INTRODUCTION
Cancer is a disease caused by the accumulation of genetic defects within human cells, which culminates in an uncontrolled proliferative state. Current therapy for cancers includes radiation, surgical removal, and pharmacological treatments with various anticancer agents. Rigorous research in the discovery and development of anticancer agents has led to the establishment of many anticancer agents ranging from conventional chemotherapeutic agents to targeted therapy using small-molecule drugs and monoclonal antibodies. However, despite the availability of a vast collection of effective anticancer agents, the acquisition of a drug-resistant phenotype (Holohan et al., 2013; Housman et al., 2014) and serious adverse reactions associated with certain chemical entities still limit the outcome of anticancer pharmacotherapy. Thus, the development of novel anticancer compounds which will add to the variety of treatment options is likely to confer benefits to cancer patients.
Although the pathogenesis of cancers is widely accepted to be heterogenic in nature, driven by an astounding repertoire of genetic and epigenetic defects, the deregulation of cell cycle progression and programmed cell death pathways holds a central place in cancer pathogenesis (Evan and Vousden, 2001). Many anticancer agents used clinically and in clinical trials have been shown to directly or indirectly affect cell cycle progression or apoptosis in cancer cells (Sun et al., 2021; Wong, 2011). Therefore, the assessment of the activity of putative anticancer compounds in the interference with cell cycle progression or apoptosis in cancer cells should serve as an additional test to verify cytotoxicity and reveal the anticancer potential of novel therapeutic entities.
Euphorbiaceae is a large botanical family encompassing over 2,000 species of unique flora with distinctive milky latex and flower shape. The medicinal property of Euphorbia has been documented in both traditional medicinal records and contemporary pharmaceutical research. There are over 100 publications documenting the cytotoxic activity of extracts and pure compounds isolated from over 60 species of Euphorbia (Wongrakpanich and Charoensuksai, 2018). Phytochemical research of Euphorbia has led to the isolation of over 500 compounds, especially terpenoids (Shi et al., 2008). Many of these compounds have been shown to exert anticancer activity through the induction of apoptosis (Wongrakpanich and Charoensuksai, 2018).
Endemic to tropical regions of the world and abundantly found in Thailand, Euphorbia lactea Haw. (E. lactea) is a succulent plant with a distinctive triangular stem. Despite the documented anticancer properties of other Euphorbia species, only a few publications have shed light on the anticancer activities of E. lactea. First, El-Manawaty et al. (2013) reported that the methanolic extracts of E. lactea exhibited cytotoxic activity against hepatocellular carcinoma cell line HepG2 and colorectal cancer cell line HCT116. This observation was further echoed by a report from another group describing the cytotoxic effects of the E. lactea crude methanolic extract in HepG2 and breast cancer cell line MCF7 (El-Hawary et al., 2020). Our lab previously reported the inhibitory effect of the crude ethanolic extract of E. lactea against the viability and migration of HN22 cancer cells (Wongprayoon and Charoensuksai, 2018). Taken together, the anticancer activity of the E. lactea extract has been documented through several independent reports; nevertheless, the chemical entities responsible for the anticancer activity of E. lactea are yet to be identified.
MATERIALS AND METHODS
Plant materials
The E. lactea specimen used in this experiment was propagated from cuttings collected from a mother plant and cultivated in our greenhouse in Bangkok, Thailand. Plant collection was carried out in May 2019. The species identification was made by a comparison of morphologies between the specimen and pictures of E. lactea in plant databases such as http://www.eol.org and https://www.cabi.org. A voucher specimen (SUPY.PC1) was deposited at the Herbarium of the Faculty of Pharmacy, Silpakorn University, Thailand.
Chemicals and reagents
The common solvents, including ethanol, n-hexane, ethyl acetate (EtOAc), and n-butanol (n-BuOH), for extraction and chromatography were purchased from Merck (Germany). Deuterated chloroform (CDCl3, 99.98% D) for nuclear magnetic resonance (NMR) analysis and cerium (IV) sulfate were purchased from Sigma-Aldrich (St. Louis, MO). Column chromatography was carried out on silica gel (230–400 mesh, Merck). Aluminum sheets of silica gel (60 F254, Merck) were used for thin-layer chromatography (TLC).
Head-and-neck cancer cell line HN22, hepatocellular carcinoma cell line HepG2, colorectal cancer cell line HCT119, and cervical cancer cell line HeLa were kindly provided by Prof. Praneet Opanasopit, Faculty of Pharmacy, Silpakorn University, Thailand. Immortalized human keratinocyte cell line HaCaT was given by Assist. Prof. Dr. Veerawat Teeranachaidekul, Department of Pharmacy, Faculty of Pharmacy, Mahidol University, Thailand. Dulbecco’s modified Eagle’s medium (DMEM), minimum essential media (MEM), fetal bovine serum (FBS), nonessential amino acids, GlutaMAX, and penicillin/streptomycin solution were purchased from Gibco (Waltham, MA)
Dimethyl sulfoxide (DMSO), Triton-X 100, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide (MTT) were purchased from Sigma-Aldrich. Hydroxypropyl-β-cyclodextrin (CAVASOL W7 HP Pharma) from Wacker Chemie AG (Munich, Germany) was given by Prof. Praneet Opanasopit. Irinotecan (Irinotel) was purchased from Fresenius Kabi Oncology, India. DNase-free RNase A was purchased from Bio Basic (Amherst, NY). Propidium iodide was purchased from Life Technologies (Carlsbad, CA). FITC-conjugated annexin-V A13199 was purchased from Thermo Fischer (Waltham, MA).
Extraction and isolation
The aerial parts of E. lactea were cut into thin slices and left to air-dry in a parabola dome solar drying chamber for 5 days. The dried specimens (2.68 kg) were crushed and extracted with 95% ethanol at room temperature (7 l × 3 times). The extract was concentrated under reduced pressure to give a dark brown residue (613.40 g). The residue was resuspended in water (2 l) and then extracted with n-hexane, EtOAc, and n-BuOH (each solvent 1,400 ml × 3), successively. The respective solvents were evaporated to dryness at 40°C under reduced pressure to yield the n-hexane (94.02 g), EtOAc (33.11 g), and n-BuOH (36.82 g) fractions. The remaining aqueous phase was concentrated to give the H2O fraction (428.46 g). A portion of the n-hexane fraction (46.15 g) was subjected to flash column chromatography over silica gel and eluted with n-hexane-EtOAc (gradient from 100% n-hexane to 100% EtOAc) to afford 16 fractions (H1–H16). Fraction H6 (0.6030 g) was recrystallized from ethanol to give 1 (0.1069 g). Fraction H8 (0.3297 g) was further subjected to a silica gel column, eluted with n-hexane–EtOAc (99:1–97:3) to yield 2 (0.2006 g). Fraction H12 (0.5303 g) was recrystallized from ethanol to give 3 (0.1879 g). Further purification of fraction H16 (0.5584 g) by column chromatography over a silica gel, using n-hexane–EtOAc (99:1–95:5), afforded 4 (0.1534 g).
Structural identification
The chemical structures of pure compounds 1–4 were determined by mass spectrometry and NMR spectroscopy. High-resolution electrospray ionization mass spectrometry (HRESIMS) experiments were performed on a Micro TOF Brüker Daltonics mass spectrometer. The 1H and 13C-NMR data were recorded at 300 MHz and 75 MHz on a Brüker AVANCE 300 NMR spectrometer using tetramethylsilane as an internal reference. Column chromatography was carried out using silica gel (230–400 mesh, Merck). TLC was performed, and spots were detected under ultraviolet light at 254 nm or by spraying with a solution of 1% cerium(IV) sulfate in 10% H2SO4 followed by heating.
By comparison of 1H-NMR, 13C-NMR, and mass spectral data of each compound (Supplementary Figs. S1–S12) with those reported in the literature, the isolated compounds were identified as pentacyclic triterpenoids including friedelin [1], friedelan-3β-ol [2], taraxerol [3], and friedelan-3α-ol [4]. Physical and spectroscopic data of these compounds are shown as follows.
Friedelin [1] was obtained as white crystals with melting point 254°C–258°C; 1H-NMR (Supplementary Fig. S1) (300 MHz, CDCl3) δ ppm: 2.40 (1H, m, H-6b), 2.25 (1H, q, J = 6.3 Hz, H-4), 1.97 (1H, m, H-1b), 1.75 (1H, m, H-6a), 1.69 (1H, m, H-1b), 1.18 (3H, s, H-28), 1.05 (3H, s, H-27), 1.01 (3H, s, H-26), 1.00 (3H, s, H-30), 0.95 (3H, s, H-29), 0.89 (3H, d, J = 6.3 Hz, H-23), 0.87 (3H, s, H-25), and 0.73 (3H, s, H-24); 13C-NMR (Supplementary Fig. S2) (75 MHz, CDCl3) δ ppm: 213.3 (C-3), 59.5 (C-10), 58.2 (C-4), 53.1 (C-8), 42.8 (C-18), 42.1 (C-5), 41.5, (C-2), 41.3 (C-6), 39.7 (C-13), 39.3 (C-22), 38.3 (C-14), 37.4 (C-9), 36.0 (C-16), 35.6 (C-11), 35.3 (C-19), 35.0 (C-29), 32.8 (C-21), 32.4 (C-15), 32.1 (C-28), 31.8 (C-30), 30.5 (C-12), 30.0 (C-17), 28.2 (C-20), 22.3, (C-1), 20.3 (C-26), 18.7 (C-27), 18.2 (C-7), 17.9 (C-25), 14.7 (C-24), and 6.8 (C-23); HRESIMS (Supplementary Fig. S9) m/z 449.3757 [M+Na]+ (calculated mass for C30H50ONa, 449.3760).
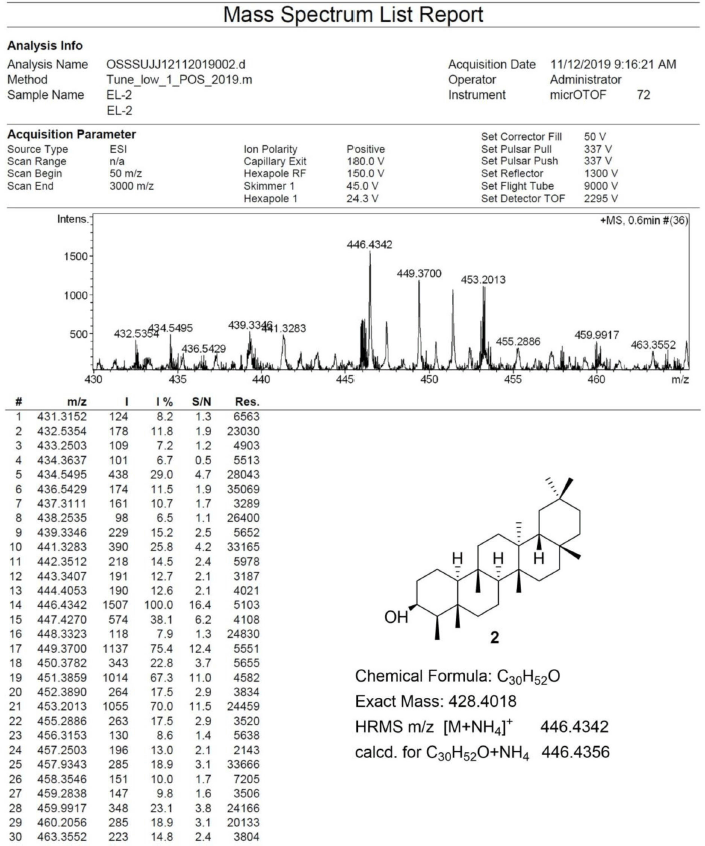
Friedelan-3β-ol [2] was obtained as white amorphous powder with melting point 274°C–280°C; 1H-NMR (Supplementary Fig. S3) (300 MHz, CDCl3) δ ppm: 3.73, (1H, m, H-3), 1.90 (1H, m, H-2b), 1.73 (1H, dt, J = 9.6, 3.3 Hz, H-6b), 1.22 (1H, m, H-2a), 1.17 (3H, s, H-28), 1.01 (3H, s, H-27), 1.00 (3H, s, H-30), 0.99 (3H, s, H-26), 0.98 (1H, m, H-6a) 0.96 (3H, s, H-24), 0.95 (3H, s, H-29), 0.93 (3H, d, J = 6.0 Hz, H-23), and 0.86 (3H, s, H-25); 13C-NMR (Supplementary Fig. S4) (75 MHz, CDCl3) δ ppm: 72.8 (C-3), 61.3 (C-10), 53.2 (C-8), 49.2 (C-4), 42.8 (C-18), 41.7 (C-6), 39.7 (C-14), 39.3, (C-22), 38.4 (C-13), 37.8 (C-5), 37.1 (C-9), 36.1 (C-16), 35.6 (C-11), 35.3 (C-19), 35.2 (C-2), 35.0 (C-29), 32.8 (C-21), 32.3 (C-15), 32.1 (C-28), 31.8 (C-30), 30.6 (C-12), 30.0 (C-17), 28.2 (C-20), 20.1 (C-26), 18.7 (C-27), 18.3 (C-25), 17.6, (C-7), 16.4 (C-24), 15.8 (C-1), and 11.6 (C-23); HRESIMS (Supplementary Fig. S10) m/z [M+NH4]+ 446.4342 (calculated mass for C30H52O+NH4+, 446.4356).
Taraxerol [3] was obtained as white crystals with melting point 269°C–272°C; 1H-NMR (Supplementary Fig. S5) (300 MHz, CDCl3) δ ppm: 5.53 (1H, dd, H-15, J = 8.2, 3.2 Hz), 3.19 (1H, m, H-3), 2.03 (1H, dt, J = 12.5, 3.2 Hz, H-7b), 1.96 (1H, dd, J = 14.7, 3.1 Hz, H-16b), 1.63 (1H, m, H-16a), 1.34 (1H, m, H-7a), 1.09 (3H, s, H-26), 0.98 (3H, s, H-23), 0.95 (3H, s, H-29), 0.93 (3H, s, H-25), 0.90 (6H, s, H-27, H-30), 0.82 (3H, s, H-28), and 0.80 (3H, s, H-24); 13C-NMR (Supplementary Fig. S6) (75 MHz, CDCl3) δ ppm: 158.1 (C-14), 116.9 (C-15), 79.1 (C-3), 55.5 (C-5), 49.3 (C-9), 48.7 (C-18), 41.3 (C-7), 39.0 (C-8), 38.8 (C-4), 38.0 (C-10), 37.7 (C-1, C-16), 37.6 (C-13), 36.7 (C-16), 35.8 (C-17), 35.1 (C-22), 33.7 (C-21), 33.4 (C-29), 33.1 (C-12), 29.9 (C-30), 29.8 (C-28), 28.8 (C-20), 28.0 (C-23), 27.1 (C-2), 25.9 (C-26), 21.3 (C-27), 18.8 (C-6), 17.5 (C-11), 15.5 (C-24), and 15.4 (C-25); HRESIMS (Supplementary Fig. S11) m/z 449.3768 [M+Na]+ (calculated mass for C30H50ONa, 449.3760).
Friedelan-3α-ol [4] was obtained as white amorphous powder with melting point 294°C–298°C; 1H-NMR (Supplementary Fig. S7) (300 MHz, CDCl3) δ ppm: 3.35, (1H, m, H-3), 2.06 (1H, m, H-2b), 1.77 (1H, dt, J = 12.8, 3.2 Hz, H-6b), 1.22 (1H, m, H-2a), 1.17 (3H, s, H-28), 1.03 (1H, m, H-6a), 1.01 (3H, s, H-27), 0.99 (3H, s, H-30), 0.99 (3H, s, H-26), 0.94 (3H, s, H-29), 0.89 (3H, d, J = 6.6 Hz, H-23), 0.81 (3H, s, H-25), and 0.77 (3H, s, H-24); 13C-NMR (Supplementary Fig. S8) (75 MHz, CDCl3) δ ppm: 72.2 (C-3), 60.1 (C-10), 53.2 (C-4), 53.0 (C-8), 42.8 (C-18), 41.4 (C-6), 39.7 (C-14), 39.3, (C-22), 38.3 (C-13), 38.1 (C-5), 37.0 (C-9), 36.7 (C-2), 36.1 (C-16), 35.5 (C-11), 35.3 (C-19), 35.0 (C-29), 32.8 (C-21), 32.4 (C-15), 32.1 (C-28), 31.8 (C-30), 30.6 (C-12), 30.0 (C-17), 28.2 (C-20), 20.2 (C-26), 19.6 (C-1), 18.7 (C-27), 18.1 (C-25), 17.8 (C-7), 14.6 (C-24), and 9.9 (C-23); HRESIMS (Supplementary Fig. S12) m/z 451.3855 [M+Na]+ (calculated mass for C30H52ONa, 451.3916).
Cell culture
HN22, HepG2, and HCT116 were maintained in DMEM supplemented with 10% FBS and 1% GlutaMAX. HeLa was maintained in MEM supplemented with 10% FBS, 1% nonessential amino acids, and 1% GlutaMAX. HaCaT was maintained in DMEM supplemented with 10% FBS. All culture media contained 100 units/mL penicillin and 100 µg/mL streptomycin. Cells were cultured in a humidified atmosphere containing 5% CO2 and temperature-controlled at 37°C.
Determination of cell viability by MTT assay
The crude extract (n-hexane, EtOAc, and n-BuOH) and H2O fractions were dissolved in DMSO. The pure compounds 1–4 were dissolved in a mixture solution of DMSO containing 30% w/v hydroxypropyl-β-cyclodextrin (DMSO-HPBCD).
Cell viability determination by the MTT assay was performed following an established protocol (Kumar et al., 2018) with some modifications. Briefly, 8,000 cells were seeded into each well of a 96-well plate and allowed to attach overnight. Due to the limited solubility of the extract, fractions, and pure compounds in the dissolving vehicle, the maximal concentrations investigated were 500 µg/ml for extracts and fractions and 110 µM for pure compounds. Cells were then incubated with varying concentrations of the extracts (500–1.953 µg/ml final concentration) or pure compounds (110–0.176 µM) for 72 hours. 100 µM irinotecan was used as a positive control. The vehicles used to dissolve the test agents, i.e., DMSO for plant extract and fractions or DMSO-HPBCD for 1–4, were used as negative controls. The final concentration of DMSO and DMSO-HPBCD was maintained at 0.5% w/v for all test conditions. Afterward, 25 µl of 5 mg/ml MTT dissolved in phosphate buffer saline (PBS) was added to each well and incubated for 4 hours. Culture media were then discarded, and 100 µl of DMSO was added to each well to dissolve formazan crystals. Absorbance at 550 nm was measured using a microplate reader (Model No. AOPUS01 and A153601; Packard BioScience Company, CT). All experiments were performed in triplicate.
Cell cycle analysis by flow cytometry
The analysis of cell cycle distribution by propidium iodide staining and flow cytometry was performed according to an established protocol (Crowley et al., 2016a) with some modifications. Briefly, HN22 cells were cultured in the presence of 110 µM of 2 or vehicle control for 72 hours. Cells were then washed with PBS, harvested, and fixed with 70% ice-cold ethanol. Afterward, cells were washed twice with ice-cold PBS and treated with 100 µg/mL of DNase-free RNase A in PBS containing 0.1% v/v Triton-X 100 for 5 minutes at room temperature. Cells were then stained with 20 µg/ml propidium iodide in PBS containing 0.1% v/v Triton-X 100 for 15 minutes at room temperature in the dark. Cell cycle distribution profiles were obtained with a flow cytometer (Attune NxT, Thermo Fischer). Data were analyzed with Attune NxT Software (Thermo Fischer). All experiments were carried out in triplicate.
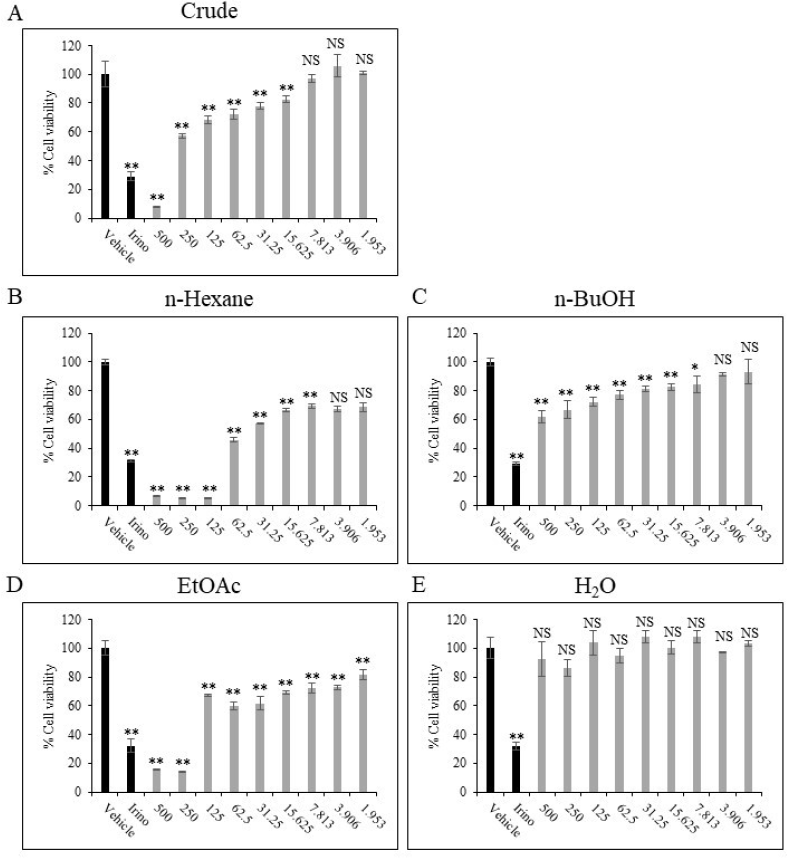 | Figure 1. Cytotoxic activity of E. lactea extracts toward HN22 cells. HN22 cells were treated with DMSO (vehicle control), 100 µM irinotecan, or extracts at concentrations ranging from 500 to 1.953 µg/ml for 72 hours as described Materials and Methods in the section. ** p < 0.01, * p < 0.05, NS p ≥ 0.05 compared to vehicle control. [Click here to view] |
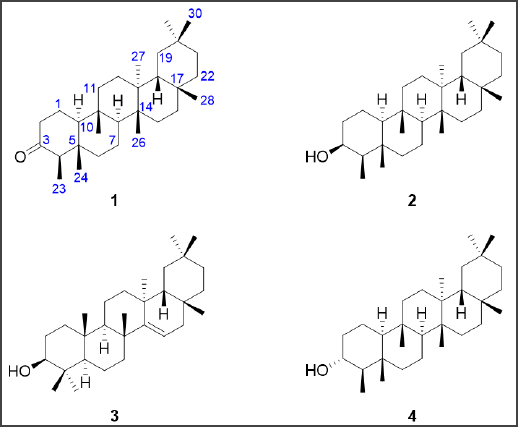 | Figure 2. Chemical structures of friedelin [1], friedelan-3β-ol [2], taraxerol [3], and friedelan-3α-ol [4] isolated from the n-hexane fraction of E. lactea. [Click here to view] |
Analysis of apoptotic cells by flow cytometry
The flow cytometry analysis of cells undergoing apoptosis by annexin-V and propidium iodide double staining was carried out following an established protocol (Crowley et al., 2016b) with some modifications. Briefly, HN22 cells were cultured in the presence of 110 µM of 2, 20 µM Irinotecan for positive control, or vehicle for negative control for 72 hours. Cells were then collected, resuspended in PBS, and counted. For each condition, 1 × 105 cells were collected to stain with 5 µl of FITC-conjugated annexin-V in binding buffer (0.1 M HEPES, 1.5 M NaCl, 50 mM MgCl2, 50 mM KCl, and 18 mM CaCl2, pH 7.4) for 15 minutes at room temperature while protected from light. Afterward, propidium iodide was added to a final concentration of 5 µg/ml and incubated for 15 minutes at room temperature while protected from light. Then, the cells were analyzed by a flow cytometer (Attune NxT, Thermo Fischer). Data were analyzed with Attune NxT Software (Thermo Fischer).
Statistical analysis
One-way analysis of variance with Tukey’s HSD post-hoc test was used to analyze the statistical significance of cell viability experiments of the extract, fractions, and pure compounds. Student’s t-test test was used for cell cycle experiments. The software GraphPad Prism version 7 (GraphPad Software Inc., La Jolla, CA) was used for statistical analysis. p < 0.05 was considered statistically significant.
RESULTS AND DISCUSSION
Our group previously reported the cytotoxic activity of the crude hydroalcoholic extract of fresh E. lactea against HN22 cells (Wongprayoon and Charoensuksai, 2018). This prompted us to ask whether E. lactea contains lead compound(s) with potent anticancer activity. First, we sought to study the cytotoxic activity of the crude extract collected from the extraction of dried and ground E. lactea with 95% ethanol. Indeed, the crude extract of E. lactea exhibited a dose-dependent cytotoxic activity against HN22 cells with statistical significance in all concentrations from 15.625 µg/ml and above (Fig. 1A). Next, we sought to further investigate the cytotoxic effect by sequentially partitioning the crude extract with n-hexane, EtOAc, and n-BuOH to collect refined extracts with different polarities. The cytotoxic effect of n-hexane, EtOAc, n-BuOH, and the H2O fractions was then studied using the same concentration interval of the crude extract. The result revealed that the cytotoxic activity was markedly increased in the n-hexane and EtOAc fractions, while such effect was diminished in the n-BuOH and negligible in the H2O fraction (Fig. 1).
As the n-hexane fraction exhibited the most potent cytotoxic effect, this fraction was subjected to further purification to obtain pure chemical entities. Purification of the n-hexane fraction by chromatography techniques resulted in the isolation of four triterpenoids as the major constituents. The triterpenoids were identified as friedelin [1], friedelan-3β-ol [2], taraxerol [3], and friedelan-3α-ol [4] (Fig. 2). The chemical structures of all compounds were elucidated based on their 1D and 2D NMR spectroscopic data and by comparison of their physical and spectroscopic data with literature reports (Govindachari et al., 1967; Jamal et al., 2009; Koay et al., 2013; Ndwigah et al., 2013). In addition, the molecular mass of all isolated compounds agreed with the measured m/z analyzed by HRESIMS. The isolation of these compounds has been reported in other Euphorbia species. For example, 1 was previously isolated from Euphorbia tortilis (Anju et al., 2018) and Euphorbia geniculata (Farozi et al., 2015). 2 was previously detected in E. neriifolia (Anjeneyulu et al., 1973) and Euphorbia antiquorum (Min et al., 1989). 3 was previously purified from E. antiquorum (Min et al., 1989), Euphorbia myrsinites (Aynehchi et al., 1972), and E. neriifolia (Anjeneyulu et al., 1973). 4 was previously isolated from E. neriifolia (Anjeneyulu et al., 1973). However, to our knowledge, our report describes the first detection and isolation of these compounds from the species E. lactea. These compounds share a core pentacyclic triterpenoid structure, which has been shown to have various pharmacological effects, including anticancer activity (Ghante and Jamkhande, 2019).
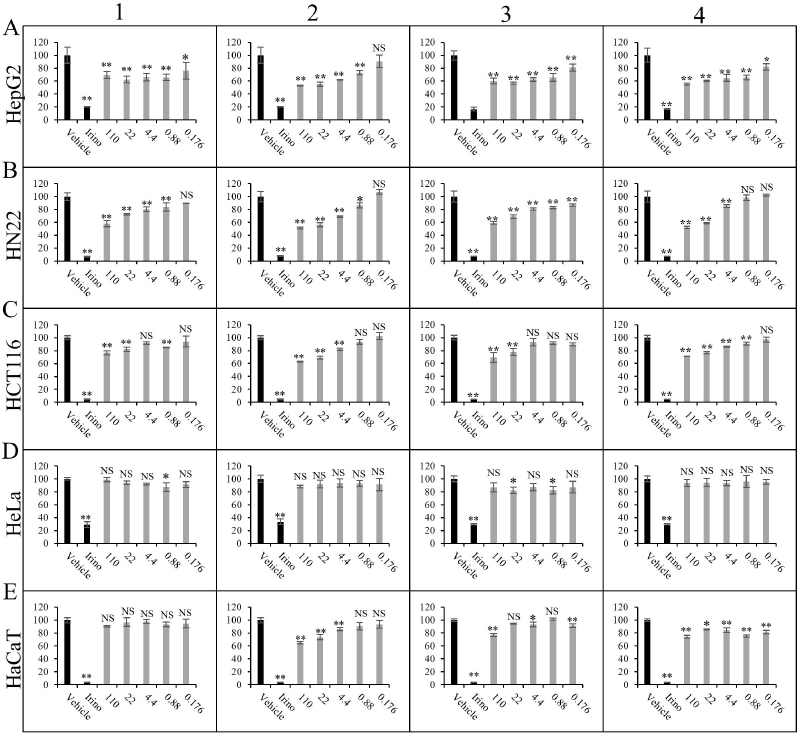 | Figure 3. Cytotoxic activity of 1–4 toward cancer cell lines. (A) HepG2, (B) HN22, (C) HCT116, (D) HeLa, and (E) immortalized human keratinocyte cell line HaCaT were treated with vehicle control, 100 µM irinotecan, or 1–4 at concentrations ranging from 110 to 0.176 µM for 72 hours as described in the Materials and Methods section. ** p < 0.01, * p < 0.05, NS p ≥ 0.05 compared to vehicle control. [Click here to view] |
Cytotoxic activities of the isolated compounds 1–4 were then investigated in four cancer cell lines, namely HepG2, HN22, HCT116, and HeLa, while HaCaT was used as a representative noncancerous cell line (Fig. 3). Compounds 1–4 exhibited a dose-dependent cytotoxic effect on HepG2, HN22, and HCT116 with more effect on cancer cells than untransformed cells. Among the four compounds, 2 appeared to be the most potent, with the strongest inhibition effect on HN22 and HepG2 cells. Interestingly, all compounds exhibited marginal or no effect on HeLa cells, suggesting that the cytotoxic effect of these triterpenoids is likely dependent on cell type.
Given that 2 exhibited the most prominent cytotoxic effect, we then sought to further explore the underlying molecular mechanism likely associated with such activity, i.e., the induction of cell cycle arrest and apoptosis. First, the effect of 2 on the cell cycle distribution of HN22 and HepG2 cells was studied. No change in cell cycle distribution was detected in HepG2 cells treated with 2 (Fig. 4A and B). However, treatment of HN22 cells with 2 is accompanied by a statistically significant increase in cells at the S-phase, indicative of the induction of an S-phase cell cycle arrest (Fig. 4C and D). Next, an apoptosis assay by flow cytometry analysis of cells stained with propidium iodide and FITC-conjugated annexin-V was performed on HN22 cells treated with 2. While apoptotic cells were markedly increased upon treatment with the positive control irinotecan (Fig. 5), the percentage of early and late apoptotic cells remained unchanged upon treatment with 2, suggesting that the cytotoxic activity of 2 toward HN22 cells was not mediated through apoptosis, at least at the concentration and exposure time we investigated.
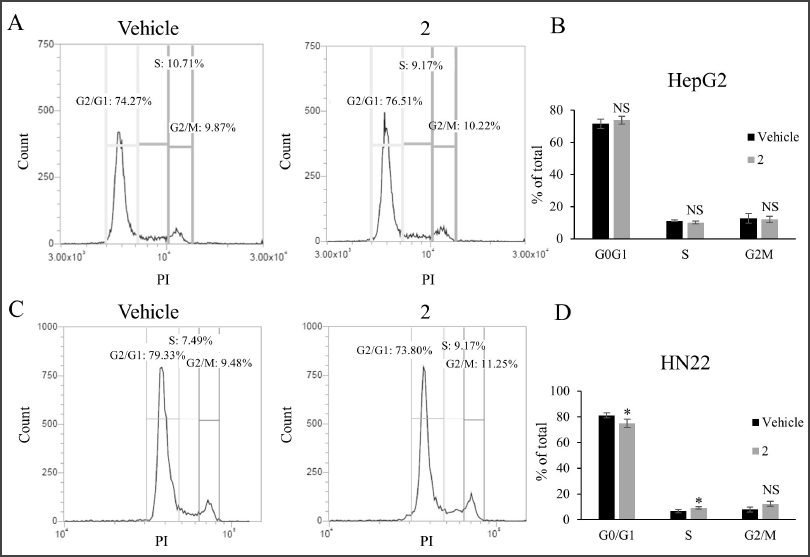 | Figure 4. Cell cycle distribution analysis of cancer cell lines treated with 2. (A) Histogram showing cell cycle distribution of HepG2 cells treated with 110 µM of 2 or vehicle control. (B) Quantitation of HepG2 cells treated with 110 µM of 2 or vehicle control in the G0/G1, S, and G2/M phases of the cell cycle. (C) Histogram showing cell cycle distribution of HN22 cells treated with 110 µM of 2 or vehicle control. (D) Quantitation of HN22 cells treated with 110 µM of 2 or vehicle control in the G0/G1, S, and G2/M phases of the cell cycle. * p < 0.05, NS p ≥ 0.05 compared to vehicle control. [Click here to view] |
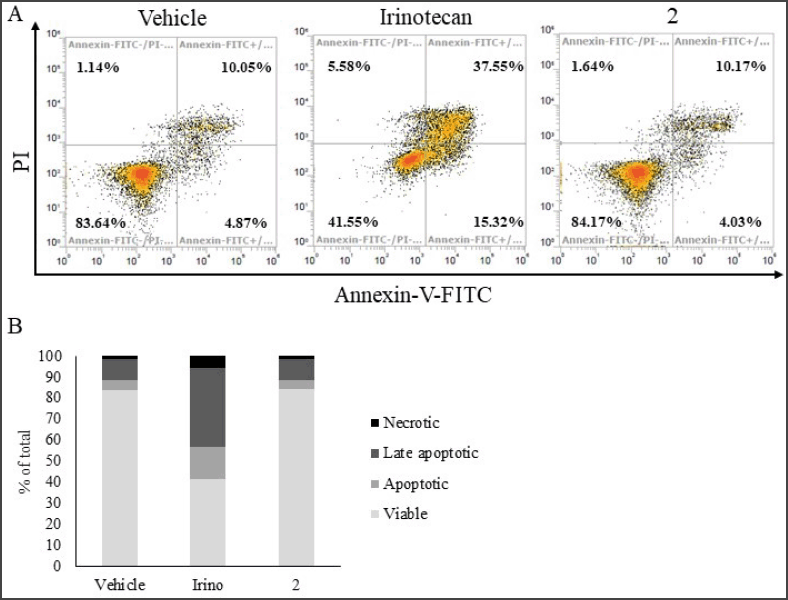 | Figure 5. Analysis of apoptotic cells by flow cytometry. (A) Density plot of HN22 cells treated with vehicle control, 20 µM irinotecan (positive control), and 110 µM of 2 at 72 hours. (B) Quantitation of viable, apoptotic, late apoptotic, and necrotic cells as shown in (A). [Click here to view] |
Indeed, the cytotoxic activity of 2 has previously been tested against several cancer cell lines. While it generally exhibited mild cytotoxic activity against many cancer cell lines (Monkodkaew et al., 2009; Oliveira et al., 2012; Su et al., 2009), 2 markedly suppressed the viability of certain types of cancer. First, Martucciello et al. (2010) reported that 2 inhibited the cell viability of the Kaposi sarcoma cell line by ~30% at 20 µM. Later, Yessoufou et al. (2015) reported that, among the five cancer types tested, including HeLa, MCF7, Jurkat, HT-29, and T24, 2 exhibited the strongest cytotoxic activity against the T24 bladder cancer cell line with an IC50 of 15.61 µg/mL (~35 µM). In line with this observation, we detected >40% reduction in cell viability in HN22 and HepG2 cells treated with 2 at 22 µM, suggesting that the cytotoxic activity of 2 may be more robust toward these types of cancer. Indeed, these observations need to be confirmed in a controlled test. Moreover, the molecular mechanism underlying the anticancer activity of 2 was largely unexplored. Previous reports utilizing molecular docking analysis suggested that 2 may be able to interact with DNA methyltransferase 1 (Wilaputraka et al., 2017) and HER2 and EGFR (Perumal et al., 2016). Our result thus shed some light on the mechanism of action of 2 in that its anticancer effect was mediated through interference with the cell cycle, particularly a cell cycle arrest at the S-phase, and not through the induction of apoptosis like many anticancer compounds (Pistritto et al., 2016). Therefore, it is of particular interest to study the effect of 2 when used in combination with other anticancer agents in susceptible cancer types.
CONCLUSION
This work reported the first isolation of four triterpenoidal compounds, namely friedelin [1], friedelan-3β-ol [2], taraxerol [3], and friedelan-3α-ol [4], from the n-hexane fraction of E. lactea. Cytotoxic activity of these compounds was observed in the HN22, HepG2, and HCT116 cell lines. Among the four compounds, 2 exhibited a prominent anticancer effect against HN22 cells. Subsequent analysis of cell cycle distribution and cells undergoing apoptosis using flow cytometry revealed that treatment with 2 was associated with an increase in cells at the S-phase in HN22 while apoptosis was unperturbed. In summary, our results highlighted E. lactea as a plant with anticancer activity and identified 2 as a chemical constituent harboring anticancer activity in E. lactea.
ACKNOWLEDGMENTS
The authors thank the Faculty of Pharmacy, Silpakorn University, for research facilities and instruments. They also thank Prof. Serm Janjai for his assistance in the dehydration of the plant specimen by using the parabola dome solar drying chamber. The authors thank Prof. Praneet Opanasopit for providing the cell lines and reagents. They also thank Asst. Prof. Dr. Nattiya Kapol for the irinotecan.
FUNDING
This publication is a part of research project “The development of natural products for the treatment of age-related diseases” (SURIC 62/01/43) funded by the National Research Council of Thailand through Silpakorn University Research, Innovation and Creativity Administration Office (SURIC).
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
ABBREVIATIONS
n-BuOH: n-Butanol
DMEM: Dulbecco’s modified Eagle’s medium
DMSO: Dimethyl sulfoxide
DMSO-HPBCD: 30% w/v hydroxypropyl-β-cyclodextrin in DMSO
EtOAc: Ethyl acetate
E. lactea: Euphorbia lactea Haw.
FBS: Fetal bovine serum
HPBCD:Hydroxypropyl-β-cyclodextrin
HRESIMS: High-resolution electrospray ionization mass spectrometry
MEM: Minimum essential media
MTT: 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazoliumbromide
NMR: Nuclear magnetic resonance
PBS: Phosphate buffer saline.
REFERENCES
Anjeneyulu ASR, Row LR, Subrahmanyam C, Murty KS. Crystalline constituents from euphorbiaceae-XIII. The structure of a new triterpene from Euphorbia nerifolia L. Tetrahedron, 1973; 29(23):3909–14. CrossRef
Anju V, Singh A, Shilpa G, Kumar B, Priya S, Sabulal B, Rameshkumar KB. Terpenes and biological activities of Euphorbia tortilis. Lett Org Chem, 2018; 15(3):221–5. CrossRef
Aynehchi Y, Mojtabaii M, Yazdizadeh K. Chemical examination of Euphorbia myrsinitis linn. J Pharm Sci, 1972; 61(2):292–3. CrossRef
Crowley LC, Chojnowski G, Waterhouse NJ. Measuring the DNA content of cells in apoptosis and at different cell-cycle stages by propidium iodide staining and flow cytometry. Cold Spring Harb Protoc, 2016a; 2016(10). CrossRef
Crowley LC, Marfell BJ, Scott AP, Waterhouse NJ. Quantitation of apoptosis and necrosis by annexin V binding, propidium iodide uptake, and flow cytometry. Cold Spring Harb Protoc, 2016b; 2016(11). CrossRef
El-Hawary SS, Mohammed R, Tawfike AF, Lithy NM, AbouZid SF, Amin MN, et al. Cytotoxic activity and metabolic profiling of fifteen Euphorbia species. Metabolites, 2020; 11(1). CrossRef
El-Manawaty M, Fayad W, El-Fiky N, Wassel G, El-Menshawi B. High-throughput screening of 75 euphorbiaceae and myrtaceae plant extracts for in-vitro antitumor and pro-apoptotic activities on human tumor cell lines, and lethality to brine shrimp. Int J Pharm Pharm Sci, 2013; 5:178–83.
Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature, 2001; 411(6835):342–8. CrossRef
Farozi A, Banday JA, Shah SA. Genicunolide A, B and C: Three new triterpenoids from Euphorbia geniculata. Beilstein J Org Chem, 2015; 11:2707–12. CrossRef
Ghante MH, Jamkhande PG. Role of pentacyclic triterpenoids in chemoprevention and anticancer treatment: an overview on targets and underling mechanisms. J Pharmacopunct, 2019; 22(2):55–67. CrossRef
Govindachari TR, Viswanathan N, Pai BR, Rao R, Srinivasan M. Triterpenes of Calophyllum inophyllum Linn. Tetrahedron, 1967; 23(4):1901–10. CrossRef
Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer, 2013; 13(10):714–26. CrossRef
Housman G, Byler S, Heerboth S, Lapinska K, Longacre M, Snyder N, et al. Drug resistance in cancer: an overview. Cancers, 2014; 6(3):1769–92. CrossRef
Jamal A, Yaacob W, Din LB. Triterpenes from the root bark of Phyllanthus Columnaris. Aust J Basic Appl Sci, 2009; 3:1428–31.
Koay Y, Wong K, Osman H, Eldeen IMs, Asmawi M. Chemical Constituents and Biological Activities of Strobilanthes crispus L. Rec Nat Prod, 2013; 7:59–64.
Kumar P, Nagarajan A, Uchil PD. Analysis of cell viability by the MTT assay. Cold Spring Harb Protoc, 2018; 2018(6). CrossRef
Martucciello S, Balestrieri ML, Felice F, Estevam Cdos S, Sant’Ana AE, Pizza C, et al. Effects of triterpene derivatives from Maytenus rigida on VEGF-induced Kaposi’s sarcoma cell proliferation. Chem Biol Interact, 2010; 183(3):450–4. CrossRef
Min Z-d, Mizuo M, Toshiyuki T, Munekazu I, Xu G-Y, Huang Q-J. A diterpene from Euphorbia antiquorum. Phytochemistry, 1989; 28:553–5. CrossRef
Monkodkaew S, Loetchutinat C, Nuntasaen N, Pompimon W. Identification and antiproliferative activity evaluation of a series of triterpenoids isolated from Flueggea virosa (Roxb. ex Willd.). Am J Appl Sci, 2009; 6(10):1800–6. CrossRef
Ndwigah SN, Thoithi GN, Mwangi JW, Amugune BK, Mugo HN, Kibwage IO. Phytosterols from Dombeya torrida (J. F. Gmel.) East Cent Afr J Pharm Sci, 2013; 16:44–8.
Oliveira JPC, Ferreira ELF, Chaves MH, Militão GCG, Gerardo MV, Costa AM, Pessoa C, de Moraes MO, Costa-Lotufo LV. Chemical constituents of Lecythis pisonis and cytotoxic activity. Rev Bras Farmacogn, 2012; 22(5):1140–4. CrossRef
Perumal PC, Sowmya S, Velmurugan D, Sivaraman T, Gopalakrishnan VK. Assessment of dual inhibitory activity of epifriedelanol isolated from Cayratia trifolia against ovarian cancer. Bangladesh J Pharmacol, 2016; 11(2):545–51. CrossRef
Pistritto G, Trisciuoglio D, Ceci C, Garufi A, D’Orazi G. Apoptosis as anticancer mechanism: function and dysfunction of its modulators and targeted therapeutic strategies. Aging, 2016; 8(4):603–19. CrossRef
Shi Q-W, Su X-H, Kiyota H. Chemical and pharmacological research of the plants in genus Euphorbia. Chem Rev, 2008;108(10):4295–327. CrossRef
Su M, Wu X, Chung HY, Li Y, Ye W. Antiproliferative activities of five Chinese medicinal herbs and active compounds in Elephantopus scaber. Nat Prod Commun, 2009; 4(8):1025–30.
Sun Y, Liu Y, Ma X, Hu H. The influence of cell cycle regulation on chemotherapy. Int J Mol Sci, 2021; 22(13):6923. CrossRef
Wilaputraka IGNRB, Azminah A, Erlina L, Syahdi RR, Yanuar A. Virtual screening of indonesian herbal database for dna methyltransferase inhibitors. Asian J Pharm Clin Res, 2017; 10(Special Issue October):153–7. CrossRef
Wong RS. Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res, 2011; 30(1):87. CrossRef
Wongprayoon P, Charoensuksai P. Cytotoxic and anti-migratory activities from hydroalcoholic extract of Euphorbia lactea Haw. against HN22 cell line. Thai Bull Pharm Sci, 2018; 13(1):69–77.Wongrakpanich A, Charoensuksai P. Induction of apoptosis in cancer cells by plants in the genus euphorbia. Thai Bull Pharm Sci, 2018; 13(2):1–11.
Yessoufou K, Elansary HO, Mahmoud EA, Skalicka-Wo?niak K. Antifungal, antibacterial and anticancer activities of Ficus drupacea L. stem bark extract and biologically active isolated compounds. Ind Crops Prod, 2015; 74:752–8. CrossRef
SUPPLEMENTARY MATERIAL
The online version of this article contains Supplementary Materials: 1H-NMR, 13C-NMR, and HRESIMS spectra of 1–4.
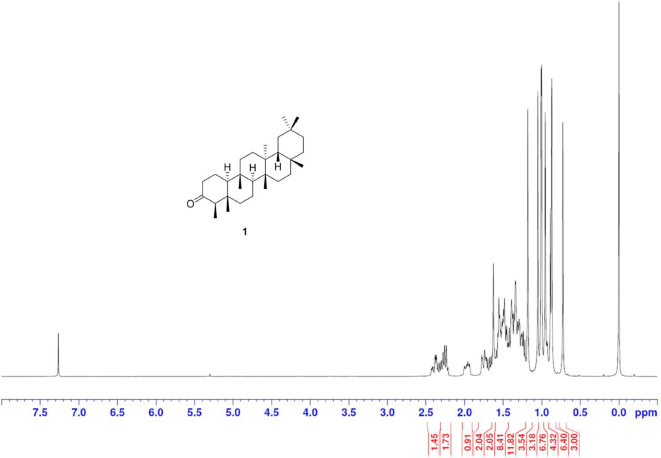 | S1. 1H NMR spectrum of 1 (300 MHz, CDCl3) [Click here to view] |
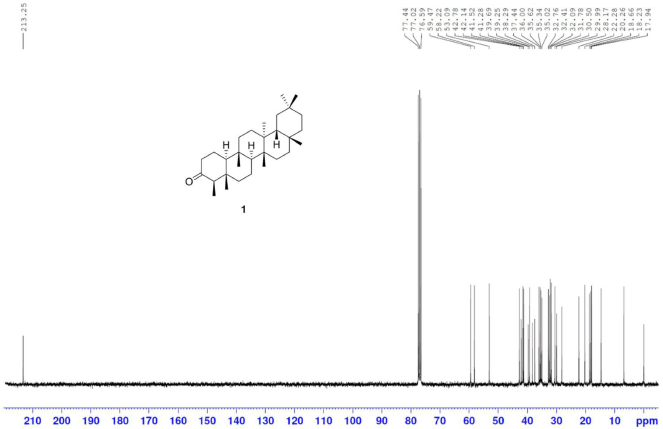 | S2. 13C NMR spectrum of 1 (75 MHz, CDCl3) [Click here to view] |
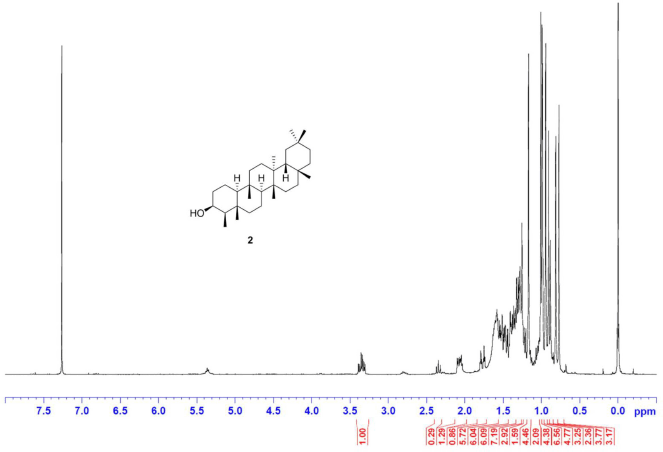 | S3. 1H NMR spectrum of 2 (300 MHz, CDCl3) [Click here to view] |
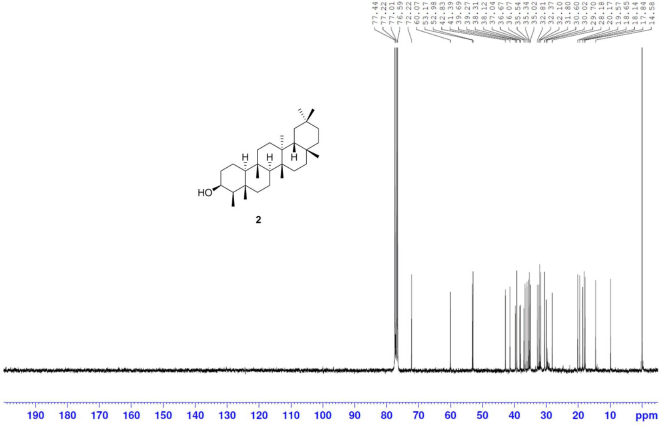 | S4. 13C NMR spectrum of 2 (75 MHz, CDCl3) [Click here to view] |
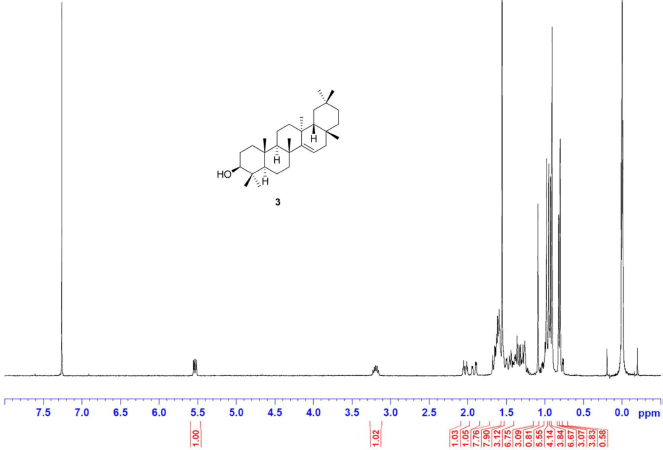 | S5. 1H NMR spectrum of 3 (300 MHz, CDCl3) [Click here to view] |
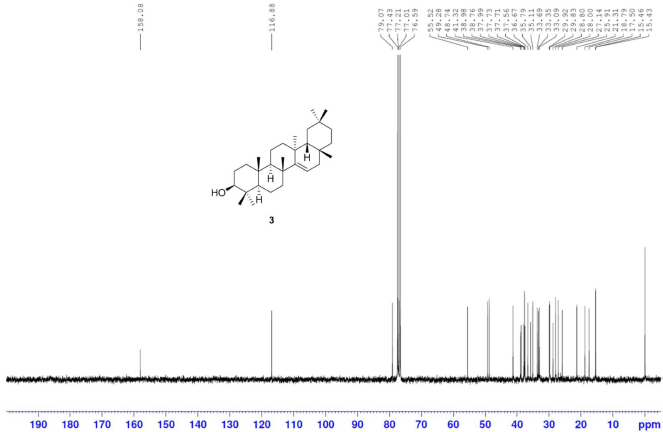 | S6. 13C NMR spectrum of 3 (75 MHz, CDCl3) [Click here to view] |
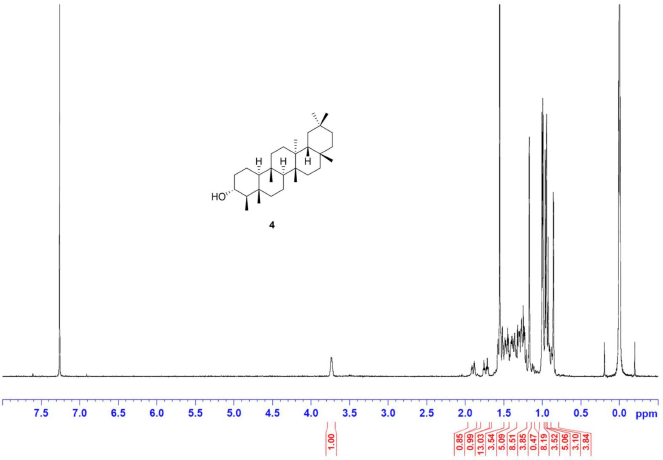 | S7. 1H NMR spectrum of 4 (300 MHz, CDCl3) [Click here to view] |
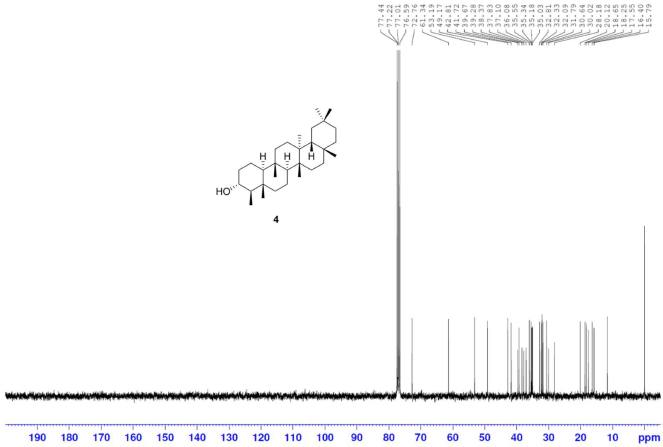 | S8. 13C NMR spectrum of 4 (75 MHz, CDCl3) [Click here to view] |
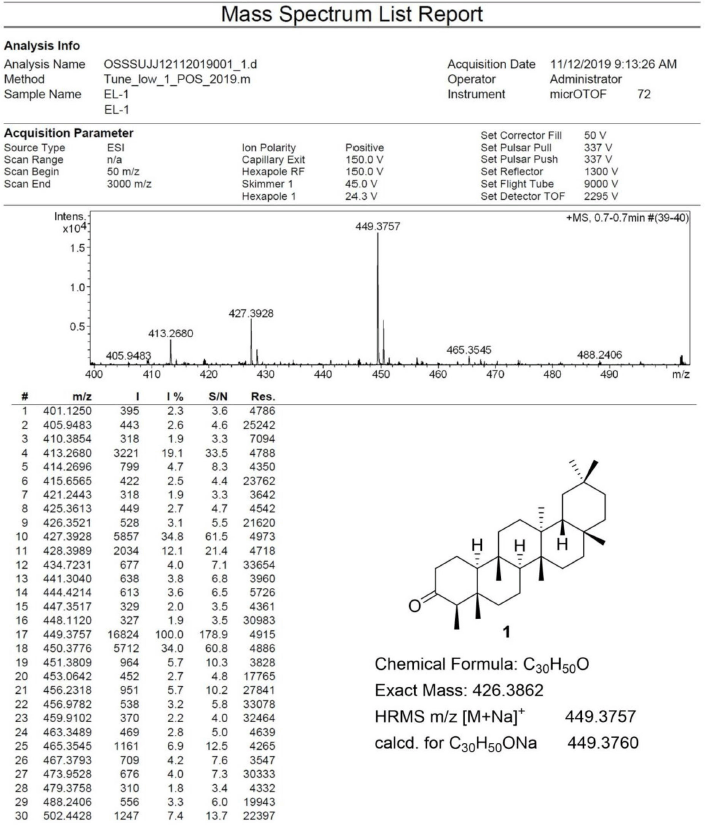 | S9. HRESIMS spectrum of 1 [Click here to view] |
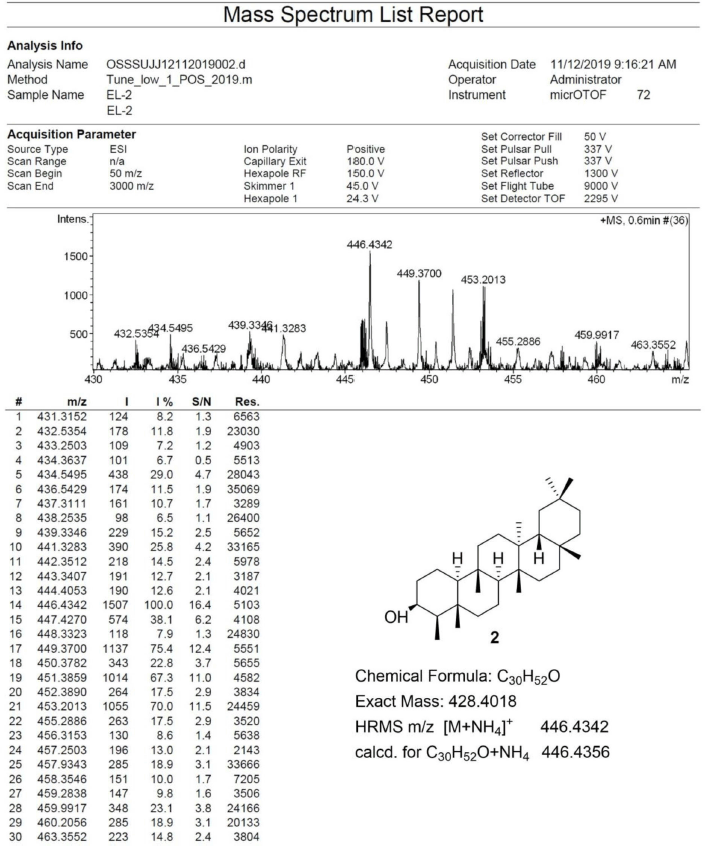 | S10. HRESIMS spectrum of 2 [Click here to view] |
 | S11. HRESIMS spectrum of 3 [Click here to view] |
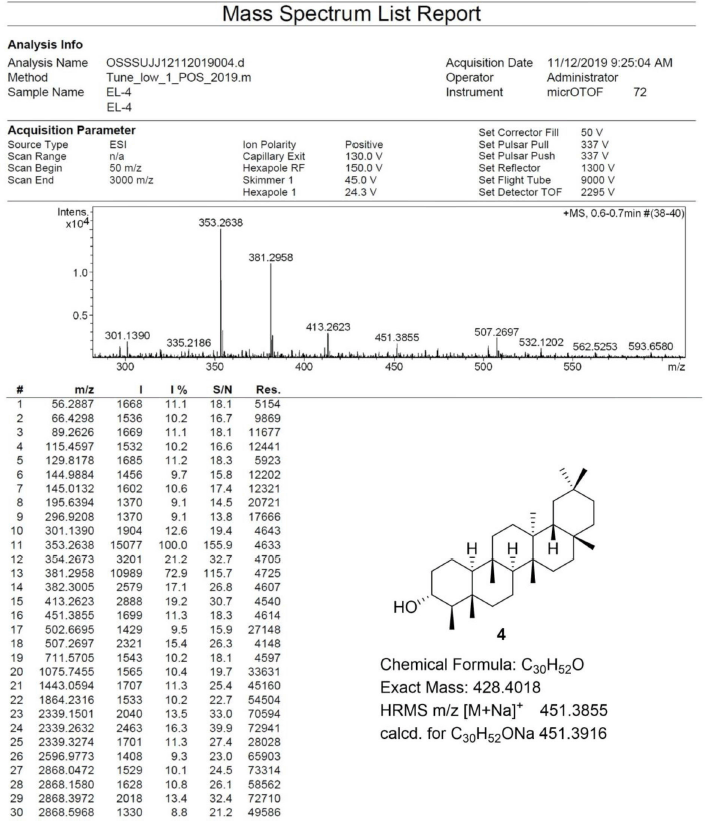 | S12. HRESIMS spectrum of 4 [Click here to view] |