INTRODUCTION
In October 2019, China announced the emergence of a new severe acute respiratory syndrome coronavirus (SARS-CoV-2) in Wuhan city, Hubei province. A contagious outbreak of the viral disease aroused a new pandemic spread worldwide, and the disease was named “coronavirus disease-19 (COVID-19)” (Chen et al., 2020). Simultaneously, exponential increases in incidence and mortality rates have been reported by the ministries of health all over the world (Abbas et al., 2021; Al-Domi et al., 2021; Bendavid et al., 2021; Brune et al., 2021). The clinical manifestations of the infection varied from mild pharyngitis to severe respiratory distress syndrome. Governments gathered their forces to contain the outbreak and to hamper the ubiquitous dissemination by implementing a bundle of imperative rules. Concomitantly, scientists applied the utmost efforts to endow conceivable explanations about the notorious virus. Researchers sought different aspects such as the structure of the virus, route of transition, the clinical manifestation of infected patients, and prognostic vaccines for the disease (Tai et al., 2020). The real-time reverse transcriptase-polymerase chain reaction (RT-PCR) technique is considered the golden standard for sensitive and precise identification of COVID-19 (Alandijany and Faizo, 2021). Additionally, RT-PCR was implemented to monitor the incidence rates of the disease and to control the spread of the virus abroad through epidemiological investigation teams (Rakotonanahary et al., 2021). On the other hand, screening of immunoglobulins IgM and IgG against SARS-CoV-2 was implemented to determine the prevalence and incidence rates during the disease outbreak in different countries (Faller et al., 2021; Zaidi et al., 2021). Epidemiological studies have acquired valuable information about the prevalence of COVID-19 and offered infrastructure for politicians and decision-makers to confront the virus spread (Dick et al., 2021).
Early in March 2020, the Jordanian Ministry of Health announced the incidence of the first case of COVID-19 in a citizen who arrived from Italy (Sughayer et al., 2020). Afterward, an exponential increase was demonstrated in the number of incidences of COVID-19 until the Jordanian government confronted the outbreak with a comprehensive lockdown (Basheti et al., 2021).
Early in 2021, the Jordanian Ministry of Health approved the vaccination program against COVID-19 for citizens. The immunization plan prioritized vaccination of risk groups such as healthcare workers, military forces, and the elderly above 60 years (Ministry of Health, 2020). Later, the plan was expanded to include the remaining categories of citizens according to two criteria: age and career. Consequently, a large number of citizens underwent a serum screening test for IgG antibodies in medical laboratories against COVID-19 to determine antibodies level before exposure to vaccination shots, whereas other citizens underwent the screening test to evaluate the immune response to COVID-19 after acquiring one or two vaccine shots. Data deduced from COVID-19 antibodies in the serum of citizens combined with the demographic and clinical factors could highlight the prevalence of infection in different communities. The concomitant variables such as gender, age, health status, education, and residence play a crucial role in the biological systems development and the immune response at a certain stage (Walid, 2016). These confounders could contribute to a hyperactivity of the immune response or even on the contrary hinder the immune activity (Al-Majali et al., 2017). In addition, this could emphasize the factors that contribute to the durability of COVID-19 antibodies acquired during the infection period or through vaccination.
The objectives of this study were to determine the prevalence of COVID-19 antibodies among Jordanian citizens and to highlight the correlation between demographic and clinical factors and serum IgG levels.
MATERIALS AND METHODS
This cross-sectional study was conducted on 412 Jordanian citizens in the period from January 2021 to December 2021 in cooperation with Al-Safwa Medical Laboratories and Al-Hakeem Medical Laboratories. Participation in the current study was voluntary, the procedure was described to the participants in detail before conducting the study, and a consent form was filled out by the participants. The form included information about demographic data of age, gender, level of education, residence, smoking, previous infection with COVID-19, number of vaccine shots, and the type of vaccine. Blood phlebotomy was conducted by a certified research assistant from the cubital vein in a blood phlebotomy room at the medical laboratories. Participant information, such as the name and phlebotomy date, was documented on the blood collection tubes. 5 ml of venous blood was collected using a 5 cc syringe. 2 ml of the collected blood was dispensed in an ethylenediaminetetraacetic acid (EDTA) tube, and 3 ml was dispensed in a plain tube. The EDTA tube was used for the determination of the ABO system and Rh group, whereas the plain tube was used for serum separation and IgG determination. The serum was separated by centrifugation at 4,000 rpm for 10 minutes and then collected and stored in a −20°C freezer for further investigation. The study was approved by the IRB Committee in the University of Al-Balqa Applied University No. IRB 26/3/1525/2022.
Using the enzyme-linked immunosorbent assay (ELISA) technique, the S1 domain of the COVID-19 spike protein was utilized for the quantitative determination of IgG against SARS-CoV-2. ELISA kits were purchased from EUROIMMUN AG (Lübeck, Germany). The 96-well plates were coated with a COVID-19 antigen of spike protein, and the procedure was performed according to the company instructions. Later, the 96-well plates were measured using a Mindray reader (Shenzhen, China) at the Biochemistry Department. For the determination of the blood group, both forward and reverse blood grouping were performed for each participant using the ABO antibodies, whereas anti-D antibody was used for the determination of the Rh group.
The data generated were subjected to a statistical analysis using SPSS software version 25. The chi-squared test was used to study the correlation between the categorical variables and IgG levels. The multinomial regression model was used to study the effect of the demographic and clinical covariants on IgG levels of SARS-CoV-2. A p value of <0.05 was considered statistically significant.
RESULTS
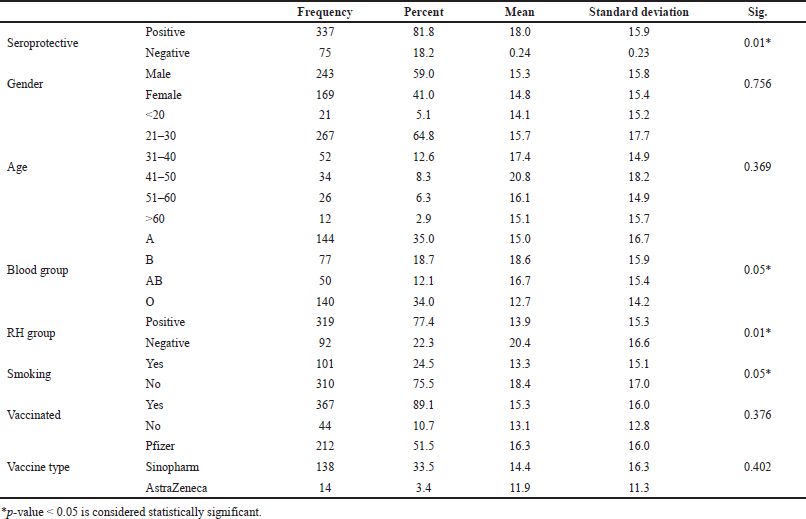
The demographic and clinical data of 412 participants are summarized in Table 1. There was a higher participation of males in the study as there were 242 (59%) males and 169 (41%) females. The seropositive ratio against COVID-19 was 81.8% among all participants. The mean seropositive level for the study population was 15.17 IU/ml. The total smoking percentage was 24.6% in the participant group, and the smoking habit was higher among males than females (62% versus 38%, respectively) (Fig. 1). Smokers showed significantly lower levels of IgG levels, as presented in Table 1. IgG serum levels were significantly higher in participants with the B blood group or negative Rh group. There was no statistical significance between the seropositive levels when appended to the participants’ gender, age, education, residence, vaccination, and vaccine type.
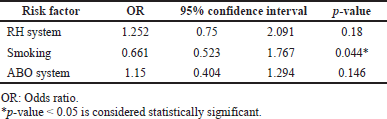
Pearson’s correlation showed a lower seroprevalence of COVID-19 antibodies among smokers and participants in the O blood group, whereas a higher seroprevalence of COVID-19 antibodies was demonstrated among participants in the B blood group or negative Rh group. Multinomial regression analysis for statistically significant variables showed a nonsignificant correlation of blood groups and Rh, whereas smoking sustained the solo significant negative covariant affecting IgG levels with an odds ratio of 0.661 (Table 2).
DISCUSSION
Jordan witnessed the earliest reported cases of COVID-19 infection in March 2020. At that time, Sughayer et al. (2020) reported a seroprevalence of zero for SARS-CoV-2 antibodies at the beginning of the pandemic in a study conducted in Amman, the capital of Jordan (Sughayer et al., 2020). Then, after 4 months of the comprehensive lockdown imposed in March 2020, the seroprevalence for COVID-19 antibodies was 14.5% for blood donors in Irbid city, a northern city in Jordan (Elnasser et al., 2021). Later, the percentage of seroprevalence showed ascertainment up to 27.4% in early 2021 among healthy blood donors in Amman (Sughayer et al., 2021a). Following that, a study conducted by Sughayer et al. (2021b) demonstrated a high prevalence of 74.4% for COVID-19 antibodies in healthy blood donors in Amman (Sughayer et al., 2021a). Several studies all over the world declared an escalation in the percentage of positive seroprevalence of COVID-19 (Alsuwaidi et al., 2021; Balou et al., 2021; Soeorg et al., 2022; Tunheim et al., 2022). Additionally, a study conducted on 1,443,519 blood donation specimens in the United States of America showed a seroprevalence of 83.3% for participants for combined infection- and vaccine-induced antibodies in May 2021 (Jones et al., 2021). In the current study, there was a high seroprevalence of positive COVID-19 antibodies of 81.8% with a mean IgG level of 15.17 IU/ml for the combined subcategories of vaccinated and infected participants. This high ratio of positive prevalence could be attributed to one of two factors: either the increase in the number of citizens subjected to vaccination shots or the acquisition of a COVID-19 infection before the study conduction. Specifically, 89.9% of the study population reported having one or two shots of vaccine. Additionally, we could not attribute the high seroprevalence of COVID-19 antibodies to the subjection to a prior infection since 61% of the study population reported they do not know if they were infected with COVID-19 or have not been infected ever. This level of seroprevalence is concomitant with the results of a multicenter study of antibody seroprevalence of COVID-19 antibodies in Iran that showed a conversion of seroprevalence percentage of 79.5% when measured for combined vaccinated and COVID-19-infected participants (Javadinia et al., 2022). On the other hand, the type of vaccine did not significantly affect the IgG levels in the current study, and all vaccines conferred relevant levels of immunoglobulin. The vaccines reported by the participants were Pfizer, Sinopharm, and AstraZeneca. Likewise, Wei et al. (2021) reported a nonsignificant difference in immunoglobulin levels among the study groups when subjected to different vaccine kinds. Specifically, they concluded that the major difference in IgG levels is attributed to the durability of IgG immunoglobulin and the time required for reaching the highest immune response (Wei et al., 2021).
 | Table 1. Demographic and clinical data of the study population. [Click here to view] |
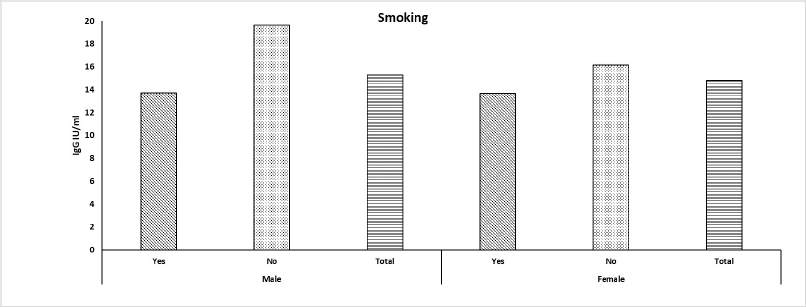 | Figure 1. Immunoglobulin level according to smoking habit among males and females. The IgG levels were classified according to smokers, nonsmokers, and the total level of both smokers and nonsmokers. [Click here to view] |
 | Table 2. Multinomial regression analysis for the demographic and clinical factors. [Click here to view] |
The current study showed a higher incidence of smoking among males than females. Smoking habits showed a paradox for the level of IgG of COVID-19, regardless of gender. There was a significant decrease in seroprotective levels of smoking COVID-19 patients. This point has been noticed by several scientists who reported a decrease in the levels of seroprotective levels in smokers’ blood (Kahlert et al., 2021; Maraqa et al., 2021; Michos et al., 2021). Congruently, Warszawski et al. (2022) reported a statistically significantly lower likelihood of IgG antibodies against COVID-19 in daily smokers versus nonsmokers. Similarly, a study conducted in Iraq on diabetics and smokers as comorbidities for corona infection reported smoking as a less significant virulence cofactor than diabetes which conferred lower immunoglobulins in patients’ serum (Abbas et al., 2021). The plausible explanation for such levels is the anti-inflammatory effect of nicotine found in cigarettes which suppresses the adaptive immune activity and hinders antibody formation (Usman et al., 2021).
The average smoking rate of the participant population was lower than in the neighboring countries; for instance, the rates were higher in both Lebanon and Turkey at 42.3% and 29.3%, respectively (Abdulrahim and Jawad, 2018; Özer et al., 2018), whereas the smoking rate in the current study is consistent with rates reported in the United States and Italy at 25.1% and 23.40%, respectively (Gorini et al., 2020; Jeong et al., 2021).
In the current study, the male smoking rate was extremely higher than the female smoking rate. This note was congruent in other countries as males showed higher smoking rates than females in Turkey, Iraq, and Lebanon (Abdulrahim and Jawad, 2018; Ibrahim et al., 2018; Özer et al., 2018; Walid, 2016).
Remarkably, there was no significant difference in IgG levels between males and females or in the different age groups in the current study. Zeng et al. (2020) reported in a study conducted on 331 patients, with confirmed SARS-CoV-2 infection, a nonsignificant correlation of the IgG immunoglobulin levels among males and females and between young and elderly patients. They concluded that the major difference they noted in IgG levels is attributed to the state of disease severity (Zeng et al., 2020).
Several research groups clarified the relationship between the blood group and the COVID-19 seroprevalence; for instance, Valenti et al. (2021) reported in a study conducted in Italy on blood donors the highest prevalence for seroprotective IgG was demonstrated in donors of the O and A blood groups. Likewise, Sughayer et al. (2021a) showed a high prevalence of seroprotective immunoglobulins in the O blood groups, whereas the same author reported in a previous study the seroprevalence of IgG in the A blood group donor. In the current study, there was a significant prevalence for the B blood group with a higher seroprevalence for negative Rh participants.
In the current study, there was no significant correlation between the different blood and Rh groups on the IgG levels when the data was subjected to a multinomial analysis. Statistical analysis confirmed the dominancy of smoking over other covariates as a blood group on IgG results. These findings are consistent with a study from Saudi predictors of SARS-COV-2 infection among blood donors in Saudi Arabia (Banjar et al., 2021) and UAE (Alsuwaidi et al., 2021).
One of the limitations of this study is that these studies are subjected to variation in the population type and size. For instance, several studies from the Middle East, Saudi Arabia, Pakistan, China, Italy, Brazil, US, England, Scotland, and Spain have concluded a variation in the seroprevalence rates that were directly or proportionally correlated to numerous factors such as the demographic data, sample size, gender, study period, lockdown procedures, and the onset of the outbreak wave (Abouzid et al., 2021; Alserehi et al., 2021; Davis et al., 2021; Naiyar et al., 2021; Qin et al., 2021).
CONCLUSION
Based on the sample of participants in Jordan from January 2021 to December 2021, demographic factors including gender, age, education, and residence did not influence the immunoglobulin level, whereas smoking and blood group affected the levels negatively.
ACKNOWLEDGMENTS
The author wishes to thank Al-Safwa Medical Laboratories and Al-Hakeem Medical Laboratories for their endless efforts.
CONFLICT OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
FUNDING
There is no funding to report.
ETHICAL APPROVAL
This study was approved by the Ethical Committee of the Al-Balqa Applied University, Al-Salt, Jordan (Ethical Approval No. 26/3/1/525/2022).
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abbas HM, Nassir KF, Al Khames Aga QA, Al-Gharawi AA, Rasheed JI, Al-Obaidy MW, Al Jubouri AM, Jaber AS, Al Khames Aga LA. Presenting the characteristics, smoking versus diabetes, and outcome among patients hospitalized with COVID-19. J Med Virol, 2021; 93(3):1556–67. CrossRef
Abdulrahim S, Jawad M. Socioeconomic differences in smoking in Jordan, Lebanon, Syria, and Palestine: a cross-sectional analysis of national surveys. PloS One, 2018; 13(1):e0189829. CrossRef
Abouzid M, El-Sherif DM, Eltewacy NK, Dahman NBH, Okasha SA, Ghozy S, Islam SMS, Collaborators E. Influence of COVID-19 on lifestyle behaviors in the Middle East and North Africa Region: a survey of 5896 individuals. J Transl Med, 2021; 19(1):129. CrossRef
Al-Domi H, Al-Dalaeen A, Al-Rosan S, Batarseh N, Nawaiseh H. Healthy nutritional behavior during COVID-19 lockdown: a cross-sectional study. Clin Nutr ESPEN, 2021; 42:132–7. CrossRef
Al-Majali IS, Al-Oran SA, Hassuneh MR, Al-Qaralleh HN, Rayyan WA, Al-Thunibat OY, Mallah E, Abu-Rayyan A, Salem S. Immunomodulatory effect of Moringa peregrina leaves, ex vivo and in vivo study. Cent Eur J Immunol, 2017; 42(3):231–8. CrossRef
Alandijany TA, Faizo AA. Development of serological assays and seroprevalence studies of the new coronavirus 2019 (COVID-19): reports from Saudi Arabia. Healthcare (Basel), 2021; 9(12):1730. CrossRef
Alserehi HA, Alqunaibet AM, Al-Tawfiq JA, Alharbi NK, Alshukairi AN, Alanazi KH, Bin Saleh GM, Alshehri AM, Almasoud A, Hashem AM, Alruwaily AR, Alaswad RH, Al-Mutlaq HM, Almudaiheem AA, Othman FM, Aldakeel SA, Abu Ghararah MR, Jokhdar HA, Algwizani AR, Almudarra SS, Albarrag AM. Seroprevalence of SARS-CoV-2 (COVID-19) among healthcare workers in Saudi Arabia: comparing case and control hospitals. Diagn Microbiol Infect Dis, 2021; 99(3):115273. CrossRef
Alsuwaidi AR, Al Hosani FI, Al Memari S, Narchi H, Abdel Wareth L, Kamal H, Al Ketbi M, Al Baloushi D, Elfateh A, Khudair A, Al Mazrouei S, Al Humaidan HS, Alghaithi N, Afsh K, Al Kaabi N, Altrabulsi B, Jones M, Shaban S, Sheek-Hussein M, Zoubeidi T. Seroprevalence of COVID-19 infection in the Emirate of Abu Dhabi, United Arab Emirates: a population-based cross-sectional study. Int J Epidemiol, 2021; 50(4):1077–90. CrossRef
Balou HA, Yaghubi Kalurazi T, Joukar F, Hassanipour S, Shenagari M, Khoshsorour M, Mansour-Ghanaei F. High seroprevalence of SARS-CoV-2 (COVID-19)-specific antibodies among healthcare workers: a cross-sectional study in Guilan, Iran. J Environ Public Health, 2021; 2021:9081491. CrossRef
Banjar A, Al-Tawfiq JA, Alruwaily A, Alserehi H, Al-Qunaibet A, Alaswad R, Almutlaq H, Almudaiheem A, Khojah AT, Alsaif F. Seroprevalence of antibodies to SARS-CoV-2 among blood donors in the early months of the pandemic in Saudi Arabia. Int J Infect Dis, 2021; 104:452–7. CrossRef
Basheti IA, Mhaidat QN, Mhaidat HN. Prevalence of anxiety and depression during COVID-19 pandemic among healthcare students in Jordan and its effect on their learning process: a national survey. PLoS One, 2021; 16(4):e0249716. CrossRef
Bendavid E, Mulaney B, Sood N, Shah S, Bromley-Dulfano R, Lai C, Weissberg Z, Saavedra-Walker R, Tedrow J, Bogan A, Kupiec T, Eichner D, Gupta R, Ioannidis JPA, Bhattacharya J. COVID-19 antibody seroprevalence in Santa Clara County, California. Int J Epidemiol, 2021; 50(2):410–9. CrossRef
Brune B, Korth J, Fessmann K, Stappert D, Nohl A, Lembeck T, Standl F, Stang A, Dittmer U, Witzke O, Herrmann A, Dudda M. SARS-CoV-2 IgG seroprevalence in personnel of the extraclinical fight against the COVID-19 pandemic. Notf Rett Med, 2021:1–9; doi:10.1007/s10049-021-00948-z
Chen W, Lan Y, Yuan X, Deng X, Li Y, Cai X, Li L, He R, Tan Y, Deng X. Detectable 2019-nCoV viral RNA in blood is a strong indicator for the further clinical severity. Emerg Microb Infect, 2020; 9(1):469–73. CrossRef
Davis G, York AJ, Bacon WC, Lin SC, McNeal MM, Yarawsky AE, Maciag JJ, Miller JLC, Locker KCS, Bailey M, Stone R, Hall M, Gonzalez J, Sproles A, Woodle ES, Safier K, Justus KA, Spearman P, Ware RE, Cancelas JA, Jordan MB, Herr AB, Hildeman DA, Molkentin JD. Seroprevalence of SARS-CoV-2 infection in Cincinnati Ohio USA from August to December 2020. PLoS One, 2021; 16(7):e0254667. CrossRef
Dick DW, Childs L, Feng Z, Li J, Rost G, Buckeridge DL, Ogden NH, Heffernan JM. COVID-19 seroprevalence in Canada modelling waning and boosting COVID-19 immunity in Canada a Canadian immunization research network study. Vaccines (Basel), 2021; 10(1):17. CrossRef
Elnasser Z, Obeidat H, Amarin Z, Alrabadi N, Jaradat A, Alomarat D, BaniSalem M, Almomani R. Prevalence of COVID-19 among blood donors: the Jordan University of Science and Technology experience. Medicine (Baltimore), 2021; 100(41):e27537. CrossRef
Faller E, Wyse A, Barry R, Conlon K, Everard C, Finnegan P, Foran C, Herlihy E, Kerr G, Lapthorne S, McGreal-Bellone A, Morrissey E, O’Sullivan D, O’Sullivan G, Eustace JA, Spillane D, Dempsey C, Benson J, Prentice M, Gallagher J, MacSharry J, Fanning LJ, O’Riordan S, Horgan M, Sadlier C. Seroprevalence study of SARS-CoV-2 antibodies in healthcare workers following the first wave of the COVID-19 pandemic in a tertiary-level hospital in the south of Ireland. BMJ Open, 2021; 11(6):e051415. CrossRef
Gorini G, Gallus S, Carreras G, De Mei B, Masocco M, Faggiano F, Charrier L, Cavallo F, Spizzichino L, Galeone D. Prevalence of tobacco smoking and electronic cigarette use among adolescents in Italy: Global Youth Tobacco Surveys (GYTS), 2010, 2014, 2018. Prevent Med, 2020; 131:105903. CrossRef
Ibrahim BA, Al-Humaish S, Al-Obaide MA. Tobacco smoking, lung cancer, and therapy in Iraq: current perspective. Front Public Health, 2018; 6:311. CrossRef
Javadinia SA, Ariamanesh M, Nabavifard M, Porouhan P, PeyroShabany B, Fazilat-Panah D, Hatami F, Ghasemi A, Lyman GH, Welsh JS, Ashkar Tizabi S, Dehghani M. Multicenter study of antibody seroprevalence against COVID-19 in patients presenting to Iranian cancer centers after one year of the COVID-19 pandemic. Cancer Invest, 2022; 40(2):115–23. CrossRef
Jeong HW, Chang HH, Kim EJ, Kim YK, Kim SM, Kim EH, Kim YI, Casel MAB, Kim SG, Rollon R, Jang SG, Yu KM, Kim HS, Park HS, Park SJ, Kim YD, Kim EG, Choi YK. Differences in seroprevalence between epicenter and non-epicenter areas of the COVID-19 outbreak in South Korea. J Microbiol, 2021; 59(5):530–3. CrossRef
Jones JM, Stone M, Sulaeman H, Fink RV, Dave H, Levy ME, Di Germanio C, Green V, Notari E, Saa P, Biggerstaff BJ, Strauss D, Kessler D, Vassallo R, Reik R, Rossmann S, Destree M, Nguyen K-A, Sayers M, Lough C, Bougie DW, Ritter M, Latoni G, Weales B, Sime S, Gorlin J, Brown NE, Gould CV, Berney K, Benoit TJ, Miller MJ, Freeman D, Kartik D, Fry AM, Azziz-Baumgartner E, Hall AJ, MacNeil A, Gundlapalli AV, Basavaraju SV, Gerber SI, Patton ME, Custer B, Williamson P, Simmons G, Thornburg NJ, Kleinman S, Stramer SL, Opsomer J, Busch MP. Estimated US infection- and vaccine-induced SARS-CoV-2 seroprevalence based on blood donations, July 2020-May 2021. JAMA, 2021; 326(14):1400–9. CrossRef
Kahlert CR, Persi R, Gusewell S, Egger T, Leal-Neto OB, Sumer J, Flury D, Brucher A, Lemmenmeier E, Moller JC, Rieder P, Stocker R, Vuichard-Gysin D, Wiggli B, Albrich WC, Babouee Flury B, Besold U, Fehr J, Kuster SP, McGeer A, Risch L, Schlegel M, Friedl A, Vernazza P, Kohler P. Non-occupational and occupational factors associated with specific SARS-CoV-2 antibodies among hospital workers—a multicentre cross-sectional study. Clin Microbiol Infect, 2021; 27(9):1336–44. CrossRef
Maraqa B, Basha W, Khayyat R, Abdul-Hadi AR, Jabareen J, Al-Shakhra K, Al-Kaila M, Nazzal Z. Prevalence of SARS-CoV-2 antibodies in the Palestinian population: a primary health center-based cross-sectional study. PLoS One, 2021; 16(10):e0258255. CrossRef
Michos A, Tatsi EB, Filippatos F, Dellis C, Koukou D, Efthymiou V, Kastrinelli E, Mantzou A, Syriopoulou V. Association of total and neutralizing SARS-CoV-2 spike -receptor binding domain antibodies with epidemiological and clinical characteristics after immunization with the 1(st) and 2(nd) doses of the BNT162b2 vaccine. Vaccine, 2021; 39(40):5963–7. CrossRef
Ministry of Health. COVID-19 updates in Jordan. Ministry of Health, 2020. Available via https://corona.moh.gov.jo/en
Naiyar I, Anjum AF, Khalid AM, Noor I, Abdullah MS, Anwar MZ. Seroprevalence of COVID-19 and associated factors in a medical institution in Pakistan. J Taibah Univ Med Sci, 2021; 16(4):619–23. CrossRef
Özer N, K?l?çkap M, Tokgözo?lu L, Göksülük H, Karaaslan D, Kay?kç?o?lu M, Y?lmaz MB, Barç?n C, Abac? A, ?ahin M. Data on smoking in Turkey: systematic review, meta-analysis and meta-regression of epidemiological studies on cardiovascular risk factors. Turk Kardiyol Dern Ars, 2018; 46(7):602–12. CrossRef
Qin X, Shen J, Dai E, Li H, Tang G, Zhang L, Hou X, Lu M, Wu X, Duan S, Zhang J, Tsoi MF, Jiang P, Li Y. The seroprevalence and kinetics of IgM and IgG in the progression of COVID-19. BMC Immunol, 2021; 22(1):14. CrossRef
Rakotonanahary RJL, Andriambolamanana H, Razafinjato B, Raza-Fanomezanjanahary EM, Ramanandraitsiory V, Ralaivavikoa F, Tsirinomen’ny Aina A, Rahajatiana L, Rakotonirina L, Haruna J, Cordier LF, Murray MB, Cowley G, Jordan D, Krasnow MA, Wright PC, Gillespie TR, Docherty M, Loyd T, Evans MV, Drake JM, Ngonghala CN, Rich ML, Popper SJ, Miller AC, Ihantamalala FA, Randrianambinina A, Ramiandrisoa B, Rakotozafy E, Rasolofomanana A, Rakotozafy G, Andriamahatana Vololoniaina MC, Andriamihaja B, Garchitorena A, Rakotonirina J, Mayfield A, Finnegan KE, Bonds MH. Integrating health systems and science to respond to COVID-19 in a model district of rural madagascar. Front Public Health, 2021; 9:654299. CrossRef
Soeorg H, Jogi P, Naaber P, Ottas A, Toompere K, Lutsar I. Seroprevalence and levels of IgG antibodies after COVID-19 infection or vaccination. Infect Dis (Lond), 2022; 54(1):63–71. CrossRef
Sughayer MA, Mansour A, Al Nuirat A, Souan L, Ghanem M, Siag M. COVID-19 seroprevalence rate in healthy blood donors from a community under strict lockdown measures. 2020.
Sughayer MA, Mansour A, Al Nuirat A, Souan L, Ghanem M, Siag M. Dramatic rise in seroprevalence rates of SARS-CoV-2 antibodies among healthy blood donors: The evolution of a pandemic. Int J Infect Dis, 2021a; 107:116–20. CrossRef
Sughayer MA, Mansour A, Nuirat AA, Souan L, Abdel-Razeq R, Siag M. A second dramatic rise in seroprevalence rates of SARS-CoV-2 antibodies among adult healthy blood donors in Jordan; have we achieved herd immunity? medRxiv, 2021b; doi:10.1101/2021.08.15.21261584 CrossRef
Tai W, He L, Zhang X, Pu J, Voronin D, Jiang S, Zhou Y, Du L. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol Immunol, 2020; 17(6):613–20. CrossRef
Tunheim G, Ro GOI, Tran T, Kran AB, Andersen JT, Vaage EB, Kolderup A, Vaage JT, Lund-Johansen F, Hungnes O. Trends in seroprevalence of SARS-CoV-2 and infection fatality rate in the Norwegian population through the first year of the COVID-19 pandemic. Influ Respir Virus, 2022; 16(2):204–12. CrossRef
Usman MS, Siddiqi TJ, Khan MS, Patel UK, Shahid I, Ahmed J, Kalra A, Michos ED. Is there a smoker’s paradox in COVID-19? BMJ Evid-Based Med, 2021; 26(6):279–84. CrossRef
Valenti L, Bergna A, Pelusi S, Facciotti F, Lai A, Tarkowski M, Lombardi A, Berzuini A, Caprioli F, Santoro L, Baselli G, Ventura CD, Erba E, Bosari S, Galli M, Zehender G, Prati D, Covid-19 Donors Study n. SARS-CoV-2 seroprevalence trends in healthy blood donors during the COVID-19 outbreak in Milan. Blood Transfus, 2021; 19(3):181–9. CrossRef
Walid AR. Influence of smoking duration on cadmium deposition in blood and scalp hair among University Students in Jordan. Iran J Public Health, 2016; 45(2):266–7.
Warszawski J, Beaumont AL, Seng R, de Lamballerie X, Rahib D, Lydie N, Slama R, Durrleman S, Raynaud P, Sillard P, Beck F, Meyer L, Bajos N, group Es. Prevalence of SARS-Cov-2 antibodies and living conditions: the French national random population-based EPICOV cohort. BMC Infect Dis, 2022; 22(1):41. CrossRef
Wei J, Stoesser N, Matthews PC, Ayoubkhani D, Studley R, Bell I, Bell JI, Newton JN, Farrar J, Diamond I, Rourke E, Howarth A, Marsden BD, Hoosdally S, Jones EY, Stuart DI, Crook DW, Peto TEA, Pouwels KB, Eyre DW, Walker AS, Lambert A, Thomas T, Black R, Felton A, Crees M, Jones J, Lloyd L, Sutherland E, Pritchard E, Vihta K-D, Doherty G, Kavanagh J, Chau KK, Hatch SB, Ebner D, Ferreira LM, Christott T, Dejnirattisai W, Mongkolsapaya J, Cameron S, Tamblin-Hopper P, Wolna M, Brown R, Cornall R, Screaton G, Lythgoe K, Bonsall D, Golubchik T, Fryer H, Cox S, Paddon K, James T, House T, Robotham J, Birrell P, Jordan H, Sheppard T, Athey G, Moody D, Curry L, Brereton P, Jarvis I, Godsmark A, Morris G, Mallick B, Eeles P, Hay J, VanSteenhouse H, Lee J, the C-ISt. Antibody responses to SARS-CoV-2 vaccines in 45,965 adults from the general population of the United Kingdom. Nat Microbiol, 2021; 6(9):1140–9. CrossRef
Zaidi S, Rizwan F, Riaz Q, Siddiqui A, Khawaja S, Imam M, Naz A, Waheed S, Shamsi T. Seroprevalence of anti-SARS-CoV-2 antibodies in residents of Karachi-challenges in acquiring herd immunity for COVID 19. J Public Health (Oxf), 2021; 43(1):3–8. CrossRef
Zeng F, Dai C, Cai P, Wang J, Xu L, Li J, Hu G, Wang Z, Zheng F, Wang L. A comparison study of SARS-CoV-2 IgG antibody between male and female COVID-19 patients: a possible reason underlying different outcome between sex. J Med Virol, 2020; 92(10):2050–4. CrossRef