radicals known as lipid peroxidation “malondialdehyde (MDA)” (Ito et al., 2019). Leptin may contribute to the regulation of many physiological functions of the body, such as regulation of blood pressure, inflammation, nutrition, appetite, insulin and glucose metabolism, lipid metabolism, coagulation, and apoptosis (Gelen et al., 2021). In a hyperlipidemic rat model, oxidative stress is associated with the development of inflammation and has been shown to mediate the development of atherosclerotic abnormalities (Monguchi et al., 2017). The frequent and recurrent mechanisms of oxidative alteration and inflammatory processes, which turn into a chronic form, aggravate the development of atherosclerosis (Reaven et al., 2004). Lipid contents, notably low-density lipoprotein (LDL) cholesterols, are extremely permeable to the subendothelial layer when there is an excess of dietary fat. Phenolic compounds inhibit inflammation by inhibiting cytosolic multiprotein complexes that assemble in response to cytosolic pathogen-associated molecular patterns and damage-associated molecular patterns to form active forms of interleukin (IL)-1β and IL-18 (Gelen, 2021). The oxidative alteration proceeded, resulting in the conversion of the LDL to the oxidized form. As a result, MDA generation increases, prompting additional macrophages to absorb excess lipids as part of the flushing process (Othman et al., 2021).
Obesity is closely related to free radical-induced stress, which is associated with a wide range of inflammatory and metabolic disease states (Reaven et al., 2004). It is strongly correlated to the cumulative body damage caused by free radicals that are not adequately neutralized by antioxidant compounds (Valdecantos et al., 2009). It has been shown that free radicals have a negative impact on cell viability due to membrane damage caused by oxidative lipid, protein, and permanent alteration of DNA (Mishra, 2004). Indicators of oxidative damage to reactive oxygen species (ROS) include lipid peroxidation, such as thiobarbituric acid reactive substances and levels of hydroperoxides, as well as indicators of protein oxidation, including carbonyl proteins (Uzun et al., 2007). Thiols improve oxidative stress-related diseases (Yaman and Ayhanci, 2021). In addition, under situations associated with oxidative stress, the activity of antioxidant enzymes, such as superoxide dismutase, catalase, glutathione S-transferase, and glutathione peroxidase, which act as free radical scavengers, is reduced, which exacerbates oxidative damage (Noeman et al., 2011).
Hyperglycemia and changes in glucose uptake in diabetic patients have been linked to oxidative stress and mitochondrial dysfunction, as well as to an inflammatory response characterized by increased proinflammatory cytokines, such as tumor necrosis factor-alpha (TNFα).. Both conditions may be a precursor to coronary heart disease and other cardiomyopathies. Some researchers also discovered that an 8-week high-fat diet (HFD) induced elevated oxidative stress (Sun et al., 2016) in obese rats, leading to vasoactive and endothelial dysfunction of the coronary arteries (Effting et al., 2019).
Extensive research has been carried out on the ingredients of natural supplements that primarily help clients fight obesity. Various antiobesity herbal products, functional fatty acids, and natural additional nutritional ingredients have been used. Due to the increased health awareness in the consumer, products derived from natural herbs are expected to be potential components for creating nature-derived antiobesity drugs in the weight loss category (Sun et al., 2016).
The rate of obesity has risen in many industrialized and developing countries in recent years, more than doubling in 73 countries since 1980 (Afshin et al., 2017). Obesity is expected to affect “12% of adults (603.7 million) and 5% of children (107.7 million) worldwide” (Saboo et al., 2010). Obesity is becoming increasingly prevalent over the world, indicating the need for more research into this risk factor in the future.
The objective of this study was to investigate the effect of goldenberry (GB) (Physalis peruviana) extract (fruits with husk) supplementation on fat accumulation, blood fat profile, hepatic oxidative damage, hepatic fat deposition, inflammation, and hepatic scarring, as well as metabolic syndrome in obese rats.
MATERIALS AND METHODS
Plant material
Goldenberry fruits were collected from a market in Cairo, Egypt, during their off-season in February/March 2020. The identification of the plants was confirmed by the Herbarium of the Department of Botany, Faculty of Science, Cairo University. The voucher samples were preserved in the herbarium of the National Research Centre, Cairo, Egypt.
Preparation of extract
Fresh fruits with their husk (5 kg) were washed in the laboratory with running tap water, stirred vigorously with a blender, then soaked in 70% MeOH, warmed at 40°C, and filtered (using Whatman No.1 filter paper) in another sterile container. Extraction processes were performed repeatedly three times at 40°C. Then, the resulting liquid was collected, filtered, and reduced through evaporation under vacuum by a rotary evaporator (Heidolph, Germany) at 45°C, and the crude extract was put into lyophilization to give a dry residue (60 g).
Reagents
Folin–Ciocalteu and AlCl3 reagents were supplied by Sigma (St. Louis, MO) and were used as they were received.
Estimation of total phenolics and flavonoid contents
The total phenolic content (TPC) of fresh goldenberry fruit with husk was determined using Folin–Ciocalteu colorimetric assay described by Mandal and Madan (2013), with minor modifications, using gallic acid as a standard. The results were calculated using the standard curve of gallic acid at known concentrations (2.5–50 μg ml–1). TPC was expressed as mg gallic acid equivalent (GAE)/g of a plant extract. Total flavonoid concentration (TFC) was measured using an AlCl3 colorimetric assay. The TFC of the extract was determined according to the reported procedure (Kumaran and Joel Karunakaran, 2007). TFC was expressed as mg rutin equivalent (RE)/g plant extract.
Animal care and treatments
Thirty-two adult female Wistar rats weighing 150–170 g (National Research Centre, Animal House, Egypt) stayed at a stable room temperature (25°C) with 12 hours light/dark cycles and free access to food and water. The animals were given a week to adapt. Eight rats were kept as a control, and others were given a HFD and tap water with 25% sucrose for 12 weeks to develop obesity. HFD contains carbohydrate 42.3%, protein 17%, fat 22.50%, fiber 3%, 2%, minerals 5%, and moisture 10%. Normal rats were fed free standard chow pellets. Animals were handled in accordance with the recommendations of the National Institute of Health Guide for the Care and Use of Laboratory Animals. It was approved by the Research Ethics Committee of the National Research Centre number 19161.
The rats were separated into four groups:
Group I: normal control rats served were fed a free standard chow diet for 8 weeks (n = 8).
Group II: rats were given a HFD and water from the tap with 30% sucrose for 12 weeks to develop obesity (n = 8).
Group III: obese rats fed with HFD-treated oral gavage with a low dose of 200 mg/kg b.w. (body weight) of GB extract for 8 weeks (n = 8).
Group IV: obese rats fed with HFD-treated oral gavage with a high dose of 400 mg/kg body weight (b.w.) of GB extract for 8 weeks (n = 8).
Anthropometric measures
Body weight (g) and nasal–anal length (NAL) (cm) of rats in the normal control group and experimental groups were assessed at the beginning of the experiment, as well as after 2, 4, 6, and 8 weeks. The circumference of the waist was measured. BMI (g/cm2) was calculated according to the following formula: “BMI = body weight (g)/NAL (cm2).” Obesity was defined as a BMI of 0.68 g/cm2 or higher, as reported previously by Novelli et al. (2007).
Samples’ collection
Three months later, blood was then collected in heparin-filled tubes from the retroorbital plexus under local anesthesia by diethyl ether and plasma isolated by centrifugation at 3,000 g for 15 minutes. The supernatant was isolated and stored at −80°C until assay. Animals were killed under ether anesthesia, and organs were removed for pathology and molecular biology investigations, as well as adipose tissue collected for biochemistry.
Adiponectin and leptin determination
Rat adiponectin and leptin were quantified quantitively by enzyme-linked immunosorbent assay (ELISA) using plasma ELISA using “SinoGeneClon Biotech Co. Kit, China.”
Liver function and lipid profile tests
The gamma-glutamyl transferase (GGT) was kinetically tested using kits provided by an Egyptian biotechnology company,while plasma aspartate aminotransferase (AST), alanine aminotransferase (ALT), and lipid profiles were evaluated calorimetrically using kits from Salucea Company, The Netherlands. The lipid profile was determined by calorimetry using blood determination kits from “Salucea Company, The Netherlands.” The lipid profile contains total cholesterol (TC), triglyceride (TG), LDL, and high-density lipoprotein (HDL).
Insulin resistance parameters
Blood glucose was determined calorimetrically following the instructions of the Salucea Company kit. Plasma insulin was assayed by immunoassay (ELISA) according to the instructions of Sunlong Biotech Co. Kit (China). The insulin resistance index was estimated from the following equation: homeostatic model assessment for insulin resistance = fasting glucose (mg/dl) × fasting insulin (mIU/ml)/ 405.
Oxidative stress and antioxidant markers analysis
One gram of rat liver was ground in 10 ml of phosphate buffer (pH 7.4) and rotated for 15 minutes at 4°C at 10,000 rpm. The supernatant was collected and used to perform protein and enzyme analysis. The detection principle of MDA in catabolite from lipid peroxide can react with thiobarbituric acid and produce a red compound, which has a maximum absorption peak of 532 nm (Kei, 1978). Superoxide dismutase (SOD) activity was measured in tissue homogenates using a previously proven technique (Nishikimi et al., 1972). For 1 minute, changes in absorbance at 480 nm were measured at 15 s intervals. At the same time, a control reaction containing all components except the enzyme was examined. One unit of enzyme activity was defined as 50% suppression of epinephrine’s antioxidant activity in the test method. The presented technique was used to determine reduced glutathione levels (Beutler et al., 1963). The absorbance of the mixture was measured immediately with a “spectrophotometer at 420 nm” once the yellow hue was formed.
Inflammatory parameters
TNF-α, C-reactive protein (CRP), IL2, and IL6 levels were determined by the ELISA technique (Sunlong Biotech Co. Kit, China).
Histopathological evaluations of liver
The livers of the separated groups were excised, fixed in 10% formalin saline, then stained with hematoxylin and eosin in 5-micron thick paraffin slices (Carleton et al., 1980), and examined under a light microscope.
Statistical analysis
Values were stated as “mean ± SE,” and the variances between groups were tested for significance using “analysis of variance (ANOVA), followed by Tukey’s comparison test estimated by Graph Prism, version 9.2.” The level of “statistical significance was at p < 0.05.”
RESULTS
Total phenolics and flavonoid contents
The TPC and TFC of Physalis peruviana fruit with husk extract are shown in Table 1. The TPC is calculated as GAE/g of plant extract, and TFC is calculated as rutin equivalent (RE)/ g of dried extract.
Anthropometric measures of obese rats
Table 2 presents time-dependent alterations in body weight and BMI in the four groups over 2, 4, 6, and 8 weeks. At the start of goldenberry extract supplementation, there was a significant increase in body weight and BMI in obese, low-dose (200 mg/kg b.w.), and high-dose (400 mg/kg b.w.) rats compared to the normal control group. In the 4–8-week period marked by the observation of the opposite, there was a significant decrease in BMI for the low and high GB doses compared to the obese rats. Moreover, there are advanced changes in the waist in the four groups over a period of 2, 4, 6, and 8 weeks. This means that the body mass index (BMI) of the two administrated GB groups (low and high dose GB) showed a decrease in BMI values after discontinuation of the trial (after 8 weeks of oral GB) compared to the obesity group, which showed an increase in BMI values by a highly significant difference (p < 0.05).
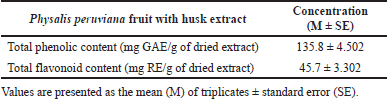 | Table 1. Total phenolic and flavonoid contents in Physalis peruviana fruit with husk extract. [Click here to view] |
Effect of goldenberry supplementation on adiponectin and leptin hormones of obese rats
Adiponectin and leptin for the obese, obese + low-dose GB extract, and obese + high-dose GB extract groups as compared to the control group are shown in Figure 1. Adiponectin was significantly decreased in HFD rats compared with control rats (p < 0.05). Otherwise, leptin was significantly increased in obese rats in contrast to the normal control group. Otherwise, GB supplementation at low or high doses significantly increased adiponectin and decreased leptin levels compared to obese rats (p < 0.05).
Effects of goldenberry supplementation on lipid profiles of obese rats
Table 3 shows the lipid profile of obese rats that took the GB supplement. In this study, a substantial increase in plasma TG and TC levels (p < 0.05) was noticed in obese rats compared to the normal control group. GB supplementation markedly reduced the levels of plasma TC and TG in obese rats (p < 0.05) as viewed in Table 3 and significantly lowered the level of HDL cholesterol in obese rats (p < 0.05) compared to the normal control group. GB supplementation groups significantly improved HDL compared to obese rats (p < 0.05). Furthermore, the level of LDL cholesterol was significantly amplified (p < 0.05) in the obese group compared to the normal control group. GB group of low or high dose markedly reduced the LDL level (p < 0.05).
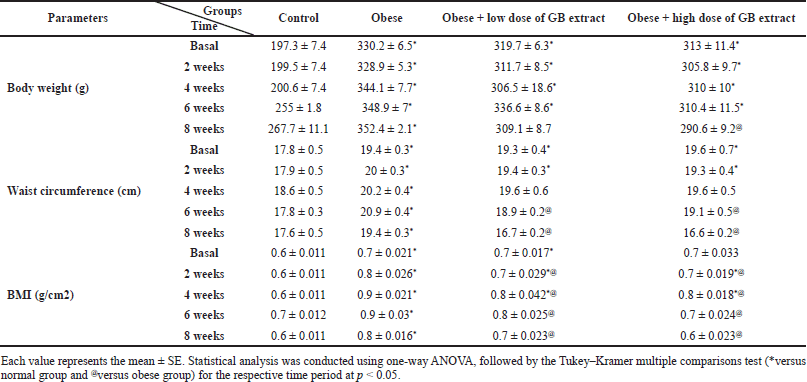 | Table 2. Effect of GB extract on anthropometric measures of obese rats. [Click here to view] |
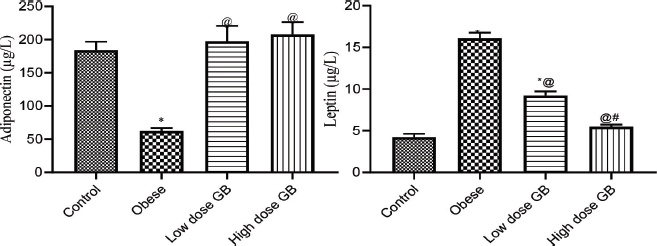 | Figure 1. Adiponectin and leptin of obese, obese + low dose of GB extract, and obese + high dose of GB extract groups in comparison with the control. Each bar represents the mean ± SE. Statistical analysis was performed using one-way ANOVA, followed by the Tukey–Kramer multiple comparisons test for the respective time period (*versus control group and @versus obese group) at p < 0.05. [Click here to view] |
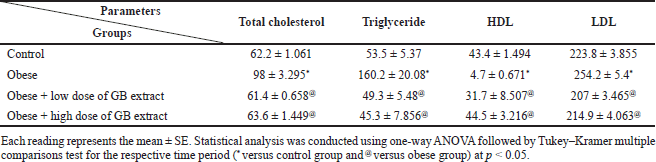 | Table 3. Lipid profile parameters of obese, obese + low dose of GB extract, and obese + high dose of GB extract groups compared to control. [Click here to view] |
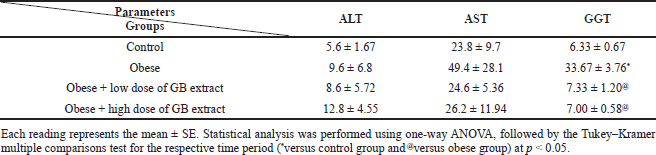 | Table 4. Liver function parameters for obese, obese + low dose of GB extract, and obese + high dose of GB extract groups compared to control. [Click here to view] |
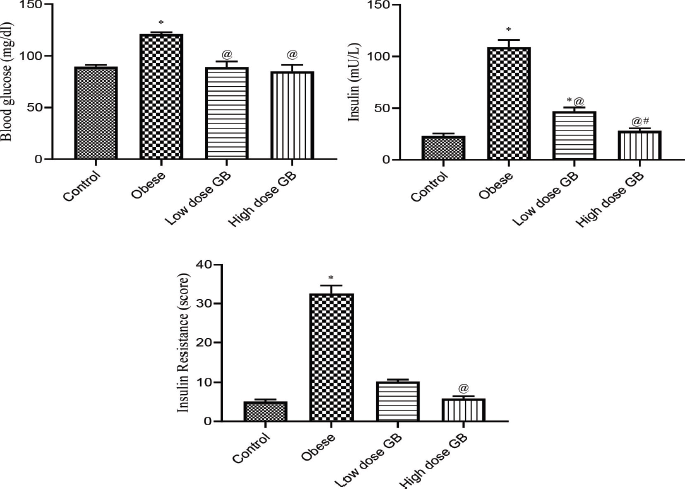 | Figure 2. Blood glucose, insulin, and insulin resistance of Obese, Obese+ low dose of GB Extract, and Obese+ high dose of GB Extract groups as compared to control. Each bar represents the mean ± se. Statistical analysis was conducted using one-way ANOVA followed by Tukey-Kramer multiple comparisons test for the respective time period. (* vs control group and @ vs obese group) at p<0.05. [Click here to view] |
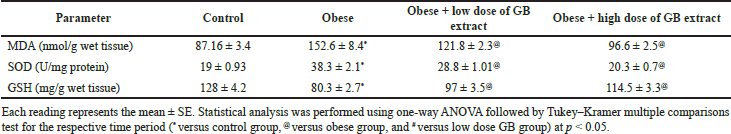 | Table 5. Antioxidant and oxidative stress parameters in the liver of obese, obese + low dose of GB extract, and obese + high dose of GB extract groups compared to control. [Click here to view] |
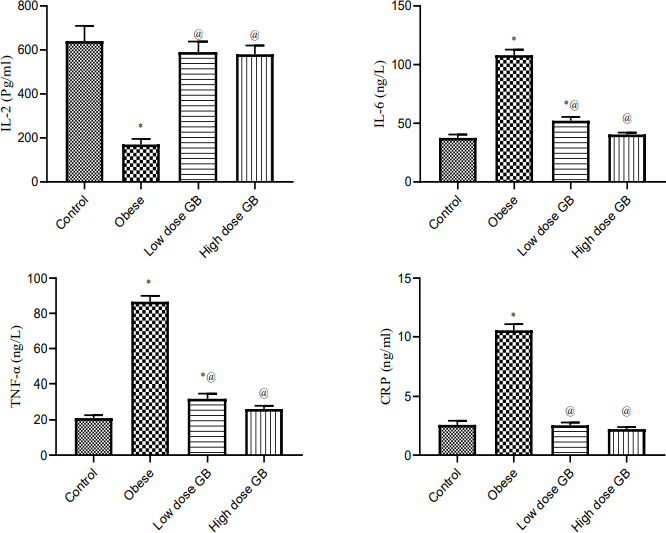 | Figure 3. Anti-inflammatory markers Il-2, interleukin-2; Il6, interleukin-6; TNF- α, tumor necrosis factor- alpha; and CRP, C-reactive protein of Obese, Obese+ low dose of GB Extract, and Obese+ high dose of GB Extract groups as compared to control. Each bar represents the mean ± se. Statistical analysis was performed using one-way ANOVA followed by Tukey-Kramer multiple comparisons test at respective time interval. (* vs control group and @ vs obese group) at p<0.05. [Click here to view] |
Effects of goldenberry supplementation on liver function of obese rats
Animals fed a high-fat diet developed steatosis and hepatic impairment, as a result of fat buildup in the liver. The activity of enzyme markers of liver function was used to measure hepatic damage. When liver damage occurs, serum ALT, AST, and GGT activities rise, therefore we examined these enzymes in control animals fed a high-fat diet. Table 4 shows the changes in liver enzyme functioning. In the high-fat-fed diet rats, plasma AST activity was substantially higher (p < 0.05) than in the control rats. Compared with obese rats, oral goldenberry supplementation for 8 weeks restored AST activity in the obese + low-dose GB extract and obese + high-dose GB extract rats (p < 0.05). In rats fed a high-frequency diet, plasma ALT and GGT activities were also elevated twofold to threefold compared to the control group (p < 0.05), respectively. In the obese + low-dose GB extract and obese + high-dose GB extract rats, oral supplementation with GB also normalized the activities of these liver enzymes by decreasing ALT and GGT activities compared to obese rats. (p < 0.05).
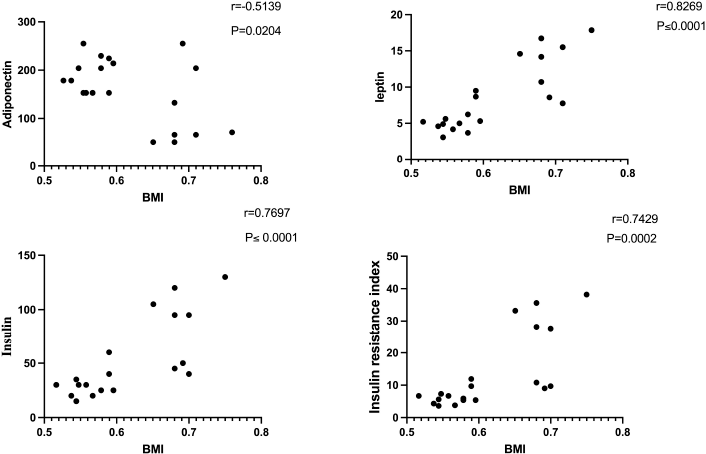 | Figure 4. Correlation of BMI with adiponectin, leptin, insulin, and insulin resistance in obese rats. [Click here to view] |
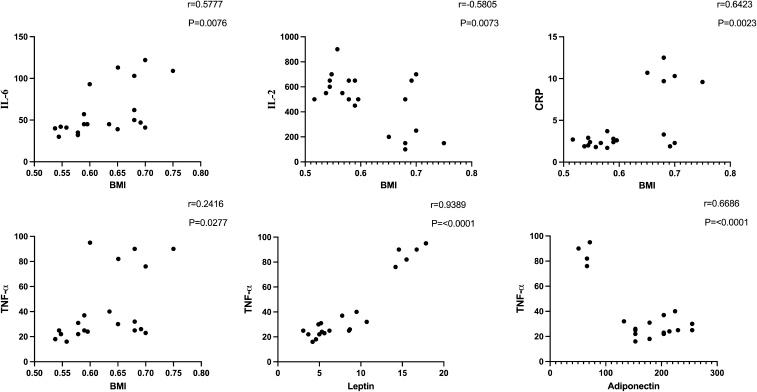 | Figure 5. Correlation of BMI with TNF-α, IL-6, CRP, and IL-2 and correlation of leptin and adiponectin with TNF-α in obese rats. [Click here to view] |
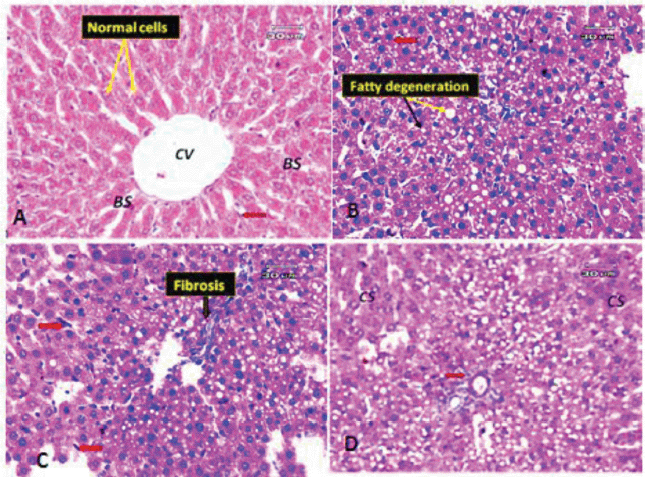 | Figure 6. (A) Photomicrograph of a liver section of a control rat showing the general appearance of the hepatic lobules (normal cells). Notice the CV, normal BS, and Kupffer cells (red arrow). (B) Liver section of obese rat showing macrovesicular and microvesicular steatosis (fatty change); black arrow indicates the microvesicles and yellow arrow indicates the macrovesicles. Some hepatocytes appeared pyknotic (red arrow). (C) Liver section of obese rat (another filed) showing minimal fibrosis and hypertrophy of Kupffer cells (red arrow). (D) Liver section of obese rat (another filed) demonstrating hepatocytes with cloudy swelling or hydropic degeneration (CS), few inflammatory infiltrate around portal tract (red arrow). [Click here to view] |
Effect of goldenberry supplementation on insulin resistance parameters in obese rats
Figure 2 illustrates the blood glucose, insulin, and insulin resistance of obese, obese + low-dose GB extract, and obese + high-dose GB extract groups as compared to control (p < 0.05). Treatment of obese rats with a low or high dose of goldenberry extract attenuated the increase in plasma insulin or glucose concentration and in the insulin resistance index (Fig. 2). The insulin level of high-dose GB extracts is significantly lower than that of a low dose (p < 0.05), while there is no significant difference between doses of GB extract on glucose or IR level (p < 0.05).
Effect of goldenberry supplementation on the liver’s antioxidant and oxidative stress parameters in obese rats
Table 5 presents the antioxidant enzymes and oxidative stress parameters in the liver of the obese, obese + low-dose GB extract, and obese + high-dose GB extract groups compared to the control group. The content of MDA in the liver is used to determine reactive oxygen species when compared to control rats since animals on the HF diet have a substantially higher lipid peroxidation in the liver (p > 0.05). When compared to obese rats, GB supplementation improved lipid peroxidation (p > 0.05). Reduced glutathione (GSH) and SOD are two naturally occurring cellular antioxidants that help reduce oxidative stress. The capabilities of cellular antioxidant were reduced in the HF diet-fed rats due to increased oxidative stress, as shown in Table 5 (p < 0.05). In this investigation, SOD activity was considerably elevated (p < 0.05) in the liver of HF diet-fed rats compared to the control group, whereas GSH was dramatically reduced in the liver of obese rats compared to normal rats. Compared with obese rats, GB supplementation significantly (p < 0.05) restored SOD activity and GSH levels in the obese + low-dose and obese + high-dose GB extract groups.
Effect of goldenberry supplementation on the plasma inflammatory markers in obese rats
Anti-inflammatory markers IL-2, IL-6, TNF-α, and CRP for obese, obese + low-dose GB extract, and obese + high-dose GB extract groups compared to the control group are shown in Figure 3. IL-2 was significantly decreased in HFD rats compared with control rats (p < 0.05). Otherwise, IL-6, TNF-α, and CRP were significantly increased in obese rats in contrast to the normal control group. GB supplementation at low or high doses significantly improved all levels of anti-inflammatory markers compared to obese rats (p < 0.05). Correlation results (Figs. 4 and 5) revealed a positive correlation between BMI and leptin, insulin, CRP, TNF-α, IL6, or IR. On the other hand, a negative correlation of BMI with both adiponectin and IL2 was recorded.
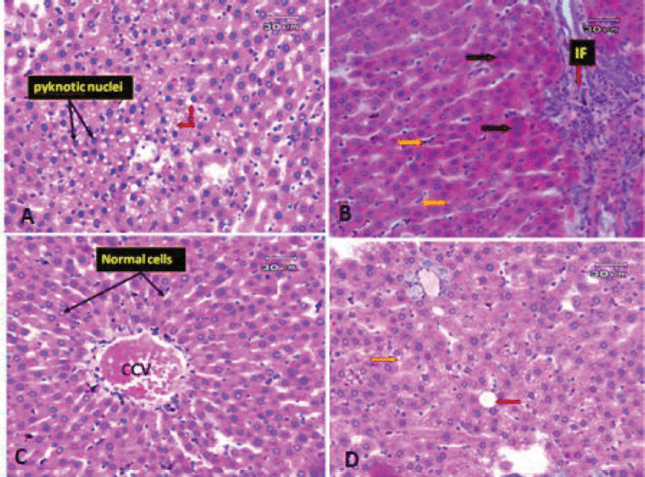 | Figure 7. Photomicrographs of rat liver. (A) Liver section of obese rat subjected to extract at low dose showing some improvement in pathological changes in the form of no steatosis, but the liver tissue still suffers from some changes in the form of some hepatocyte appeared pyknotic (black arrow) and microvacuolar degeneration (red arrow). (B) Liver section of obese rat subjected to extract at a low dose (another filed) showing an increase inflammatory infiltrate around portal vein (red arrow) and hyperplasia of Kupffer cells (orange arrow). (C) Liver section of obese rat subjected to extract at high dose revealed more or less preserved hepatic architecture. The hepatocytes are arranged in cords radiating from CCV and separated by normal BS and Kupffer cells. (D) Liver section of obese rat subjected to extract at high dose (another filed) showing some macro- (red arrow) and microvacuolation (yellow arrow) still present. [Click here to view] |
Effect of goldenberry supplementation on the histological assessment of the liver
The liver, which has two lobes, is one of the largest organs in the human body. The plates of hepatocytes sprout from a terminal branch of the hepatic vein—the central vein—split each lobe into hexagonal hepatic lobules. Six portal tracts and one or more bile ductless surround each hepatic lobule on average. Portal triads allow blood to enter, travel through the sinusoids between hepatocyte plates, and drain into the CV. Kupffer cells and endothelial cells border the blood sinusoids (BS) (Fig. 6A). Liver tissues of obese rats were histopathologically examined and revealed fatty changes with micro- and macrovesicular steatosis. There was a little fibrosis; some hepatocytes that seemed pyknotic; and hypertrophied Kupffer cells. There were few inflammatory infiltrates surrounding the portal vein, as well as hazy swelling injuries (Figs. 6B–D). Although minute vacuolar degeneration, some pyknotic hepatocytes, increased inflammatory infiltrate around the portal vein, and hyperplasia of Kupffer cells were still present, the histological changes in liver sections of obese rats exposed to low-dose goldenberry extract led to some improvement in pathological changes in the form of no steatosis (Figs. 7A and B). Histopathological changes in liver tissues of obese rats exposed to a high dose of the extract revealed a reduction in hepatic lesions and degenerative changes (e.g., no steatosis, no pyknotic cells, and no cloudy swelling), the liver lobules regained their regular architecture with normal hepatocytes, and although the CV was still congested, some macro- and microvacuolation was still present (Figs. 7C and D).
DISCUSSION
Health problems related to being overweight are increasing in many countries around the world. A high-fat, high-energy diet is believed to be the primary contributor to the development of these issues. Our aim in this research is to study the effect of goldenberry extract on insulin resistance, oxidative stress, inflammation, and fatty liver induced by obesity. In our research, treating obese rats with goldenberry significantly reduced BMI, waist circumference, and body weight gain. Our results are in agreement with Hassan et al. (2019). BMI changes have been linked to hyperlipidemia and oxidative stress in rat serum, suggesting that BMI may predict these negative effects of obesity in rats (Novelli et al., 2007).
The results obtained indicated that the levels of cholesterol, TGs, and LDL were significantly amplified in obese rats compared with the control, whereas HDL levels were considerably lower. Our findings are consistent with previous studies (Hassan et al., 2019; Jiang et al., 2021). Goldenberry fruits with husk extract have hypercholesterolemia-lowering effects in obese rats. Goldenberry administration also keeps the liver from oxidative stress and decreases the amount of fatty liver that develops as a result of the high-fat diet (Ramadan, 2012). It has been proposed that people with coronary atherosclerosis should take goldenberry to prevent the condition from progressing (Ramadan, 2011). Goldenberry fruit has been reported to be highly nutritious, as it contains high levels of vitamins A, B, and C. The main active ingredient of vitamin A in the fruit is a-carotene, ß-carotene, and ß-kryptoxanthin. Phytosterols are of great interest because of their antioxidant capacity and impact on both TC and LDL cholesterol (Sun et al., 2016). Supplementation of goldenberry extract (the fruit with husk) increased HDL cholesterol levels. Since HDL is involved in the transfer of cholesterol from peripheral cells to the liver, elevated blood HDL cholesterol levels obtained from dietary goldenberry extract are thought to be beneficial in reducing the risk of cardiovascular disease. Our study of the fruit and husk as a single extract reveals the full extent of their antiobesity potential. The obese rats had significantly higher blood levels of leptin and lower levels of adiponectin compared to the control group. Goldenberry extract supplementation (low or high dose) significantly reduced the increase in leptin levels, brought them to near-normal levels, and improved adiponectin levels. Our results are in agreement with previous research (Al-Rasheed et al., 2017; Stoica et al., 2021). Obesity is characterized by a wide range of circulating adipokine levels due to the aberrant accumulation and dysfunction of adipose tissues. Also, obesity problems and an increased risk of developing obesity-related comorbidities, including type 2 diabetes, are associated with altered levels of leptin and adiponectin. In rat models, leptin and adiponectin are also indicators of metabolic syndrome. Leptin is a neuroendocrine and energy homeostasis regulator that reflects energy reserves, fat mass, and energy deprivation. There is a positive correlation between BMI and leptin in obese rats. On the other hand, a negative correlation was recorded between BMI and adiponectin. These correlations suggest that BMI may influence the secretion of the two adipocyte hormones.
Hepatic injury or alterations in the permeability of the hepatocyte membrane are associated with increased AST, ALT, and GGT activities. Because AST is found throughout the body, particularly in the liver, muscles, and red blood cells, ALT and GGT are considered to be more specialized liver enzymes. Furthermore, a greater degree of liver function in serum is used to diagnose not only liver damage but also hepatic insulin resistance, metabolic syndrome, and type 2 diabetes (Kinasih et al., 2020). Serum ALT level and insulin resistance are intrinsically correlated, but not AST, and has been shown to be a predictor of T2D in humans. At the highest dose of the extract, oral administration of goldenberry extract to obese rats resulted in the largest decline in liver enzymes. These results are consistent with Arun and Asha’s (2007) study, where they found that Physalis extract is rich in flavonoids and has hepatoprotective or antihepatoma activity. It has been found that Physalis peruviana scavenge-free radicals generated by CCl4 raises the activity of the antioxidant defense system and increases the kidney’s vulnerability to oxidant stress. As a result, Physalis extract can be used as a dietary antioxidant to slow down aging, prevent illnesses caused by ROS, and reduce oxidative damage in tissues.
Obesity is a principal causative factor in the development of the metabolic syndrome. Insulin resistance, inadequate glucose metabolism, and finally the development of type 2 diabetes mellitus are all linked to obesity, oxidative stress, and fatty liver (Aydin et al., 2014; Hafizi Abu Bakar et al., 2015). The rise in glucose, insulin resistance, and lipid profiles is caused by the saturated fats found in the high-fat diet, as well as by sugar (Timmers et al., 2011). According to the findings, the obese rats fed a high-fat diet with sucrose had higher plasma glucose levels, insulin levels, insulin resistance index, plasma lipid levels, including cholesterol and TG levels, and oxidative stress in the liver. In obese rats, goldenberry administration reduced glucose levels, insulin resistance, and plasma lipid levels. As a result, the antiobesity efficacy of goldenberry fruit and husk extract was evaluated in the current study using an HFD-induced obesity animal model. Fruit-containing husk extract reduces blood glucose levels indicating that the extract is beneficial in maintaining glucose homeostasis. Obese mice were given the fruit and husk extract had easier insulin-stimulated glucose absorption into the peripheral organs. Insulin sensitivity was improved in obese rats treated with the fruit and husk extract, revealing that the extract has significant insulin sensitization activity in addition to enhanced glucose homeostasis, most likely due to improved pancreatic ß-cell function, as evidenced by increased plasma insulin levels. Insulin controls the activity of several metabolic enzymes to regulate metabolism by modifying glucose absorption and utilization in target organs such as the liver, skeletal muscle, and adipose tissue (Petersen and Shulman, 2018). Wet liver weights in obese rats were considerably increased after treatment with fruit extract in this study, indicating enhanced glucose use and storage. Liver MDA and SOD levels were increased dramatically, whereas GSH levels were significantly decreased in obese rats, according to the results, by replenishing cellular antioxidants, GB supplementation reduced oxidative stress, inflammatory indicators, and lipid peroxidation.
There are positive correlations between BMI and parameters of inflammation (IL-2, IL-6, CRP, and TNF-α). Moreover, there is a positive correlation between leptin and TNF-α. Leptin and the TNF-α system may be involved in the pathogenesis of obesity and insulin resistance. An increase in leptin and TNF-α leads to an increase in insulin resistance (Lee et al., 2014).
TNF-α, a proinflammatory cytokine, is one of the most prominent proinflammatory mediators implicated in the development of insulin resistance and the pathophysiology of T2DM. TNF-α is predominantly generated in adipocytes and/or peripheral tissues. TNF causes tissue-specific inflammation by generating ROS and activating a variety of transcriptionally driven pathways. TNF-α increases insulin resistance in adipocytes and peripheral tissues by affecting insulin signaling through serine phosphorylation, leading to the development of T2DM (Akash et al., 2018).
In this study, histopathological investigations of liver tissues in obese rats showed fatty changes with micro- and macrovesicular steatosis; some hepatocytes appeared pyknotic; and hypertrophied Kupffer cells could be observed. Our results agree with previous studies (Agil et al., 2015; Altunkaynak, 2005; Ramli et al., 2014). After a low dose of GB administration, the results exhibited that hepatic tissue of obese rats showed slight improvement in pathological changes, i.e., there was no steatosis, but the liver tissue still suffered from some changes in the form of some hepatocytes that appeared pyknotic and microvacuolar atrophy. These observations are similar to previous studies (Agil et al., 2015; Hatzis et al., 2013; Ramli et al., 2014), which reported having shown little infrequent steatosis of the liver cells and gentle correction of the tissue cells. Finally, high-dose GB reported that the hepatic architecture was more or less preserved. The hepatocytes are arranged in cords radiating from a congested central vein (CCV) and separated by normal BS and Kupffer cells. These are in agreement with Chinchu et al. (2020) and Mashmoul et al. (2016).
Increased lipid peroxidation was related to decreased GSH levels and increased SOD levels, indicating that oxidative stress induced by free radicals in obesity contributed to the progression of liver steatosis (fatty transformation) and fibrosis. Treatment with goldenberry extracts significantly enhanced the activity of these enzymes, indicating that goldenberry has antioxidant and antihepatoprotective properties, which is comparable to the results of Arun and Asha (2007). The antioxidant and antifibrotic effect of goldenberry may be due to the presence of quercetin (Janbaz et al., 2004). Quercetin belongs to a broad class of polyphenolic flavonoid compounds found in goldenberry (Table 1). Due to its ability to scavenge free radicals and bind transition metal ions, quercetin is a powerful antioxidant. Quercetin’s properties allow it to inhibit lipid peroxidation (Anand David et al., 2016) and it has anti-inflammatory properties (Boots et al., 2008). Goldenberry extract also contains kaempferol in addition to quercetin. Due to its ability to scavenge free radicals and active oxygen species such as singlet oxygen, superoxide anion radicals, and hydroxyl radicals, kaempferol is known as a potential antioxidant (Tatsimo et al., 2012). MDA is one of the most common lipid peroxidation products, and its high levels may reflect the severity of lipid peroxidation injury in hepatocytes (Al-Olayan et al., 2014). MDA levels are significantly reduced when goldenberry extract is supplemented, demonstrating that it has an antiperoxidative action.
CONCLUSION
Treating obese rats with goldenberry extract (fruits with husk) showed an efficient therapeutic result in alleviating the symptoms and complications of obesity, such as insulin resistance, type 2 diabetes, and fatty liver, presumably by reducing oxidative stress, lipids, and inflammatory markers or improving the antioxidant defense system. Ultimately, goldenberry may reduce the complications of obesity via controlling adipocyte hormones and capturing free radicals.
ACKNOWLEDGMENT
The authors are grateful to National Research Centre, Cairo, Egypt, for funding this work.
AUTHORS’ CONTRIBUTIONS
Sherif Moussa and Samir Bashandy: conceptualization, methodology, data curation, writing, and visualization. Fatma Ibrahim, Samir Aziz, and Sherif Moussa: conceptualization, methodology, data curation, writing, and editing preparation. Marawan Abd Elbaset, Atef Attia, and Noha Abdellatif: conceptualization, methodology, software, visualization, and investigation. Fatma A Morsy: pathological and histochemical methodology, investigation, and writing. Sayed Toumy El and Josline Salib: preparation of the goldenberry extract and measurement of total phenolics and flavonoid contents.
CONFLICT OF INTEREST
No conflict of interest has been declared by the authors.
FUNDING
This research received a grant from National Research Centre under the project No.12060172.
ETHICAL APPROVAL
Animal experimentation was approved by the Ethics Committee of the National Research Centre for Animal Care and Use (Approval no.: 19161).
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, Marczak L, Mokdad AH, Moradi-Lakeh M, Naghavi M, Salama JS, Vos T, Abate KH, Abbafati C, Ahmed MB, Al-Aly Z, Alkerwi A, Al-Raddadi R, Amare AT, Amberbir A, Amegah AK, Amini E, Amrock SM, Anjana RM, Ärnlöv J, Asayesh H, Banerjee A, Barac A, Baye E, Bennett DA, Beyene AS, Biadgilign S, Biryukov S, Bjertness E, Boneya DJ, Campos-Nonato I, Carrero JJ, Cecilio P, Cercy K, Ciobanu LG, Cornaby L, Damtew SA, Dandona L, Dandona R, Dharmaratne SD, Duncan BB, Eshrati B, Esteghamati A, Feigin VL, Fernandes JC, Fürst T, Gebrehiwot TT, Gold A, Gona PN, Goto A, Habtewold TD, Hadush KT, Hafezi-Nejad N, Hay SI, Horino M, Islami F, Kamal R, Kasaeian A, Katikireddi SV, Kengne AP, Kesavachandran CN, Khader YS, Khang YH, Khubchandani J, Kim D, Kim YJ, Kinfu Y, Kosen S, Ku T, Defo BK, Kumar GA, Larson HJ, Leinsalu M, Liang X, Lim SS, Liu P, Lopez AD, Lozano R, Majeed A, Malekzadeh R, Malta DC, Mazidi M, McAlinden C, McGarvey ST, Mengistu DT, Mensah GA, Mensink GBM, Mezgebe HB, Mirrakhimov EM, Mueller UO, Noubiap JJ, Obermeyer CM, Ogbo FA, Owolabi MO, Patton GC, Pourmalek F, Qorbani M, Rafay A, Rai RK, Ranabhat CL, Reinig N, Safiri S, Salomon JA, Sanabria JR, Santos IS, Sartorius B, Sawhney M, Schmidhuber J, Schutte AE, Schmidt MI, Sepanlou SG, Shamsizadeh M, Sheikhbahaei S, Shin MJ, Shiri R, Shiue I, Roba HS, Silva DAS, Silverberg JI, Singh JA, Stranges S, Swaminathan S, Tabarés-Seisdedos R, Tadese F, Tedla BA, Tegegne BS, Terkawi AS, Thakur JS, Tonelli M, Topor-Madry R, Tyrovolas S, Ukwaja KN, Uthman OA, Vaezghasemi M, Vasankari T, Vlassov VV, Vollset SE, Weiderpass E, Werdecker A, Wesana J, Westerman R, Yano Y, Yonemoto N, Yonga G, Zaidi Z, Zenebe ZM, Zipkin B, Murray CJL . Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med, 2017; 377:13–27; doi:10.1056/NEJMoa1614362 CrossRef
Agil A, El-Hammadi M, Jiménez-Aranda A, Tassi M, Abdo W, Fernández-Vázquez G, Reiter RJ. Melatonin reduces hepatic mitochondrial dysfunction in diabetic obese rats. J Pineal Res, 2015; 59:70–9; doi:10.1111/jpi.12241 CrossRef
Akash MSH, Rehman K, Liaqat A. Tumor necrosis factor-alpha: role in development of insulin resistance and pathogenesis of type 2 diabetes mellitus. J Cell Biochem, 2018; 119:105–10; doi:10.1002/jcb.26174.
Al-Olayan EM, El-Khadragy MF, Aref AM, Othman MS, Kassab RB, Abdel Moneim AE. The potential protective effect of Physalis peruviana L. against carbon tetrachloride-induced hepatotoxicity in rats is mediated by suppression of oxidative stress and downregulation of MMP-9 expression. Oxid Med Cell Longev, 2014; 2014; doi:10.1155/2014/381413 CrossRef
Al-Rasheed NM, Abdelkarem HM, Fadda LM, Mohamed AM, Al-Rasheed NM, Bassiouni Y, Ali HM, Gafeer AH. Amelioration of insulin, leptin and adiponectin levels in experimental metabolic syndrome model by some drugs. Indian J Pharm Sci, 2017; 78:701–7; doi:10.4172/pharmaceutical-sciences.1000173 CrossRef
Altunkaynak BZ. Effects of high fat diet induced obesity on female rat livers (a histochemical study). Eur J Gen Med, 2005; 2:100–9; doi:10.29333/ejgm/82319 CrossRef
Anand David AV, Arulmoli R, Parasuraman S. Overviews of biological importance of quercetin: a bioactive flavonoid. Pharmacogn Rev, 2016; 10:84–9; doi:10.4103/0973-7847.194044 CrossRef
Arun M, Asha VV. Preliminary studies on antihepatotoxic effect of Physalis peruviana Linn. (Solanaceae) against carbon tetrachloride induced acute liver injury in rats. J Ethnopharmacol, 2007; 111:110–4; doi:10.1016/J.JEP.2006.10.038 CrossRef
Aydin Suleyman, Aksoy A, Aydin Suna, Kalayci M, Yilmaz M, Kuloglu T, Citil C, Catak Z. Today’s and yesterday’s of pathophysiology: biochemistry of metabolic syndrome and animal models. Nutrition, 2014; 30:1–9; doi:10.1016/j.nut.2013.05.013 CrossRef
Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med, 1963; 61:882–8.
Boots AW, Wilms LC, Swennen ELR, Kleinjans JCS, Bast A, Haenen GRMM. In vitro and ex vivo anti-inflammatory activity of quercetin in healthy volunteers. Nutrition, 2008; 24:703–10; doi:10.1016/J.NUT.2008.03.023 CrossRef
Caixàs A, Albert L, Capel I, Rigla M. Naltrexone sustained-release/bupropion sustained-release for the management of obesity: review of the data to date. Drug Des Devel Ther, 2014; 8:1419. CrossRef
Carleton HM, Drury RAB, Wallington EA. Carleton’s histological technique. Oxford University Press, Cary, NC, 1980.
Chinchu JU, Mohan MC, Prakash Kumar B. Anti-obesity and lipid lowering effects of Varanadi kashayam (decoction) on high fat diet induced obese rats. Obes Med, 2020; 17:100170; doi:10.1016/J.OBMED.2019.100170 CrossRef
Chong ETJ, Lee P-C. Prevalence of overweight and obesity in Malaysia, 2010–2016: a comprehensive meta-analysis. Southeast Asian J Trop Med Public Health, 2018; 49:859–69.
Dludla P, Nkambule B, Jack B, Mkandla Z, Mutize T, Silvestri S, Orlando P, Tiano L, Louw J, Mazibuko-Mbeje SE. Inflammation and oxidative stress in an obese state and the protective effects of gallic acid. Nutrients, 2018; 11(1):23; doi:10.3390/nu11010023 CrossRef
Effting PS, Brescianini SMS, Sorato HR, Fernandes BB, Fidelis GDSP, Silva PRL da, et al. Resistance exercise modulates oxidative stress parameters and tnf-α content in the heart of mice with diet-induced obesity. Arq Bras Cardiol, 2019; 112:545–52; doi:10.5935/abc.20190072 CrossRef
Gelen V. Can polyphenols be used as anti-inflammatory agents against COVID-19 (SARS-CoV-2)-induced inflammation? In: Kükürt A (ed.). IntechOpen, Rijeka, Croatia, Ch. 17, 2021; doi:10.5772/intechopen.98684. CrossRef
Gelen V, Kükürt A, ?engül E, Devec? HA. Leptin and its role in oxidative stress and apoptosis: an overview. Role Obes Hum Heal Dis, 2021:143. CrossRef
Hafizi Abu Bakar M, Kian Kai C, Wan Hassan WN, Sarmidi MR, Yaakob H, Zaman Huri H. Mitochondrial dysfunction as a central event for mechanisms underlying insulin resistance: the roles of long chain fatty acids. Diabetes Metab Res Rev, 2015; 31:453–75; doi:10.1002/dmrr.2601. CrossRef
Hassan SM, El-Kholie EM, Khedr AM. Anti-obesity effect of gooseberry (Physalis peruviana) fruits in-induced obese rats. J Specif Educ Stud Res, 2018; 4(1):565–90. CrossRef
Hatzis G, Ziakas P, Kavantzas N, Triantafyllou A, Sigalas P, Andreadou I, Ioannidis K, Chatzis S, Filis K, Papalampros A, Sigala F. Melatonin attenuates high fat diet-induced fatty liver disease in rats. World J Hepatol, 2013; 5:160–9; doi:10.4254/wjh.v5.i4.160 CrossRef
Ito F, Sono Y, Ito T. Measurement and clinical significance of lipid peroxidation as a biomarker of oxidative stress: oxidative stress in diabetes, atherosclerosis, and chronic inflammation. Antioxidants, 2019; 8; doi:10.3390/antiox8030072 CrossRef
Janbaz KH, Saeed SA, Gilani AH. Studies on the protective effects of caffeic acid and quercetin on chemical-induced hepatotoxicity in rodents. Phytomedicine, 2004; 11:424–30; doi:10.1016/J.PHYMED.2003.05.002 CrossRef
Jiang H, Zhang W, Li X, Xu Y, Cao J, Jiang W. The anti-obesogenic effects of dietary berry fruits: a review. Food Res Int, 2021; 147:110539; doi:10.1016/j.foodres.2021.110539 CrossRef
Kei S. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin Chim Acta, 1978; 90:37–43; doi:10.1016/0009-8981(78)90081-5 CrossRef
Kinasih LS, Djamiatun K, Al-Baarri AN. Golden berry (Physalis peruviana) juice for reduction of blood glucose and amelioration of insulin resistance in diabetic rats. J Gizi Dan Pangan, 2020; 15:37–44; doi:10.25182/jgp.2020.15.1.37-44 CrossRef
Kumaran A, Joel Karunakaran R. In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. LWT—Food Sci Technol, 2007; 40:344–52; doi:10.1016/j.lwt.2005.09.011 CrossRef
Lee SM, Choi HJ, Oh CH, Oh JW, Han JS. Leptin increases TNF-α expression and production through phospholipase D1 in raw 264.7 cells. PLoS One, 2014; 9:1–9; doi:10.1371/journal.pone.0102373 CrossRef
Mandal B, Madan S. Preliminary phytochemical screening and evaluation of free radical scavenging activity of Stevia rebaudiana Bertoni from different geographical sources. J Pharmacogn Phytochem, 2013; 2:14–9.
Mashmoul M, Azlan A, Mohtarrudin N, Mohd Yusof BN, Khaza’ai H, Khoo HE, Farzadnia M, Boroushaki MT. Protective effects of saffron extract and crocin supplementation on fatty liver tissue of high-fat diet-induced obese rats. BMC Complement Altern Med, 2016; 16:1–7; doi:10.1186/s12906-016-1381-9 CrossRef
Mishra KP. Cell membrane oxidative damage induced by gamma-radiation and apoptotic sensitivity. J Environ Pathol Toxicol Oncol, 2004; 23(1):61–6. CrossRef
Monguchi T, Hara T, Hasokawa M, Nakajima H, Mori K, Toh R, Irino Y, Ishida T, Hirata KI, Shinohara M. Excessive intake of trans fatty acid accelerates atherosclerosis through promoting inflammation and oxidative stress in a mouse model of hyperlipidemia. J Cardiol, 2017; 70:121–7. CrossRef
Nishikimi M, Appaji Rao N, Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun, 1972; 46:849–54; doi:10.1016/S0006-291X(72)80218-3 CrossRef
Noeman SA, Hamooda HE, Baalash AA. Biochemical study of oxidative stress markers in the liver, kidney and heart of high fat diet induced obesity in rats. Diabetol Metab Syndr, 2011; 3:1–8; doi:10.1186/1758-5996-3-17. CrossRef
Novelli ELB, Diniz YS, Galhardi CM, Ebaid GMX, Rodrigues HG, Mani F, Fernandes AA, Cicogna AC, Novelli Filho JL. Anthropometrical parameters and markers of obesity in rats. Lab Anim, 2007; 41:111–9; doi:10.1258/002367707779399518. CrossRef
Othman ZA, Zakaria Z, Suleiman JB, Ghazali WSW, Mohamed M. Anti-atherogenic effects of orlistat on obesity-induced vascular oxidative stress rat model. Antioxidants, 2021; 10:251. CrossRef
Petersen MC, Shulman GI. Mechanisms of insulin action and insulin resistance. Physiol Rev, 2018; 98:2133–223; doi:10.1152/physrev.00063.2017 CrossRef
Ramadan MF. Physalis peruviana pomace suppresses high-cholesterol diet-induced hypercholesterolemia in rats. Grasasyaceites, 2012; 63:4. CrossRef
Ramadan MF. Bioactive phytochemicals, nutritional value, and functional properties of cape gooseberry (Physalis peruviana): an overview. Food Res Int, 2011; 44:1830–6; doi:10.1016/j.foodres.2010.12.042 CrossRef
Ramli NS, Brown L, Ismail P, Rahmat A. Effects of red pitaya juice supplementation on cardiovascular and hepatic changes in high-carbohydrate, high-fat diet-induced metabolic syndrome rats. BMC Complement Altern Med, 2014; 14; doi:10.1186/1472-6882-14-189 CrossRef
Reaven G, Abbasi F, McLaughlin T, hormone TM-R progress in, 2004 undefined. Obesity, insulin resistance, and cardiovascular disease. Recent Prog Horm Res, 2004; 59:207–24. CrossRef
Saboo S, Tapadiya R, Khadabadi SS, Deokate UA. In vitro antioxidant activity and total phenolic, flavonoid contents of the crude extracts of Pterospermum acerifolium wild leaves (Sterculiaceae). J Chem Pharm Res, 2010; 2:417–23.
Skolnik NS, Ryan DH. Pathophysiology, epidemiology, and assessment of obesity in adults. J Fam Pract, 2014; 63.
Souza Cruz EM, Bitencourt de Morais JM, Dalto da Rosa CV, da Silva Simões M, Comar JF, de Almeida Chuffa LG, Seiva FRF. Long-term sucrose solution consumption causes metabolic alterations and affects hepatic oxidative stress in Wistar rats. Biol Open, 2020; 9:bio047282. CrossRef
Stoica L, Gadea R, Navolan DB, Lazar F, Duta C, Stoian D, Tarta C, Olaru F, Isaic A, Dobrescu A. Plasma ghrelin, adiponectin and leptin levels in obese rats with type 2 diabetes mellitus after sleeve gastrectomy and gastric plication. Exp Ther Med, 2021; 21:264; doi:10.3892/etm.2021.9695. CrossRef
Sun N-N, Wu T-Y, Chau C-F. Natural dietary and herbal products in anti-obesity treatment. Molecules, 2016; 21; doi:10.3390/molecules21101351 CrossRef
Tatsimo SJN, Tamokou JDD, Havyarimana L, Csupor D, Forgo P, Hohmann J, Kuiate JR, Tane P. Antimicrobial and antioxidant activity of kaempferol rhamnoside derivatives from Bryophyllum pinnatum. BMC Res Notes, 2012; 5:1–6; doi:10.1186/1756-0500-5-158/TABLES/2. CrossRef
Timmers S, De Vogel-Van Den Bosch J, De Wit N, Schaart G, Van Beurden D, Hesselink M, van der Meer R, Schrauwen P. Differential effects of saturated versus unsaturated dietary fatty acids on weight gain and myocellular lipid profiles in mice. Nutr Diabetes, 2011; 1:e11–9; doi:10.1038/nutd.2011.7 CrossRef
Uzun H, Konukoglu D, Gelisgen R, Zengin K, Taskin M. Plasma protein carbonyl and thiol stress before and after laparoscopic gastric banding in morbidly obese patients. Obes Surg, 2007; 17(10):1367–73; doi:10.1007/s11695-007-9242-8. CrossRef
Valdecantos MP, Pérez-Matute P, Martínez JA. Obesity and oxidative stress: role of antioxidant supplementation. Rev Investig Clin, 2009; 61:127–39.
Yaman SO, Ayhanci A. Lipid peroxidation. In: Atukeren P (ed.). Accent lipid peroxidation, IntechOpen, London, UK, p 1, 2021. ; doi:10.5772/intechopen.95802.