INTRODUCTION
The immune system is responsible for protecting the body from unusual conditions. Of several certain unusual conditions mediated by the immune system, some may be difficult to treat, for example, autoimmune disorders, infectious diseases, cancer, and other chronic diseases (Childs et al., 2019). As an approach, a treatment by using an immunomodulator is often used to boost the immune system. Immunomodulators are substances that were used to restore the balance of the immune system, which has been disrupted by upregulation (immunostimulant) or downregulation (immunosuppression) mechanisms (Catanzaro et al., 2018; Krensky et al., 2012). Some synthetic drugs have been used as immunomodulatory agents. However, some of them have some inconveniences, including side effects as well as bioavailability and stability issues. For that reason, an immunomodulatory agent from natural sources is preferred (Jantan et al., 2015).
The Pepolo plant (Bischofia javanica Blume) is a type of tree in the Euphorbiaceae family widely used as a traditional medicine in Indonesia, specifically in Central Sulawesi. This plant can grow in the lowlands at altitudes of ± 1,500 m above sea level. Geographically, Pepolo is native to South Asia, Southeast Asia, Australia, and China, and it is widely distributed across Western India, Southern Japan, Eastern Australia, the Pacific, and the Indonesian Archipelago (Kundu et al., 2020). Several studies have demonstrated the pharmacological action of Pepolo as an anti-inflammatory (leaves), antioxidant (bark and leaves), antileukemic (leaves), antimicrobial, antiallergic, antitussive (leaves), anthelmintic (leaves), antidiarrheal (stem bark), antidiabetic, antinematode, and antimicrobial agent and as a hair growth stimulant (stem and leaf bark) an antiaging agent for the skin and for treating burns (bark) (Kundu et al., 2012; Lee et al., 2021; Lingadurai et al., 2011; Rajbongshi et al 2014).
The compounds responsible for those activities remain unclear. However, studies have shown the presence of alkaloid, flavonoid, phenolic, glycoside, tannin, and steroid/triterpenoid compounds which possibly having a relationship with pharmacological activities. More importantly, alkaloids, flavonoids, phenolics, and steroids/triterpenoids (Dhani et al., 2017; Jambak et al., 2019) have been shown to have an effect in supporting the immune response. Previous studies have proven that ethanol extract of pepolo stem bark (PSB) has a very strong antioxidant activity with an IC50 of 12.248 µg/mL (Jambak et al., 2019). There is a relationship between the antioxidants and immunomodulation, which is well known, so it takes extra antioxidants from outside the body in the form of supplement intake from herbals (Yarosz and Chang, 2018). For the aforementioned reason, we are interested in exploring the potency of the PSB as an alternative immunomodulatory agent.
Macrophages, which are found throughout the body, are the primary effector cells of innate immunity, engulfing microbes and secreting proinflammatory factors to provide the first line of defense against invading pathogens. Macrophages also play an important role in bridging the innate and adaptive systems. The presence of Staphylococcus aureus influences macrophage polarization and cytokine exudation toward either proinflammatory or anti-inflammatory activities. Macrophages possibly destroy S. aureus either intracellularly or extracellularly. Therefore, stimulating macrophages by infecting mice using S. aureus is of interest in this project.
In this present work, the potency of the PSB extract as an immunomodulatory and anticancer agent was evaluated by observing its phagocytic activity on S. aureus-stimulated macrophages and MCF-7 cells, respectively. Then, to investigate the toxicity potential of the PSB, a brine shrimp lethality test (BSLT) was performed. Additionally, the secondary metabolite profile of the PSB extract was also analyzed using a Liquid Chromatography-Mass Spectrometry/Mass Spectrometry (LC-MS/MS) instrument to predict the compounds responsible for those activities.
MATERIALS AND METHODS
Equipment and chemicals
The equipment and chemicals used in this work are a reflux extractor set, vacuum rotary evaporator (Buchi), autoclave, blender (Philips), oven (poL-HCD Aparathra), incubator, laminar air flow, electric microscope (Olympus), spectrophotometer 20 D, ACQUITY UPLC BEH C8 (1.7 μm, 2.1 × 100 mm), biosafety cabinet, centrifuge, CO2 incubator (Thermo) and Multimode Reader, S. aureus ATCC 25293, Dimethyl sulfoxide (DMSO), phosphate buffer saline (PBS), Giemsa stain, cisplatin, PrestoBlue™ Cell Viability Reagent, Roswell Park Memorial Institute Medium, fetal bovine serum, Trypsin-Ethylenediaminetetraacetic Acid (EDTA), trypan blue, and Stimuno.
Plant material
The PSBs were collected from Sedoa Village, North Lore Subdistrict, Poso Regency in Central Sulawesi Province, Indonesia. The plant specimen was determined at the Laboratory of Plant Biosystematics, Department of Biology, Faculty of Mathematics and Natural Sciences (FMIPA) Tadulako University of Palu City, Central Sulawesi Province, Indonesia, with specimen No. 484/UN28.128/BIO/2020.
Sample processing
The fresh PSBs were selected and then scraped to remove moss and other impurities. Subsequently, the PSBs were scratched from the top circle to the bottom, thoroughly washed, and drained. Then, the PSBs were cut into small pieces (2.5 × 3.5 cm) and dried in a drying cabinet at 40°C. The dried PSBs were then powdered and sieved by using a sieve with mesh No. 40. The PSB powder was stored in a tightly sealed container before extraction and evaluation. The reflux method was used for extraction with 96% ethanol as the solvent. Subsequently, PSB Simplicia was weighed and placed in a bottom flask with a 96% ethanol solvent, which was then refluxed for 3 × 4 hours before being allowed to stand and filtered. The filtrate was concentrated using a rotary evaporator, while the thick extract was stored in a tightly sealed container.
Phytochemical screening
Phytochemical screening was carried out using color reactions and/or precipitation with specific chemical reagents to identify alkaloids, flavonoids, saponins, tannins, and steroids/triterpenoids (Safitri et al., 2020; Tiwari et al., 2011).
Experimental animals
All experiments involving animals were carried out according to the protocols that had been approved by the Medical and Health Research Ethics Committee, Faculty of Medicine, Tadulako University (Approval Tadulako University No. 1436/UN 28.1.30/Kl/2021). Healthy BALB/C male mice [body weight (BW), 20–30 g] were used in this investigation. The mice were fed, watered, and acclimatized for seven days prior to the experiment. During the experiment, the mice were divided into five groups of five animals each. Groups I–III received the PSB extract at doses of 100, 200, and 300 mg/kg BW, respectively. Group IV was a positive control and received a commercial meniran extract (Stimuno) at a dose of 19.5 mg/kg BW. Group V was the control and received 0.5% wt Na Carboxymethyl cellulose (CMC). All groups were treated once a day for seven consecutive days by gavage. Macrophages were prepared from the peritoneal fluids stimulated with S. aureus.
Immunomodulatory assay
Preparation of test bacteria
The S. aureus strain was grown overnight (24 hours) on a nutrient agar medium. The bacteria were then harvested and suspended in 0.9% NaCl. Before the infection procedure of macrophage phagocytic activity assay, the bacteria were adjusted to the desired inoculum concentration using McFarland 0.5 as a standard (OD625 = 25% T for 1.5 × 108 CFU/ml ) (Ibrahim et al., 2013).
Macrophage phagocytic activity assay
On day 8, the mice were infected intraperitoneally with 0.5 ml of S. aureus suspension and left for 1 hour. Then the mice were anesthetized by applying ether. The abdomen was dissected aseptically, and approximately 0.5 ml of peritoneal fluid containing macrophage cells (PFCMCs) from the abdomen was collected. The PFCMCs were then mixed with 1 ml of a PBS solution (pH 7.8) before being placed on the object glass. Next, the PFCMCs were fixed using methanol for 5 minutes and stained with 10% wt Giemsa stain. Before microscopic observation, the preparation was allowed to stand for 20 minutes and then rinsed with running water, and the excess water solution was drained by touching a blotting paper on one side of the slide. The microscopic observation of PFCMCs was conducted at 1,000× magnification (Yuliastri et al., 2021).
The immunomodulatory activity was determined by calculating the phagocytic activity of the PFCMCs using the ImageJ software. The phagocytic activity is expressed in percentage and calculated according to the following formula:
% phagocytic activity = (number of active macrophages) / (100 number of macrophages observed) ×100.
Then, the phagocytosis index was determined by % phagocytic activity positive control or treatment group / % phagocytic activity negative control.
When the phagocytosis index is less than 1, the activity is determined as an immunosuppressant. Meanwhile, if it is greater than 1, the activity is determined as an immunostimulant (Sagala and Murwanti, 2020).
The BSLT
To predict the toxicity of the PSB extract, the BSLT was applied. The PSB extract was dissolved in DMSO/water at varying concentrations and was incubated in vials containing brine shrimp larvae. About 10 brine shrimp larvae were placed in each vial. As a control, brine shrimp larvae were placed in a vial containing a mixture of seawater and DMSO only. After 24 hours, the nauplii were examined against a lighted background, and the average number of surviving larvae was determined. The percentage mortality was determined according to the following equation:
% mortality = (number of dead test larvae) / (total number of test larvae) × 100%.
Next, the percentage mortality was plotted against the concentrations, and the concentration killing 50% of the larvae [lethal concentration 50% (LC50)] was determined from the graph (Handayani et al., 2018).
Cytotoxic assay using MTT assay
For cytotoxic evaluation, the MCF-7 cancer cells were seeded at a concentration of 1.7 × 104 cells/well into a 96-well plate. The cells were incubated with the medium alone or with a twofold serial dilution of the PSB extract starting with the highest concentration at 1,000 μg/ml for 24 hours. Cisplatin was tested for 48 hours of incubation and served as the positive control. The Microtetrazolium (MTT) solution was added to each well and mixed. After 2 hours, the supernatant was removed and 100 μl of DMSO was added to each well to dissolve the precipitate. The cell viability percentage was calculated by measuring the absorbance at 570 nm using a multimode Enzyme-linked immunosorbent assay (ELISA) plate reader (Suzery et al., 2020).
Identification of active compound ethanol extract of PSB by LC-MS/MS
The PSB extract was then processed for the identification of bioactive secondary metabolites by LC-MS/MS analysis. The sample (1 mg) was dissolved in 1 ml methanol, and 100 µl was pipetted and made up to the 1.0 ml mark with methanol (LC-MS Chromasolv® grade). A 1 µl aliquot of the sample was injected into the column (ACQUITY UPLC BEH C8, 1.7 µm, 2.1 × 100 mm). A gradient elution method was used with 0.1% (v/v) water-formic acid as solvent A and 0.1% (v/v) acetonitrile-formic acid as solvent B and a flowing rate of 0.3 ml/minute with a 16 minutes gradient elution. It started with A:B in ratio 95:5 for the initial 1–8 minutes, 8 minutes 60:40, 11–13 minutes 0:100, and 16 minutes 95:5. Compounds were analyzed on a Water ACQUITY UPLC I-Class System with the XEVO G2-XS QTof Mass Spectrometer. The optimal conditions of analysis were as follows: column temperature, 30°C; sample temperature, 20°C; acquisition start time, 0.00–16.00 minutes; start mass, 50.00–1,200.00 m/z; scan time, 0.100 seconds; acquisition mode, ESI (+); capillary voltage, 2 kV; cone voltage, 30 V; collision energy, low CE, 10 eV and high CE, 40 eV; source temperature, 120°C; desolvation temperature, 500°C; cone gas flow, 50 l/hour; and desolvation gas flow, 1,000 l/hour. The LC-MS/MS data files were processed by the UNIFI software (version 1.8, Waters Corporation) with a screening solution workflow, which helped in automated data processing for reporting the positive identifications by comparison with a database.
Data analysis
A one-way analysis of variance and Duncan’s test were used to determine the difference in the effect of the PSB extract at different doses on the phagocytic activity of the macrophage cells. The LC50 and IC50 were calculated using probit analysis to determine the cytotoxic potential. Lastly, probit analysis was performed using the SPSS 21 program, with a significance level of 0.05 (at a 95% confidence level).
RESULTS
The extraction of 1,678.16 g PSB using ethanol as solvent yielded a 2.84% wt concentrated extract. Then, it showed that the PSB extract contains alkaloids, flavonoids, saponins, tannins, and steroids/triterpenoids, as suggested by phytochemical screening evaluation. Those results were in line with previous work reported (Pangodian et al., 2020).
As shown in Figure 1(a), exposing macrophages to the PSB extract resulted in a significant phagocytic activity at nearly all PSB extract concentrations tested at a dose of 100–200 mg/kg BW. According to Figure 1(a), the highest phagocytosis activity was observed at a dose of 100 mg/kg BW, and the response was almost similar to the group which was given a meniran commercial extract (Stimuno). Stimuno contains a meniran extract (Phyllanthus niruri L.) which is one of the original Indonesian immunomodulatory herbal phytopharmaca products and has been proven to be effective through clinical trials. The meniran extract can increase the activity of nonspecific and specific immune responses (Jantan et al., 2019; Tjandrawinata et al., 2005). The phagocytosis index as shown in Figure 1(b) suggested that, to provide an immunostimulant effect (>1), the concentration of the PSB extract should be below 200 mg/kg BW.
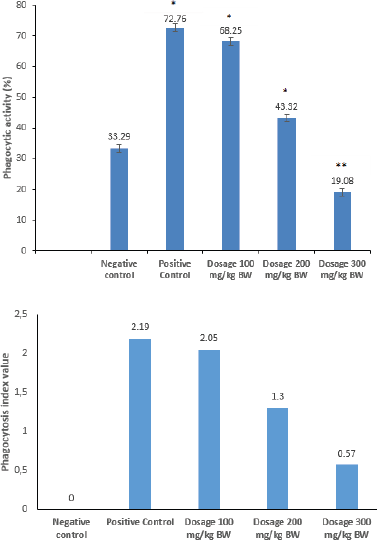 | Figure 1. Phagocytic activity. (a) Phagocytosis index value and (b) data are expressed as means ± SD (n = 5). Asterisks indicate a significant difference compared to the negative control (*p < 0.01; ** p < 0.05). [Click here to view] |
The toxicity of the herbal extract tested with the BSLT is expressed as the LC50 value. As tabulated in Table 1, it shows that, for the PSB extract at concentrations of 5–1,000 µg/ml, the % mortality varied in ranging of 6–100%. According to Meyer’s toxicity index, extracts with LC50 < 1,000 µg/ml are considered as toxic, while extracts with LC50 > 1,000 µg/ml are considered as nontoxic. According to Clarkson’s toxicity criterion, an extract with LC50 above 1,000 µg/ml is nontoxic, with LC50 of 500–1,000 µg/ml is low toxic, with LC50 of 100–500 µg/ml is medium toxic, while an extract with LC50 below 100 µg/ml is highly toxic. In accordance with the LC50 of the PSB extract and Meyer’s criterion, the PSB extract is classified as a highly toxic extract (Hamidi et al., 2014).
Then, we evaluated the potency of the PSB extract for its potential in the prevention of breast cancer. The results of the MTT assay carried out with breast cancer (MCF-7) cells depicted a certain degree of anticancer activity of the PSB extract; however, its anticancer potential varied in concentration ranges of 7.81–1,000 µg/ml (Table 2). The IC50 of the PSB extract was 362.36 µg/ml.
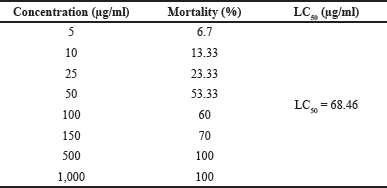 | Table 1. Cytotoxicity profile of PSB extract by BSLT method. [Click here to view] |
The secondary metabolite composition of the PSB extract was determined using an LC-MS/MS instrument. The compounds matched with the UNIFI software database are given in Table 3. The spectrum profile of LC-MS/MS of the PSB extract is shown in Figure 2. As presented, the PSB extract contained ambronal, asperulosidic acid, epigallocatechin(4β,8)-gallocatechin, stigmastan-3,6-dione, and unidentified compounds. The LC-MS/MS evaluation corroborated the phytochemical screening of the PSB extract.
DISCUSSION
The present investigation shows that the ethanol PSB extract exerted immunomodulatory potential as suggested by the phagocytic activity. Phagocytosis is an inherent function of macrophage cells, which is important for host protection and the initiation of innate and acquired immune responses (Harun et al., 2018). Therefore, peritoneal macrophages are often used in immunological studies to determine phagocytic activity (Pavlou et al., 2017). The percentage of phagocytic activity in peritoneal macrophage cells was calculated to determine how much the PSB extract increased the phagocytosis of invading foreign antigens. The results of the analysis showed that the administration of the PSB extract could significantly affect the phagocytic activity of peritoneal macrophages in vitro. Additionally, the phagocytic activity in the negative control indicates an innate (natural) immune response by macrophage cells, which protects the body from antigens that enter the body. The group that received PSB at doses of 100 and 200 mg/kg BW had higher macrophage activity than the negative control group. Furthermore, this shows that the phagocytosis of macrophages was triggered by the activation of the immune system by the stem bark extract, in addition to the natural immunity of macrophage cells. However, there was a decrease in the macrophage activity at higher doses (300 mg/kg BW) compared to other treatment groups, which shows that the administered dose can determine the immune response. If the minimum dose of an antigen is exceeded, there will be an increase in the immune response at higher doses and a decrease or elimination of the immune response at lower doses. This condition is referred to as immunogenic tolerance, which suggests that administering the extract at a higher dose may have an immunosuppressive effect (Horwitz et al., 2019). Additionally, this was supported by the results of the cytotoxic test using the BSLT method and cancer cells. The prominent increase in the cytotoxic nature of the extract at higher concentrations led to a decrease in the viability of macrophage cells, which reduced the number of actively phagocytizing macrophage cells on foreign antigens. Furthermore, the effective dose of the PSB extract that increased the phagocytic activity was 100 mg/kg BW, which was due to the antioxidant-rich compounds found in PSB (Jambak et al., 2019). The presence of antioxidants has been shown to stabilize reactive oxidative species produced by cellular activity processes, such as the phagocytosis of macrophages, which protects the macrophage cells from free radical damage. The nonexcessive production of Reactive oxygen species (ROS) improves the phagocytosis of macrophages against foreign antigens (Nur et al., 2021).
The BSLT test was used to determine the toxic effect of the extract after 24 hours of treatment based on the LC50. The Artemia salina Leach utilized in this study was 48 hours old in order to obtain the larvae at their most sensitive state with properly formed digestive tracts. Furthermore, this enabled the extract to trigger the desired effect since the cell walls of the larvae were still soft. Therefore, it could be inferred that the extract could be used as an anticancer agent if it exerted a high activity on the test animal, which was identical to cancer cells (Handayani et al., 2018). Preparation of two control media, including DMSO without samples and artificial seawater control, was conducted to determine the effect of DMSO and seawater on the cytotoxic test of the PSB extract. The BSLT method was reliable in determining the pharmacological activity of natural ingredients. If a plant extract is toxic based on the LC50 obtained from the BSLT, the plant has the potential to be used as an anticancer drug. However, if the plant is not toxic, it can be reexamined in vivo using other experimental animals larger than A. salina Leach larvae, such as mice and rats.
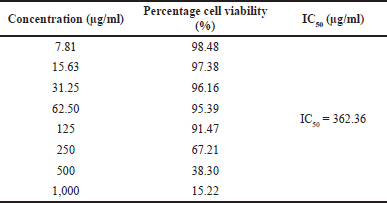 | Table 2. Cytotoxicity profile of PSB extract against MCF-7 breast cancer cells. [Click here to view] |
Table 1 shows an LC50 of 68.46 μg/ml (LC50 < 1,000 μg/ml), which implies that the PSB extract is toxic and can be used as an anticancer agent for further in vitro testing with MCF-7 breast cancer cells. Also, other studies using the BSLT method obtained similar results. Sinukaban et al. (2019), discovered that the crude methanol extract had an LC50 value of 56.92 ppm, while Manurung et al. (2020), proved that the crude ethanol extract had an LC50 value of 54,827 ppm.
The 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) method with the PrestoBlue Cell Viability Reagent was used to test anticancer activity. The IC50 value of the extract against MCF-7 cells was 362.36 μg/ml. According to the classification of cytotoxic activity against cancer cells, the extract can be classified as “very active,” “active,” or “moderately active” if the IC50 value is < 10 μg/ml, between 10 and 100, or 100–500 μg/ml, respectively (Weerapreeyakul et al., 2012). Conversely, substances are said to have no cytotoxic activity if their IC50 value is >500 µg/ml (Machana et al., 2011). The PSB extract was classified as quite active in inhibiting the growth of MCF-7 cancer cells based on the IC50. However, the cytotoxic activity of the bark extract is relatively low compared to conventional chemotherapeutic agents, such as cisplatin. The IC50 of the extract is quite promising for further development as a chemoprevention agent since the MCF-7 cells are known to be resistant to several chemotherapeutic agents. This development can be improved synergistically by the combination of the PSB extract with cisplatin on MCF-7 cells. Cisplatin is an anticancer drug in chemotherapy, which has a platinum complex form as an antiproliferative agent with side effects such as neurotoxicity, nephrotoxicity, and bone marrow suppression (Florea and Büsselberg, 2011). It was also reported that the use of cisplatin triggered resistance, which could be due to changes in cellular uptake, drug efflux, inhibition of apoptosis, and increased DNA repair. Cancer cell resistance and cisplatin side effects are triggered by high treatment doses. The use of combination chemotherapy is becoming increasingly popular. Furthermore, it involves the combination of nontoxic or less toxic chemopreventive compounds with chemotherapeutic agents to increase their efficacy and reduce their toxicity to normal tissues. One of the methods used to lower the dose of chemotherapeutic agents is through a combination with natural compounds, which produces a synergistic effect and also increases the sensitivity of the target cells to the chemotherapeutic agents (Achkar et al., 2018).
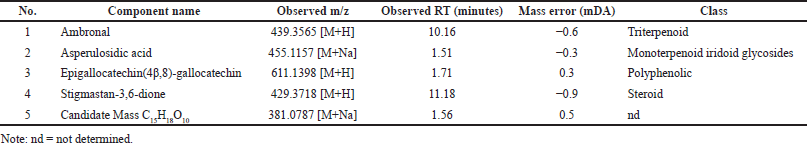 | Table 3. LC-MS/MS profile of PSB extract. [Click here to view] |
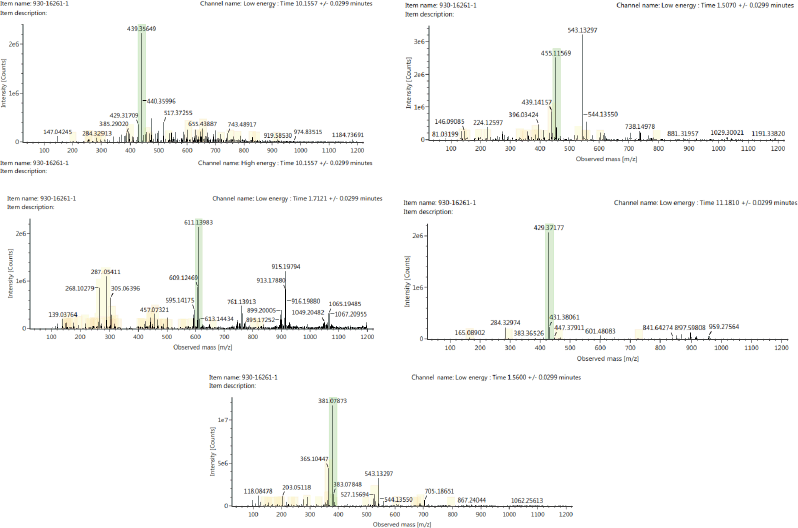 | Figure 2. Spectrum profile of five compounds after LC-MS/MS analysis from PSB extract: (a) Ambronal [+H] : (44.5 PPM) 439.3565. (b) Asperulosidic acid [+Na] : (44.5 PPM) 455.1157. (c) Epigallocatechin(4β,8)-gallocatechin [+H] : (44.5 PPM) 611.1398. (d) Stigmastan-3,6-dione [+H] : (44.5 PPM) 429.3718. (e) Candidate C15H18O10 [+Na] : (44.5 PPM) 381.0787. [Click here to view] |
The chemical profile of the extract was used as a reference to determine the mechanism of action of the extract. Several studies reported the presence of epi-fiedelanol acetate, friedelin (A), betulinate (B), and sitosterol in PSB. Meanwhile, this study found at least five compounds based on the results of LC-MS/MS analysis on the PSB extract, four of which could be identified in the literature, such as ambronal, asperulosidic acid, epigallocatechin(4,8)-gallocatechin, and stigmastan-3,6-dione. Additionally, this was due to the variation in the growing environment, which can affect the chemical content. The compounds identified in the extract were terpenoid and polyphenol compounds. The chemical content affects the mechanism of action as an immunostimulant, immunosuppressant, and anticancer agent. Epigallocatechin is a polyphenolic compound that serves as an antioxidant by inhibiting inflammatory signaling pathways, such as NF-B and AP-1, which are inducers and proinflammatory mediators. Furthermore, it has anticancer potential to prevent tumor cell angiogenesis and cell proliferation. Epigallocatechin is an immunostimulant and also an immunosuppressant by inhibiting cytokine transcription and weakening important immune system chains directly, specifically IL-2, which is required for lymphocyte multiplication and differentiation (Chourasia et al., 2021; Jantan et al., 2015; Kuo et al., 2014). The suppression of NF-B signaling pathways, mitogen-activated protein kinase, asperulosidic acid, and stigmastan are implicated in the anti-inflammatory action of the extract, which leads to the inhibition of inflammatory cytokines (TNF-, IL-6) and mediators Mitogen-activated protein kinase (MAPK). Meanwhile, the inhibition of AP-1 transactivation and cell transformation, as well as asperulosidic acid, has an antitumorigenic effect. Also, stigmastans can induce a T-helper cell response with immunomodulatory properties (Yuliastri et al., 2021). Ambronal inhibition of protein tyrosine kinase autophosphorylation might be the primary mechanism for the anticancer effect (Qian et al., 2020). The activity can result from the synergistic effect of the compounds. Therefore, the results of this study imply that the PSB extract can be investigated further to identify new resources for development as an immunomodulatory and anticancer agent. However, more studies are needed to isolate and purify these compounds with anticancer and immunomodulatory potential in in vivo studies.
CONCLUSION
The PSB extract can be used as a raw material for the development of anticancer and immunomodulatory drugs due to its high cytotoxicity, its ability to trigger phagocytosis, and its in vitro anticancer potential. Additionally, this study identifies compounds in the PSB extract which contribute to its pharmacological activity.
ACKNOWLEDGMENTS
The authors acknowledge the facilities and scientific and technical support from the Advanced Characterization Laboratories Serpong, Indonesian Institute of Sciences through E-Layanan Sains, Lembaga Ilmu Pengetahuan Indonesia, and Central Laboratory of Padjadjaran University.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
FUNDING
There is no funding to report.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
ETHICAL APPROVAL
The Medical and Health Research Ethics Committee, Faculty of Medicine, Tadulako University (Approval Tadulako University No. 1436/ UN 28.1.30/Kl/2021).
AUSTHORS’ CONTRIBUTION
YS (primary contributor) designed the study and contributed to data interpretation, data collection, data analysis, original manuscript writing, revision, and administration. S and R contributed to data acquisition, data analysis, and data collection. KK contributed to data analysis and interpretation. VS contributed to data interpretation and manuscript finalization. All authors drafted the article or revised it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Achkar IW, Abdulrahman N, Al-Sulaiti H, Jensa MJ, Uddin S, Mraiche F. Cisplatin based therapy: the role of the mitogen activated protein kinase signaling pathway. J Transl Med, 2018; 16(1):1–12; doi:10.1186/s12967-018-1471-1 CrossRef
Catanzaro M, Corsini E, Rosini M, Racchi M, Lanni C. Immunomodulators inspired by nature: a review on Curcumin and Echinacea. Molecules, 2018; 23(2778):1–17; doi:10.3390/molecules23112778 CrossRef
Childs CE, Calder PC, Miles EA. Diet and immune function. Nutrients, 2019; 11(8):1933; doi:10.3390/nu11081933 CrossRef
Chourasia M, Koppula PR, Battu A, Ouseph MM, Singh AK. EGCG, a green tea catechin, as a potential therapeutic agent for symptomatic and asymptomatic SARS-CoV-2 infection. Molecules, 2021; 26(5):1–17; doi:10.3390/molecules26051200 CrossRef
Dhani RC, Kumar AM, Pradhan M, Chhetri R, Sherpa SD, Lepcha DL. Nutraceutical potential of two edible wild fruits, Bischofia javanica Blume and Ficus cunia Buch.Ham. ex Roxb. from Sikkim Himalaya. Int J Food Sci Nutr, 2017; 2:2455–4898
Florea AM, Büsselberg D. Cisplatin as an anti-tumor drug: cellular mechanisms of activity, drug resistance and induced side effects. Cancers, 2011; 3(1):1351–71; doi:10.3390/cancers3011351 CrossRef
Hamidi MR, Jovanova B, Panovska TK. Toxicological evaluation of the plant products using Brine Shrimp (Artemia salina L.) model. Maced Pharma Bull, 2014; 60(01):9–18; doi:10.33320/maced.pharm.bull.2014.60.01.002 CrossRef
Handayani D, Wildan R, Rustini, Elmi NZ, Triana H. Cytotoxic activity screening of fungal extracts derived from the West Sumatran marine sponge Haliclona fascigera to several human cell lines: hela, WiDr, T47D and vero. J Appl Pharma Sci, 2018; 8(1):055–8; doi:10.7324/JAPS.2018.8109 CrossRef
Harun, NH, Wan Amir Nizam WA, Rapeah S. The effects of individual and combination of asiatic acid and madecassoside derived from Centella asiatica (Linn.) on the viability percentage and morphological changes of mouse macrophage cell lines (J774A.1). J Appl Pharma Sci, 2018; 8(11):109–15; doi:10.7324/JAPS.2018.81116 CrossRef
Horwitz DA, Tarek MF, Ciriaco AP, Antonio LC. Rebalancing immune homeostasis to treat autoimmune diseases. Trends Immunol, 2019; 40(10):888–908; doi:10.1016/j.it.2019.08.003 CrossRef
Ibrahim D, Lai KH, Wong CT, Lim SH. In vitro activity of methanolic extract from Lagerstroemia speciosa (Linn. ex. Murray) bark against pathogenic bacteria. J Appl Pharma Sci, 2013; 3(12):25–30; doi:10.7324/JAPS.2013.31205
Jambak K, Marline N, Aminah D. Antioxidant activity of ethanolic extract and n-hexane fraction from sikkam (Bischofia javanica Blume) stem bark. Asian J Pharma Res Devel, 2019; 7(2):1–5; doi:10.22270/ajprd.v7i2.486 CrossRef
Jantan I, Waqas A, Syed Nasir AB. Plant-derived immunomodulators: an insight on their preclinical evaluation and clinical trials. Front Plant Sci, 2015; 6(Aug):1–18; doi:10.3389/fpls.2015.00655 CrossRef
Jantan I, Haque MA, Ilangkovan M, Arshad L. An insight into the modulatory effects and mechanisms of action of Phyllanthus species and their bioactive metabolites on the immune system. Front Pharmacol, 2019; 10(August):1–19; doi:10.3389/fphar.2019.00878 CrossRef
Krensky AM, Bennett WM, Vincenti F. Immunosuppressants, tolerogens and immunostimulants (Chapter 35). In: Brunton L, Chabner B, Knollman B (eds.). Goodman & Gilman’s The Pharmacological basis of therapeutics, 12th edition, McGraw-Hill Companies, New York, NY, pp 1005–29, 2012.
Kundu M. Schmidt LH, Jorgensen MJ. Bischofia javanica Blume. Seed Leaflet. University of Copenhagen. 2012. 157
Kuo CL, Chen TS, Liou SY, Hsieh CC. Immunomodulatory effects of EGCG fraction of green tea extract in innate and adaptive immunity via T regulatory cells in murine model. Immunopharmacol Immunotoxicol, 2014; 36(5):364–70; doi:10.3109/08923973.2014.953637 CrossRef
Lee S, Ha J, Park J, Kang E, Jeon SH, Han SB, Ningsih S, Paik JH, Cho S. Antioxidant and anti-inflammatory effects of Bischofia javanica (Blume) leaf methanol extracts through the regulation of Nrf2 and TAK1. Antioxidants, 2021; 10(8):1–18; doi:10.3390/antiox10081295 CrossRef
Lingadurai S, Roy S, Joseph RV, Nath LK Antileukemic activity of the leaf extract of Bischofia javanica Blume on human leukemic cell lines. Indian J Pharmacol, 2011; 43(2):143–9; doi:10.4103/0253-7613.77348 CrossRef
Machana S, Weerapreeyakul N, Barusrux S, Nonpunya A, Sripanidkulchai B, Thitimetharoch T. Cytotoxic and apoptotic effects of six herbal plants against the human hepatocarcinoma (HepG2) cell line. Chin Med, 2011; 6(1):39; doi:10.1186/1749-8546-6-39 CrossRef
Manurung DP, Sundaryono A, Amir H. Penentuan potensi ekstak kulit batang tumbuhan Sikkam (Bischofia javanica Blume) sebagai antioksidan dengan metode DPPH dan sitotoksik dengan metode BSLT. Alotrop J Pendidik Ilmu Kim, 2020; 4(1):83–91. Available via https://ejournal.unib.ac.id/index.php/alotropjurnal/article/download/13715/6768. CrossRef
Nur S, Aisyah AN, Lukitaningsih E, Rumiyati, Juhardi RI, Andirah R, Hajar AS. Evaluation of antioxidant and cytotoxic effect against cancer cells line of Angiopteris ferox Copel tuber and its compounds by LC-MS analysis. J Appl Pharma Sci, 2021; 11(8):54–61; doi:10.7324/JAPS.2021.110808 CrossRef
Pangodian A, Nainggolan M, Dalimunthe A. Characterization and anti-inflammatory activity of ethanol extract of sikkam (Bischofia javanica Blume) stem bark. Asian J Pharma Res Devel, 2020; 8(4):16–20.
Pavlou S, Wang L, Xu H, Chen M. Higher phagocytic activity of thioglycollate-elicited peritoneal macrophages is related to metabolic status of the cells. J Inflamm (United Kingdom), 2017; 14(1):12–7; doi:10.1186/s12950-017-0151-x CrossRef
Qian P, Mu XT, Su B, Gao L, Zhang DF. Identification of the anti-breast cancer targets of triterpenoids in Liquidambaris fructus and the hints for its traditional applications. BMC Compl Med Ther, 2020; 20(1):1–15; doi:10.1186/s12906-020-03143-8 CrossRef
Rajbongshi P, Kamaruz Z, Sangeeta B, Simanti D. A review on traditional use and phytopharmacological potential of Bischofia javanica Blume. Int J Pharm Sci Rev Res, 2014; 24(2):24–9.
Safitri A, Fatchiyah F, Dewi Ratih TS, Anna R. Phytochemical screening, in vitro anti-oxidant activity, and in silico anti-diabetic activity of aqueous extracts of Ruellia tuberosa L. J Appl Pharm Sci, 2020; 10(3):101–8; doi:10.7324/JAPS.2020.103013 CrossRef
Sagala RJ, Murwanti R. The combination of ethanol extracts of Phyllanthus niruri Linn, Typhonium flagelliforme and Piper crocatum increase the macrophage phagocytosis in vitro. Majal Obat Trad, 2020; 25(2):67; doi:10.22146/mot.46705 CrossRef
Sinukaban K, Saleh C, Daniel D. Profil tumbuhan sikkam (Bischovia javanica Blume). Prosid Semin Kim, 2019:46–51. Available via http://jurnal.kimia.fmipa.unmul.ac.id/index.php/prosiding/article/view/858
Suzery M, Cahyono B, Amalina ND. Antiproliferative and apoptosis effect of hyptolide from Hyptis pectinata (L) Poit on human breast cancer cells. J Appl Pharm Sci, 2020; 10(2):1–6; doi:10.7324/JAPS.2020.102001 CrossRef
Tjandrawinata RR, Maat S, Nofiarny D. Changes in immunological parameters by standardized Phyllanthus niruri extract in the pre-clinical and clinical studies. Dexa Med, 2005; 3(18):89–93.
Tiwari P, Kumar B, Kaur M, Kaur G, Kaur H. Phytochemical screening and extraction: a review. Int Pharm Sci, 2011; 1(1):98–106; doi:10.1002/hep.29375 CrossRef
Weerapreeyakul N, Nonpunya A, Barusrux S, Thitimetharoch T, Sripanidkulchai B. Evaluation of the anticancer potential of six herbs against a hepatoma cell line. Chin Med, 2012; 7(1):1; doi:10.1186/1749-8546-7-15 CrossRef
Yarosz EL, Chang CH. Role of reactive oxygen species in regulating T cell-mediated immunity and disease. Immune Netw, 2018; 18(1):1–15; doi:10.4110/in.2018.18.e14 CrossRef
Yuliastri WO, Diantini A, Ghozali M, Sahidin I, Isrul M. Immunomodulatory activity and phytochemical analysis of Hibiscus sabdariffa L. flower fractions. J Appl Pharm Sci, 2021; 11(11):131–40; doi:10.7324/japs.2021.1101117 CrossRef