INTRODUCTION
Interruption of coronary blood flow partially or completely to a portion of the myocardium over the threshold result in to infraction. According to statement given by World Health Organization (WHO), highest mortality rate is accounted by the ischemic heart diseases (Aronow, 2006; Patel et al., 2010). Despite modern medicines are widely used to treat cardiovascular diseases but its cost, side effects, availability, people are looking in to traditional and complementary medicines has been increased to preventive medicine of human diseases (Adenipekun et al., 2018). Bioactive phytochemicals such as polyphenols, flavonoids, tannins, anthocyanins rich fruits, vegetable, medicinal plants and its extracts have been shown to beneficial in treating in heart disorders (ischemic heart disease, cardiac arrythmias, heart failure) and vascular problems (atherosclerosis, endothelial dysfunction, hypertension) (Ganapathy et al., 2020). Underlined cardioprotective mechanism involved is improving contractile function of heart, attenuation of Low-density lipoprotein oxidation, preservation of membrane bound ATPases, regulating calcium load, nitric oxide availability, mitochondrial dysfunction, myocardial inflammation (Li et al., 2016). Even several natural products rich in flavonoids, polyphenolics and its isolated compounds such as luteolin, apigenin, gallic acid, maslinic acid, rosmarinic acid, ellagic acid etc., are reported to antagonize the isoproterenol (ISO) induced oxidative stress in myocardium due to its antioxidant activity (Mohan et al., 2010). Hence discovery of novel phytochemicals having antioxidant activity are advantages in oxidative stress induced myocardial infraction (Panda, 2015). Preclinical, clinical trials and epidemiological studies highlight the pivotal role antioxidants in preventing of heart diseases (Brewer, 2011; Speer et al., 2020). Folk medicines are practiced in various indigenous cultures around the world even though the advancement of allopathic medicines happened with advanced technology and science (Thomford et al., 2018). Therefore, WHO has taken into account and established traditional medicine strategy plan for 2023 to address the issues and promote safe, effective and quality traditional medicines around the globe. India has been practising the diversified health care system which includes Allopathy, Ayurveda, Unani, Siddha and Homeopathy to deliver the possible best health care to the society. Keeping this in view India established Ministry of Ayurveda, Yoga & Naturopathy, Unani, Siddha and Homoeopathy to set standard regulations for herbal drugs and also included the herbal medicines in Indian pharmacopeia and essential drug list (Shankar and Patwardhan, 2017) Rivea ornata is a climber with cylindrical stem, broader leaves, white silky flowered and smooth surface subglobose brown colored fruits. Literature mentions its traditional importance in treating disorder of gallbladder, disease of heart, bronchitis, and fatigue. In the Konkan, its juice is used to treat piles (Kirtikar and Basu, 1918). Therefore, we try to create scientific evidence for its protective capacity by rodent model myocardial infarction (MI).
MATERIALS AND METHODS
Chemicals and equipment’s
ISO, Folin-Ciocalteu reagent, 2,2-diphenyl-1-picrylhydrazyl, vitamin C, aluminium chloride were purchased from Sigma chemicals. Gallic acid, Quercetin (TCI chemicals, India Pvt. Ltd. Chennai Tamilnadu), Ethylenediaminetetraacetic acid (EDTA), Trichloroacetic acid (TCA), 5,5 dithiobis 2-nitro benzoic acid (DTNB) Thiobarbituric acid (TBA) were purchased from Loba chemie Pvt. Ltd. Carvedilol gift sample from Connexions life sciences, Bangalore. Thin Layer Chromatography (TLC) pre-coated silica gel 60 plates (Merck), Silica gel (230–400 Mesh) from Thermo fisher scientific India Pvt.Ltd. MP30 data acquisition system (BIOPAC, Santa Barbara, CA) Rota evaporator (Aditya scientific, Hyderabad, Telangana), UV visible spectrophotometry (Labindia UV 3000), Semi-autoanylser (ERBA CHEM 7), ELISA kits (Rey biotech kits) and Cooling centrifuge (Remi).
Collection and identification of the plant material
Fresh leaves collected from flowering plant of R. ornata, were collected from Tirumala hills during between December to January and dried under shade. The plant was authenticated by Prof. K. Madhava Chetty, Taxonomist, and a voucher specimen (voucher number:0913) has been stored at S.V. University (Botany department) Tirupati, (A.P, India).
Preparation of extract
1 kg of dried leaves powder was macerated with 3 l of ethanol (99%) for 1 week. Filtrate gets concentrate using rota-evaporator, followed by drying to get the concentrate form (Senguttuvan et al., 2014).
Solvent/solvent fraction of ethanol extract of R. ornata
Hot water dissolved ethanol extract was fractionated by solvents from nonpolar to polar (n-hexane (non-polar), ethyl acetate (medium polar) and n-butanol (low polar)) respectively. After that, each fraction was concentrated using rotary evaporator. Finally, they tested for presence of flavonoids and other phytochemicals using coloration reactions reported in standard reference books and assessed the flavonoid and phenolic content (Stalikas, 2007).
Identification of flavonoids by TLC
n-Butanol fraction was tested in TLC for presence of flavonoids. Butanol fraction loaded pre-coated silica gel TLC plate was kept in closed pre-saturated chamber for 30 minutes. In the TLC method, n-hexane and ethylacetate (1:1) was used as optimized mobile phase in order to effective separation. After development of TLC chromatogram of butanol extract and standard flavonoid, plates had been removed and dried, the spots were visualized by kept in and iodine chamber (Saeed et al., 2012).
Separation of n-butanol fraction
Methanol solubilized butanol fraction (2 g) was mixed with silica gel in motor by trituration with pestle. Fraction coated silica gel placed on the top of silica gel (60–120 mesh) packed chromatography column (75 × 3.5 cm). The column was serially eluted with hexane, hexane and ethyl acetate (1:1 ration) and ethyl acetate. Total 40 sub-fractions were collected and those fractions TLC matching with standard flavonoid (quercetin) are mixed together (Etame et al., 2019).
Acute toxicity studies
It was performed as per specification of Organisation for Economic Co-operation and Development, 423 protocol. No toxic symptoms or mortality was observed until the 14 days of the period with butanol extract of R. ornata 2,000 mg/kg bw. Hence, further experimental dose was selected as one tenth and one fifth of the LD50 dose that is 200 and 400 mg/kg (Maithili et al., 2011).
Animals
The IAEC of RIPER, Anantapur has approved our protocol [878/ac/05/Committee for the Purpose of Control And Supervision of Experiments on Animals (CPCSEA)/005/2016] as per guidelines of CPCSEA, Govt of India, New Delhi.
Experimental design
Albino Wistar rats allotted as follows with minimum number (n = 6). Group 1 (Normal rats): no specific treatment, Group 2 (ISO control rats): received vehicle only (38), Group 3: Carvedilol (2 mg/kg, B.W, p.o.) (Shahzad et al., 2019), Group 4 & 5: received butanol fractions (200 and 400 mg/kg, B.W, p.o.). MI was induced to all groups (in last two days) except group 1 by ISO injection (100 mg/kg, sc).
Electrocardiogram (ECG)
Under the anesthesia (ketamine, 80 mg kg I.P and xylazine, 8 mg/kg IP), ECG was recorded with lead II position by digital physiograph (BIOPAC, Santa Barbara, CA) (Zhu et al., 2019).
Biochemical screening
On the 31st day, the blood samples [not more than 1% total body weight (B.W)] were withdrawn by non-terminal procedures (retro-orbital plexus) and clotted blood samples were centrifuged (2,500 rpm, 20 minutes) (Parasuraman et al., 2010; Upaganlawar et al., 2009). On the same day we analyzed it for quantitative estimation of Creatine kinase-MB (CK-MB), Lactate dehydrogenase (LDH), C-reactive protein (C-RP) by using semi-autoanylser ERBA CHEM (Manjunatha et al., 2020).
Estimation of myocardial tissue parameters
Myocardial tissue was separated from all animals (sacrificed by pentobarbitone injection at dose 60 mg/kg, i.p) and prepared and stored 10%w/v homogenate in ice cold 0.1 M Tris-HCL buffer (pH 7.4). Homogenate used for estimation of the followings.
Superoxide dismutase (SOD)
It was estimated according to protocol by Misra and Fridovich (1972). Briefly described, reaction mixer was prepared by combining supernatant of tissue homogenate (0.5 ml), 0.05 M carbonate buffer (1.5 ml, pH 10.2) and EDTA (0.5 ml) in a test tube. To this 0.5 ml of epinephrine (3 mM) was added and changes in absorbance was recorded for 3 minutes with 30 seconds interval at 480 nm. Its quantity was expressed as units/ mg of protein (Misra and Fridovich, 1972).
Catalase (CAT)
It was estimated according to protocol of Aebi (1984). Briefly described, in a clean test tube we taken 2 ml of Diluted tissue homogenate and 1 ml of 50 m mol/l phosphate buffer (pH 7). To this, 1,000 μl of H2O2 (30 n mol/l) just added before taking optical density at 240 nm; taken the readings for 3 minutes with 15 seconds interval. Its quantity expressed as μ mol of H2O2 evolved/mg of protein.
Reduced glutathione (GSH)
It is estimated as per protocol of Moron et al. (1979). Briefly described, in a test tube tissue homogenate(1 ml), 10% w/v TCA (1 ml) were taken and kept at 60ºC for 20 minutes on water bath and then centrifuged (at 2,000 rpm for 15 minutes). Supernatant (0.5 ml), DTNB (4 ml) and 0.2 M phosphate buffer at pH 8.0 (1.5 ml) mixed together in a test tube. The absorbance of developed yellow color solution was read against the blank at 412 nm using spectrophotometer and its quantity was noted as μg of GSH/mg of protein (Moron et al., 1979).
Malondialdehyde (MDA)
It was estimated by the protocol of Slater and Sawyer (1971). As per procedure, we added 2 ml of homogenate, 2 ml of TCA (20% w/v) in a test tube and incubate at 60ºC for 20 minutes and then cool for 15 minutes. From the centrifuged (3,000 rpm, 15 minutes) mixture, 2 ml of supernatant was taken and added with 2 ml of TBA (0.67% w/v in Tris hydrochloride, pH 7) again it was incubated at 60ºC (10 minutes). Developed pink colored solution absorbance was noted against blank at 535 nm using spectrophotometer. Units are expressed as nm of MDA/gm of tissue (Slater and Sawyer, 1971).
Estimation of other biochemical parameters
Cardiac tissue Sodium–potassium adenosine triphosphatase (Na+K+ ATPase), Calcium adenosine triphosphatase (Ca2+ATPase) (Al-Numair et al., 2015), calcium (0 - cresolphthalein complexone method), sodium (trinder method) potassium (tetra phenyl borate method) and protein (Lowry et al., 1951) contents were estimated by Erba semi-auto analyser kits (Upaganlawar and Balaraman, 2011).
Estimation of inflammation markers
Cardiac inflammatory mediators Tumour Necrosis Factor alpha (TNF-α), Interleukins (IL-6 & 10) were quantified as per instruction given by the standard ELISA kits (Rey biotech kits) (Shahzada et al., 2018).
Histopathology
Some excised hearts were fixed in fixing solution (10% buffered formalin) and processed for histopathological sections and then stained with haematoxylin and eosin.
Statistical analysis
The data generated in our study was analyzed by one-way analysis of variance followed by the Bonferroni multiple comparisons test with help of Prism, Graph pad software. We considered values were significant when p is less than 0.05.
RESULTS AND DISCUSSION
Preliminary qualitative phytochemical analysis
Active constitutes of the plant or its crude drugs are known to be responsible for their pharmacological actions. Among polyphenolic compounds such as flavonoids, stilbenes, lignans tannins are naturally occurring diverse phytochemicals available in fruits, leaves, seeds (Mondal et al., 2019). Various epidemiological studies revealed its therapeutic role in cardiovascular diseases, diabetes, cancers, neurodegenerative diseases. So, there is a much attention has been increasing to take polyphenolic rich plants or its extracts in the context of current scenario (Cory et al., 2018). These compounds attain more in polar solvents like butanol (Rita et al., 2016). Hence, observations of preliminary qualitative study have validated the presence of flavonoids and tannins in butanol fraction rather than other solvents. (Sharma and Janmeda, 2017) The results are shown in Table 1.
Qualitative tests for presence of flavonoids in butanol fraction
Butanol fraction of R. ornata showed with positive evidence for flavonoids by major flavonoids test compared to other fractions. The results of this found in Table 2. Literature stated that flavonoids rich extracts have beneficial effect in oxidative stress induced myocardial injury and these may be used as adjuvant in the stopping or prevention of development of cardiovascular diseases. (Auwal et al., 2014)
Quantification of contents of R. ornata extracts and its fraction (phenolic and flavonoid)
The above said contents were derived from gallic acid and quercetin calibration curves. Butanol fraction has shown highest phenolic and flavonoid contents (82.4 ± 4.61 mg of Gallic acid equivalents/g, 105 ± 4.09 mg of quercetin/g) compared to crude ethanol and its other fractions. Antioxidant potency is mainly depending on its phenolic and flavonoids content, being capable of donating hydrogens to highly unstable free radicals, there by that break the free radical chain reaction, so that polyphenolic rich extracts or fractions widely used in pharmaceutical, nutraceutical, medicinal and cosmetic application (Ali et al., 2018). Results of flavonoid and phenolic content shown in Table 3 and Figure 1.
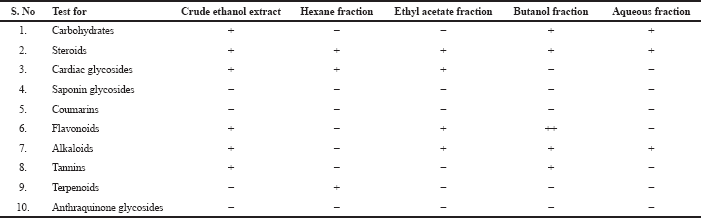 | Table 1. Phytochemical composition of extracts of R. ornata. [Click here to view] |
 | Table 2. Qualitative results of flavonoids in R. ornata plant extracts. [Click here to view] |
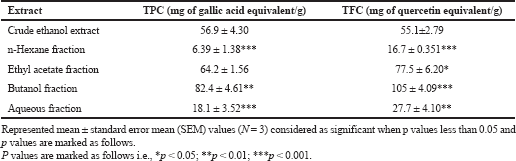 | Table 3. Total phenolic and total flavonoid content of ethanol and its fractions of R. ornate. [Click here to view] |
Identification of flavonoids in the butanol column fractions
Among 40 subfraction of butanol fraction from in the column chromatography 18, 20, 21, 22 subfraction showed same spot as that of standard flavonoid quercetin on TLC. From observation we came to know that butanol extract contains flavonoid type compounds (Gwatidzo et al., 2018). Considering of butanol fraction for in vivo cardioprotective activity than other fractions was well supported with its TLC data. TLC spots of flavonoid shown in Figure 2.
Effect of butanol fractions of R. ornata on morphological changes
B.W among all groups of animals were not changed significantly but weight of heart, ratio of heart weight (H.W) and B.W were observed as significant (**p Ë‚ 0.051) increase in ISO injected rats. Whereas butanol fraction of R. ornata dose dependently retain normal H.W/B.W ratio (*p Ë‚ 0.05 at 200 mg/kg b. wt, **p Ë‚ 0.01 at 400 mg/kg b. wt) compared to ISO injected rats. As increase in H.W/B.W ratio in ISO injected animals indicating the hypertrophy as well as inflammation. Pretreatment with butanol fraction of R. ornata reversed the morphological changes resulting in reduced hypertrophy and weight. It is supported with previous literature that flavonoids rich products reduced ISO induced deposition of collagen and inflammation, is the cause of cardiac hypertrophy (Karthikeyan et al., 2007). Table 4 gives the details of B.W changes.
Effect of butanol fractions of R. ornata on ISO induced ECG patterns
Significant (***p Ë‚ 0.001) abnormal ECG patterns such as ST segment elevation and weaking of R- wave was seen with ISO injection. These patterns were efficiently improved by butanol fraction at 400 mg/kg (*p Ë‚ 0.05, **p Ë‚ 0.01) than lower dose (200 mg/kg). Abnormal ECG patterns such as ST segment elevation, weaking of R wave are the key to diagnosing myocardial infraction. These changes are replicated in rats given with ISO injection, are associated with transmural ischemia caused by cardiac over activity, coronary ischemia and oxidative damage (Upaganlawar et al., 2012). Pathological ECG changes were comparatively alleviated in the rats treated with polyphenol rich fraction. ECG findings are shown in Figures 3 and 4.
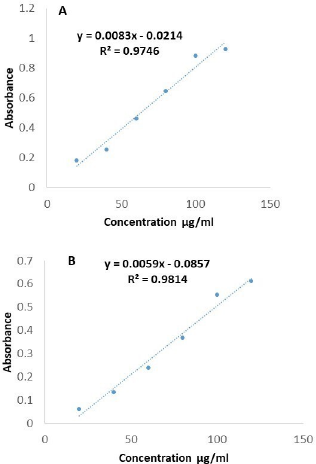 | Figure 1. Regression line of gallic acid and quercetin. [Click here to view] |
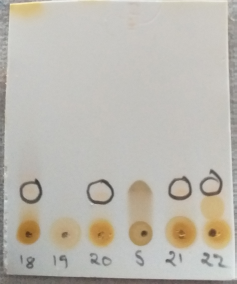 | Figure 2. TLC Identification of flavonoids in the butanol fraction. [Click here to view] |
Effect of butanol fractions of R. ornata on serum biochemical parameters
MI indicators include CK-MB, LDH and C-RP are elevated (***p Ë‚ 0.001) in the ISO injected rats compared to normal rats. Butanol fraction treated rats observed as significant reduction (CK-MB - **p Ë‚ 0.01, LDH-*p Ë‚ 0.05 and C-RP -*p Ë‚ 0.05) its levels in the serum especially at 400 mg /kg treated rats. Autooxidation of ISO and free radical formation would have destabilized the membrane myocytes which leads to leakage of LDH and CK-MB and also enhances serum levels of inflammatory marker (C-RP). It was observed that administration of polyphenol rich butanol fraction showed significant reduction in LDH, CK-MB and C-RP. This endorses its protective against ISO induced cardiac ischemia. (Govindasami et al., 2020). Results were shown in Table 5.
Effect of butanol fractions of R. ornata on cardiac tissue antioxidant parameters
Myocardial tissue levels of SOD, CAT, GSH and total proteins were significantly (***p Ë‚ 0.001) decreased but MDA levels increased (***p Ë‚ 0.001) in the ISO injected group compared with normal rats. SOD (***p Ë‚ 0.001), CAT (*p Ë‚ 0.05), GSH (***p Ë‚ 0.001), total proteins (**p Ë‚ 0.01) were increased and MDA (***p Ë‚ 0.001) was decreased by butanol fraction (especially at 400 mg/kg) compared with ISO treated rats. The degree of oxidative stress induced cardiac damage is dependents on close balance between defensive factor of oxidative stress such as SOD, CAT, GSH and cytotoxic free radicals (Mnafgui et al., 2016). Quinone metabolites of ISO are highly toxic, highly reactive interact with oxygen causes generation of superoxide ion, H2O2 and further generate highly reactive hydroxyl radicle are known to cause severe oxidative stress in myocardium (Hemalatha et al., 2016). Excessive formation of free radicals causes myocardial phospholipid peroxidation which in turn prone for cellular injury and is determined by elevation of malondialdehyde content in heart tissue of ISO group (Rathore et al., 2000). These pathological changes are significantly decreased by butanol fractions of R. ornata (200 and 400 mg/kg b.w orally) treatment when compared to ISO group. These effects are mainly due to its antioxidant rich flavonoids. Reduced glutathione protects the SH groups of tissue proteins from cytotoxic free radical or from lipid peroxides. Overconsumption of reduced glutathione or deficiency of reduced glutathione may be the cause of damaged myocardium by ISO administered rats (Sudha et al., 2013). Treatment with butanol fraction of R. ornata at 400 mg/kg was efficiently balanced reduced glutathione content, it indicates antioxidant property of the extract. Reduction of mutually defensive antioxidant proteins such as SOD and CAT is demonstrated in ISO injected rats. Overwhelming production of free radicals (super oxide and hydrogen peroxide radicals) from auto-oxidation of ISO, makes the antioxidant proteins deficiency state. Increased levels of SOD and CAT with butanol fraction of R. ornata (200 and 400 mg/kg) might be it allowed the tissue to synthesis the new defensive enzyme system while it neutralize the superoxide anion, hydroxyl radicle and H2O2 molecules (Priscilla and Prince, 2009). Antioxidant parameters results were observed in Table 6.
 | Table 4. Influence of butanol fraction of R. ornata on B.W, H.W and its ratio. [Click here to view] |
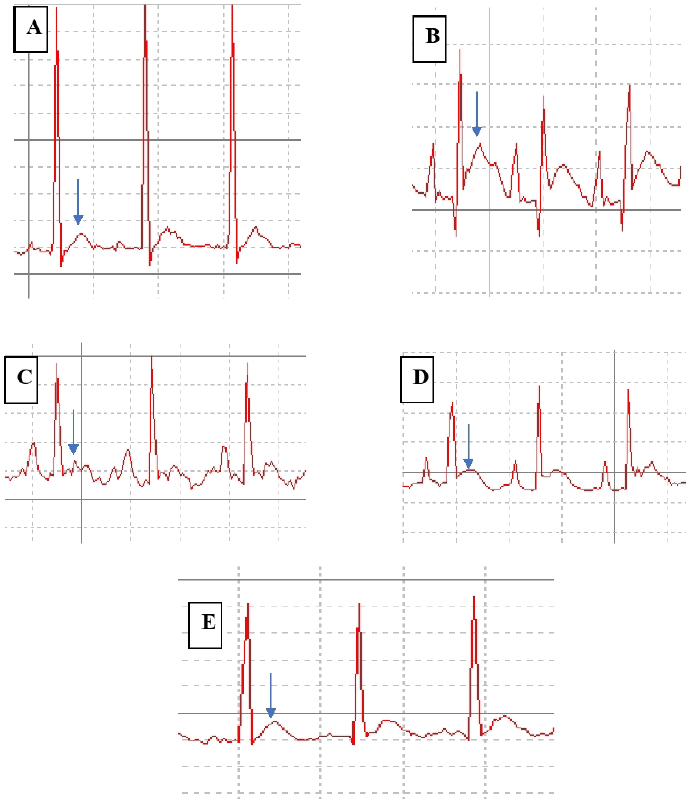 | Figure 3. Representation of ECG changes (A) Normal control (B) ISO control (C) Carvedilol treated group (D). Butanol fractions of R. ornata 200 mg/kg Pre-treated Group E. Butanol fractions of R. ornata 400 mg/kg Pre-treated group. [Click here to view] |
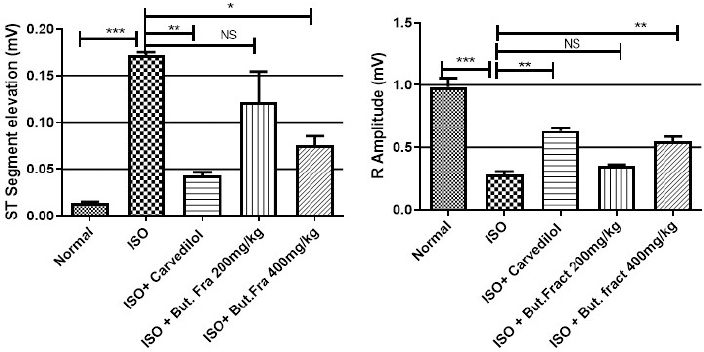 | Figure 4. ST segment & R-Amplitude changes in ECG. Represented mean ± SEM values (n = 6) considered as significant when p values less than 0.05 and all groups were compared with ISO control group. p values are marked as follows i.e., *p Ë‚ 0.05; **p Ë‚ 0.01; ***p Ë‚ 0.001. [Click here to view] |
 | Table 5. Effect of butanol fractions of R. ornata serum cardiac markers. [Click here to view] |
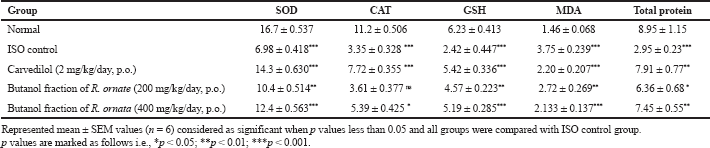 | Table 6. Effect of butanol fractions of R. ornata of cardiac antioxidants and lipid peroxidation. [Click here to view] |
Effect of butanol fractions of R. ornata on inflamation markers in cardiac tissue.
Cardiac tissue inflammation markers such as TNF-α and IL-6 increased significantly (***p Ë‚ 0.001) and IL-10 (anti-inflammatory protein) decreased significantly (***p Ë‚ 0.001) in ISO injected rats. Butanol fraction treatment attenuates the increase of TNF-α (*p Ë‚ 0.05), IL-6 (**p Ë‚ 0.01), and enhance the levels of IL-10 (*p Ë‚ 0.05) at 400 mg/kg efficiently compared with ISO injected rats. Several studies have shown that imbalance of deleterious inflammatory molecules (IL-6 and TNF-α) and protective molecules (IL-10) can occur immediately after myocardial damages, especially observed in oxidative stress condition, has significant role in impairment of myocardial contractility. Therefore, establishment of balance between inflammatory cytokines is considered as valuable option to preserve the heart function in myocardial damage. We observed increase in inflammation of cardiac tissue in ISO injected rats as is indicated by higher amount of IL-6, TNF-α & less amount of IL-10, which is also observed in the similar experiments. Polyphenolic rich butanol fraction significantly decreased inflammatory cytokine such as IL-6, TNF-α & increased in IL-10 in cardiac tissue. Anti-inflammatory property of butanol fraction may have cardio protection (Raish et al., 2019). Inflammatory markers changes were observed in Figure 5.
Effect of butanol fractions of R. ornata on cardiac ATPases and associated electrolyte changes
The cardiac membrane bound ATPases such as Na+K+ ATPase, Ca2+ATPase are significantly (***p Ë‚ 0.001) decreased in ISO administered rats compared with control animals whereas their activities were regained significantly (**p Ë‚ 0.01) by butanol fraction treatment. Along with, cardiac content of sodium, calcium is increased and potassium decreased significantly (***p Ë‚ 0.001) in ISO administered rats as that of control rats. Butanol fraction at 400 mg/kg correct the imbalance of sodium (**p Ë‚ 0.01), calcium (***p Ë‚ 0.001) and potassium (**p Ë‚ 0.01) caused by ISO. At molecular level ISO induced cardiac membrane damage was assessed by measuring cardiac ATPases. These are involved in pumping of selective ions across the cardiac membrane to maintain the ionic balance state so that cardiac cell undergoes sequential contraction and relaxation. At high dose, ISO induced free radicals attack the sensitive thiol segment of ATPases which decreases the functional capacity of membrane bound enzymes and triggers accumulation of intracellular sodium and calcium. These changes were observed in our experiment as indicated by lesser activity of sodium, potassium and calcium ATPases and greater intracellular sodium, calcium and lesser potassium levels. Polyphenolic rich butanol fraction administration was significantly regaining the activity of Na+K+ ATPase, Ca2+ATPase as well as normalization of intracellular ionic balance, this might be due to antioxidant activity protect the thiol groups of membrane bound enzymes from oxidative stress. These observations were matched with earlier reports. (Shaik et al., 2020). Membrane bound enzymes changes in treated groups are shown in Table 7 and Figure 6.
Histopathology
The Histologically ISO induced myocardial infraction has been observed as severe alteration of myocyte shape, focal fibrosis and loss of integrity of myocyte, necrotic changes. Theses pathological alteration were efficiently normalized by pre-treatment of butanol fraction of R. ornata, is indicate its protective effect on pathological changes associated with oxidative stress (Cetin, 2019). Histopathological changes in different groups are shown in Figure 7.
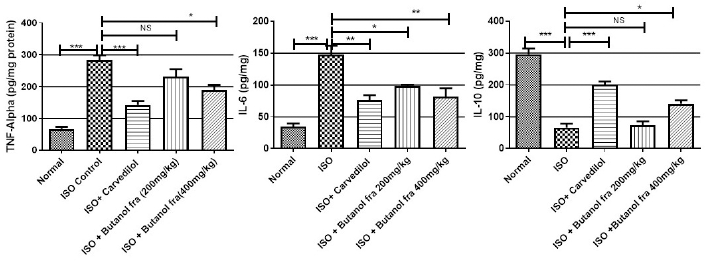 | Figure 5. Effect of butanol fractions of R. ornata on inflammatory cytokines. Represented mean ± SEM values (n = 6) considered as significant when p values less than 0.05 and all groups were compared with ISO control group. p values are marked as follows i.e., *p Ë‚ 0.05; **p Ë‚ 0.01; ***p Ë‚ 0.001. [Click here to view] |
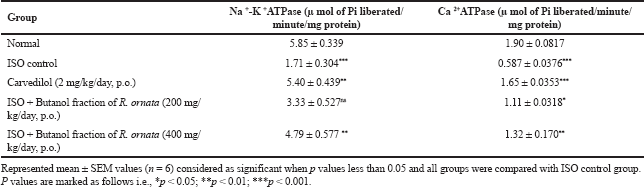 | Table 7. Effect of butanol fractions of R. ornata of membrane bound ATP ases on cardiac tissue. [Click here to view] |
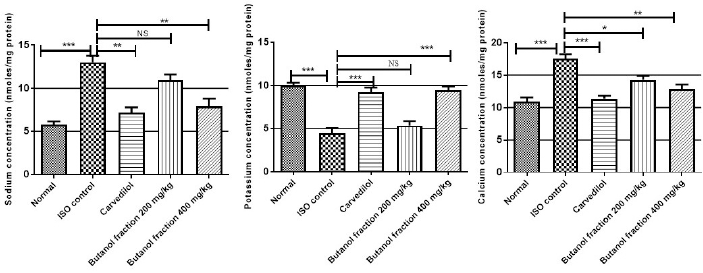 | Figure 6. Effect of butanol fraction on cardiac tissue mineral ions concertation. Represented mean ± SEM values (n = 6) considered as significant when p values less than 0.05 and all groups were compared with ISO control group. p values are marked as follows i.e., *p Ë‚ 0.05; **p Ë‚ 0.01; ***p Ë‚ 0.001. [Click here to view] |
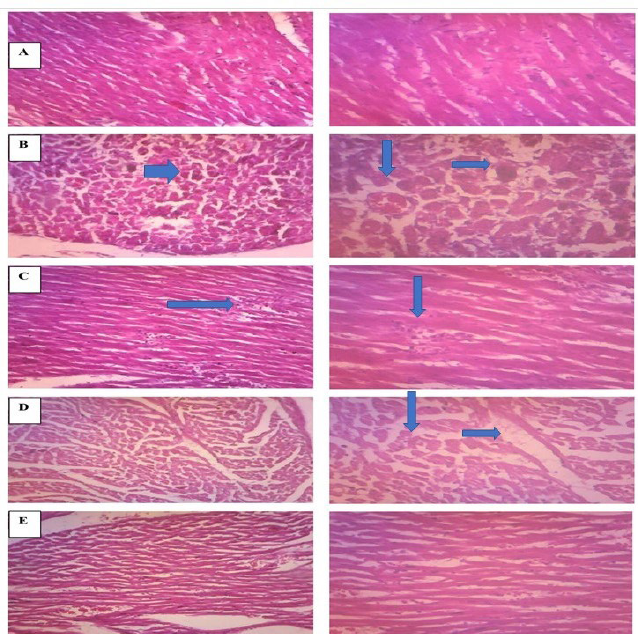 | Figure 7. Representation of histopathological changes in (A) Normal control (B) ISO (C) Carvedilol (2 mg/kg) (D) Butanol fractions of R. ornata 200 mg.kg (E) Butanol fractions of R. ornata 400 mg.kg. [Click here to view] |