INTRODUCTION
Diabetes mellitus is a degenerative disease which has become a global problem. The International Diabetes Federation (2019) reported 463 million individuals with diabetes in 2019 and expects 700 million in 2045. The hyperglycemia condition in diabetes stimulates the production of free radicals, causing oxidative stress. Oxidative stress is defined as a condition of the formation of excess free radicals in cells that are unable to be neutralized by their antioxidant defenses (Asmat et al., 2016). The most important sources of free radicals are oxygen (reactive oxygen species) and nitrogen (reactive nitrogen species) in biological systems (Kükürt et al., 2022).
The primary antioxidant defenses in the cells are enzymatic antioxidant, superoxide dismutase (SOD), glutathione peroxidase, and catalase (Cat) (Kurutas, 2016). SOD catalyzes the toxic superoxide anion (O2•−) to a less harmful substance, hydrogen peroxide (H2O2). Then, H2O2 is converted to a neutral molecule, oxygen (O2), and a water molecule (H2O) (Gandhi and Abramov, 2012). There are three isoforms of SOD: copper–zinc SOD (Cu, Zn-SOD), manganese SOD (Mn-SOD), and extracellular SOD (Ec-SOD) (Stephenie et al., 2020). Cu, Zn-SOD is present in the cell nucleus and cytoplasm and plays a crucial role in neutralizing free radicals (O2•−), leading to oxidative stress (Stephenie et al., 2020). Previous research found that Cu, Zn-SOD content decreases significantly in the pancreas, liver, and kidney of diabetic rats (Wresdiyati et al., 2010, 2015a). Therefore, the body needs to increase endogenous antioxidant defenses or supply exogenous antioxidants such as food and natural plants (Ragheb et al., 2020).
Sources of exogenous antioxidants, proven to overcome oxidative stress in diabetes, are polyphenols such as flavonoids and phenolic acids, widely contained in plants (Caro-Ordieres et al., 2020). Polyphenols are bioactive compounds that act as free radical scavengers in the body (Gelen et al., 2021). Our study has previously identified the polyphenols content in the Acalypha hispida leaves extract (Alfarisi et al., 2020a). There are 17 types of flavonoids and 5 types of phenolic acid in the A. hispida leaves extract with antihyperglycemic and antioxidant properties (Alfarisi et al., 2020a). However, there are several limitations to using herbal medicines in crude extracts, mainly through oral administration, which are high doses and poor absorption of bioactive compounds. The application of nanotechnology for phytomedicines is the best choice to increase bioavailability and efficacy (Ratheesh et al., 2018). Our previous research has successfully prepared and characterized a nanopowder of the A. hispida leaves extract through ball milling in the nanosize range 512 nm (Alfarisi et al., 2022). No research has reported the efficacy of the nanoextract of A. hispida leaves in decreasing oxidative stress and increasing antioxidant defense in diabetic rats. Furthermore, this research objective was to evaluate the effect of the nanoextracts of A. hispida leaves on antioxidant defense and microstructure of the liver and kidney in diabetic rats.
MATERIALS AND METHODS
Materials
The leaves of A. hispida were collected from the Tropical Biopharmaca Research Center, IPB University. The materials and reagents were streptozotocin (STZ) (S1030, Sigma, Saint Louis, MO), malondialdehyde (MDA) reagent, SOD reagent, Cat reagent, 4% paraformaldehyde, glucometer strip (Accu-Chek, Roche, MN), hematoxylin-eosin (HE), Cu, Zn-SOD primary antibody (S2147, Sigma, Saint Louis, MO), and Starr TrekTM Universal Link (STU700H, Biocare, CA, USA) for secondary antibody and visualization.
Nanoextract of A. hispida preparation
The extraction and preparation of the nanoextract were referred to in Alfarisi et al. (2022). The leaves were dried for 3 days in an oven at 50°C. After drying, leaves were ground and sieved in a 60-mesh sieve. Then, the leaves powder was macerated for 72 hours in 96% ethanol. The filtrate was processed into a spray dryer to get the extract. The extract was milled using planetary ball milling (FRITSCH PULVERISETTE 7, Pittsboro, NC) for 40 minutes at 5,000 rpm. The nanoextract was emulsified in 1% carboxymethyl cellulose (CMC) and then homogenized using ultrasonication for 2 minutes.
Experimental design
Adult male Sprague-Dawley rats (260–290 g; 8–12 weeks) were acclimatized for 14 days in the following conditions: temperature 25°C–27°C; humidity 60%–70%; 12 hours light and 12 hours darkness. The rats were allowed free access to water and feed. Ethical approval was approved from the Animal Ethics Committee at the Faculty of Veterinary Medicine, IPB University (No. 143/KEH/SKE/VI/2019). The sample size was determined using Federer’s (1967) formula [t (n−1) > 15], where, t is the number of treatments and n is the number of replications, and using Arifin and Zahiruddin’s (2017) formula for one-way analysis of variance (ANOVA) design: n = [(10/k) + 1], where k is the number of groups and n is the number of replications. A total of 24 rats were randomly divided into 6 groups (n = 4): (1) normal control (NLC), (2) diabetes + 1% CMC as diabetes mellitus control (DMC), (3) diabetes + metformin 88 mg/kg body weight (BW) (MET), (4) diabetes + crude A. hispida extract at 300 mg/kg BW (CAH), (5) diabetes + nanoextract of A. hispida at 30 mg/kg BW (NAH3), and (6) diabetes + nanoextract of A. hispida at 60 mg/kg BW (NAH6). STZ at 55 mg/kg BW was administered intraperitoneally to induce diabetic rats, which was considered diabetes at blood glucose levels of 300 mg/dl. The treatments were conducted for 28 days. BW was recorded at the beginning and end of treatment.
Collection of samples
All rats were anesthetized on the 29th day with a mixture of ketamine-xylazine (70:10 mg/kg BW). The blood collection was conducted via the heart and put in a vacutainer for blood biochemistry analysis. Parts of the liver and kidney were isolated for antioxidant activity and lipid peroxidation analysis. The other parts were fixed in 4% paraformaldehyde for 8 days.
Blood biochemistry
The levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine, and urea in the serum were analyzed using the Selectra Junior Auto-Analyzer (Vital Scientific). The analysis was referred to in Alfarisi et al. (2020b).
SOD and Cat activity
The activity of total SOD was evaluated using the method developed by Misra and Fridovich’s (1972) method with slight modification (Ulhusna et al., 2019). The oxidation of epinephrine at 475 nm was used to determine total SOD activity in the dialyzed supernatant. One unit of SOD activity is expressed as the quantity of enzyme necessary to reach 50% inhibition of epinephrine oxidation. The Cat activity was measured based on the conversion of the oxidation state of cobalt (II) to cobalt (III) by H2O2 reduction developed by Hadwan (2018). The change absorbance was measured at 440 nm against the reagent blank. The Cat activity was calculated using the formula [2.303/t × log(S°/S)], where t is time, S° is the absorbance of the standard tube, and S is the absorbance of the test tube.
Lipid peroxidation
Lipid peroxidation was evaluated based on the thiobarbituric acid reactive substance assay as the reaction product of MDA and thiobarbituric acid. MDA analysis was referred to in Ulhusna et al. (2019). The absorbance was recorded at 532 nm using a spectrophotometer, and 1,1,3,3-tetraethoxypropane was used as a standard solution.
Histomorphological analysis
The liver and kidney were processed as routine histology specimens. The samples were dehydrated, cleared, and embedded in paraffin using an embedding console (Tissue-Tek, SAKURA). The paraffin-embedded samples were sectioned using a rotary microtome (Yamato RV-240, Saitama, Japan) at a thickness of 5 μm. The tissue sections were stained with HE. The slides were evaluated under a light microscope (Olympus BX 31) equipped with a CCD10 USB Camera. Histomorphological analysis of liver and kidney tissue was performed using ImageJ 1.05i. The analysis was conducted in five fields of view (40×). The nuclear cell size and cytoplasm intensity of liver tissue were measured as described before (Alfarisi et al., 2020b). The kidney tissue was analyzed for the following parameters: glomerulus/Bowman’s capsule ratio, glomerular cell density, tubular necrosis score, tubular dilatation index (TDI), and interstitial volume index (IVI) (Alfarisi et al., 2020b). The tubular necrosis score was evaluated based on the following criteria: 0: normal, 1: mild (<10%), 2: moderate (10%–25%), 3: moderate to severe (25%–50%), 4: severe (50%–75%), and 5: very severe (>75%) (Bussmann et al., 2014). TDI and IVI were assessed by making a grid containing 117 (13 × 9) sampling points on an image. Then, the number of grid points superimposing TDI and IVI was recorded and expressed as a percentage of all sampling points (Fattah et al., 2019).
Immunohistochemistry analysis
Immunohistochemical staining of the Cu, Zn-SOD antioxidant was referred to in Wresdiyati et al. (2010). The liver and kidney tissue sections were deparaffined and rehydrated. Then, the sections were conducted for inactivating endogen peroxidation and blocking unspecific protein. The sections were incubated in Cu, Zn-SOD (1:200) primary antibody for 2 days. The visualization used a 3,3’-diaminobenzidine chromogen. The quantitative analysis of the liver was observed around the central vein and kidney in the nucleus of renal tubule cells in five fields of view (40×). The immunoreaction Cu, Zn-SOD product was analyzed based on the color intensity of the nucleus: strong positive (+++) indicated by a dark brown color throughout the cell nucleus, moderate positive (++) indicated by a dark brown color in some parts of the nucleus, and weak positive (+) marked with a light brown color in the nucleus and negative reactions (−) which showed a blue color in the cell nucleus.
Statistical analysis
A one-way ANOVA was used to compare the data, and statistical significance was determined at p < 0.05. Post hoc analysis was performed using Duncan’s multiple range test. Data were visualized using GraphPad Prism 7, and statistical analysis was performed using Statistical Package for the Social Sciences 25.
RESULTS
Body weight
The highest to lowest growth rates were in the NLC, MET, NAH6, CAH, NAH6, and DMC groups (Table 1). The BW of the MET and NAH6 groups increased, indicated by positive growth rates of 0.0019% and 0.0013%, respectively. A significant growth rate was only shown by the NLC group (0.0116%) compared to the other groups.
Blood biochemistry
The diabetic groups (DMC) showed significantly higher levels of ALT (916.5 mg/dl) and AST (1,119 mg/dl) than the other groups (Fig. 1A and B). The levels of AST and ALT in the MET, CAH, NAH3, and NAH6 groups were not significantly different from the NLC group (142.6 and 90 mg/dl, respectively). In addition, urea and creatinine did not change significantly in all groups (p = 0.189, p = 0.08). These results indicated that metformin and the extract and nanoextract of A. hispida maintained AST and ALT levels in diabetic rats, similar to normal rats.
SOD and Cat activity
The activities of total SOD and Cat enzymes were measured in the liver and kidney tissue (Fig. 2). The total SOD activity of the liver and kidney in the DMC group was significantly lower than in the NLC group. The NAH3, NAH6, and MET groups showed a significant increase in total SOD activity in the liver compared to the DMC group (Fig. 2A). In the kidney, in the MET, CAH, NAH3, and NAH6 groups there was an increase in total SOD activity as well as that in the NLC group (Fig. 2B).
The diabetic rats (DMC) exhibited a lower significance of Cat activity than normal groups (NLC) (Fig. 2C and D). The cat activity of CAH, NAH3, and NAH6 groups could not increase significantly in the liver compared to the MET and DMC groups (Fig. 2C). Similarly, there was no significant change in Cat activity in the MET, CAH, NAH3, and NAH6 groups compared to the DMC group (Fig. 2D). From these results, it can be concluded that metformin and the extract and nanoextract of A. hispida increased the activity of the total SOD enzyme in the liver and kidney of diabetic rats.
Lipid peroxidation
The MDA level in the liver and kidney is shown in Figure 2E and F. The diabetic rats (DMC) showed a significant increase in MDA level compared to the normal rats (NLC). A significant decrease in MDA level in the liver was demonstrated in the MET, CAH, NAH3, and NAH6 groups compared to in the DMC group (Fig. 2E). In the kidney, the MDA levels in the CAH, NAH3, and NAH6 groups decreased, but these were not significant compared to the NLC and DMC groups (Fig. 2F). The results indicated that metformin and the extract and nanoextract of A. hispida reduced MDA levels in the liver and kidney.
Histomorphological analysis
The liver histomorphological analysis was shown in Figure 3A–C. There was no significance in the mean cell nucleus area of liver tissue (p = 0.064) (Fig. 3B). In contrast, the cytoplasmic intensity between groups significantly changed (Fig. 3C). The DMC group showed significantly higher cytoplasmic intensity than the DMC group. The cytoplasmic intensity in the CAH and NAH6 groups was the same as in the NLC group.
The renal histomorphological evaluation was shown in Figure 4A–G. The DMC group showed a significantly decreased glomerular/Bowman’s capsule (B/G) area ratio compared to the NLC group (Fig. 4B). In the MET, CAH, NAH3, and NAH6 groups there was no alteration in the glomerulus/Bowman’s capsule (G/B) ratio as the NLC group. On the other hand, the MET and NAH6 groups showed the best glomerular cell density (Fig. 4C). The MET, CAH, NAH3, and NAH6 groups showed a significant decrease in tubular necrosis scores. The MET, CAH, NAH3, and NAH6 groups had the same TDI as the NLC group (Fig. 4F). There was no significant alteration of interstitial volume in all groups (Fig. 4G). Based on the data, the treatment of metformin and the extract and nanoextract of A. hispida suppressed microstructural damage to the liver and kidney in diabetic rats.
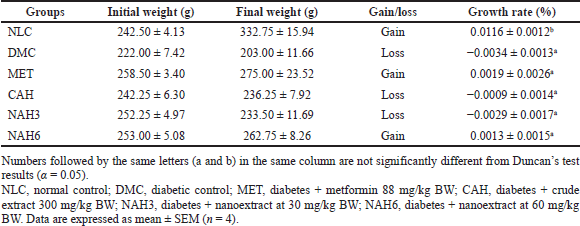 | Table 1. The effect of the nanoextract of A. hispida on the growth rate of BW. [Click here to view] |
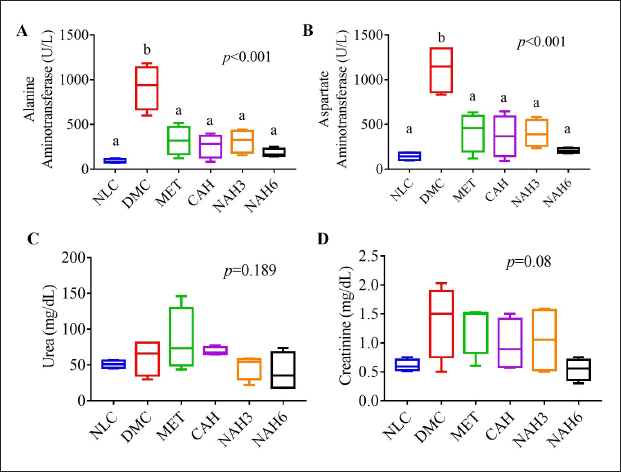 | Figure 1. Blood biochemistry profile of rats after the treatments of nanoextract of A. hispida leaves. (A) AST, (B) ALT, (C) urea, and (D) creatinine. NLC, normal control; DMC, diabetic control; MET, diabetes + metformin 88 mg/kg BW; CAH, diabetes + crude extract 300 mg/kg BW; NAH3, diabetes + nanoextract at 30 mg/kg BW; NAH6, diabetes + nanoextract at 60 mg/kg BW. Data are expressed as mean ± SEM (n = 4). The same letters (a and b) are not significantly different from Duncan’s test results (α = 0.05). [Click here to view] |
The content of Cu,Zn-SOD in the liver and kidney
The immunohistochemical staining of Cu, Zn-SOD in liver and kidney tissue is shown in Figure 5. The MET, CAH, NAH3, and NAH6 groups showed the capability to increase Cu, Zn-SOD content compared to the DMC group, as shown by the number of hepatocyte cells with a strong positive reaction of these groups higher than the DMC group (Table 2). The NAH6 group had a higher Cu, Zn-SOD content than the MET groups. It was shown that the number of hepatocyte cells in the NAH6 group with a negative reaction was significantly lower than in the MET group, but not in the NLC group. The data shows that the nanoextract 60 mg/kg BW had the best effect in maintaining Cu, Zn-SOD content.
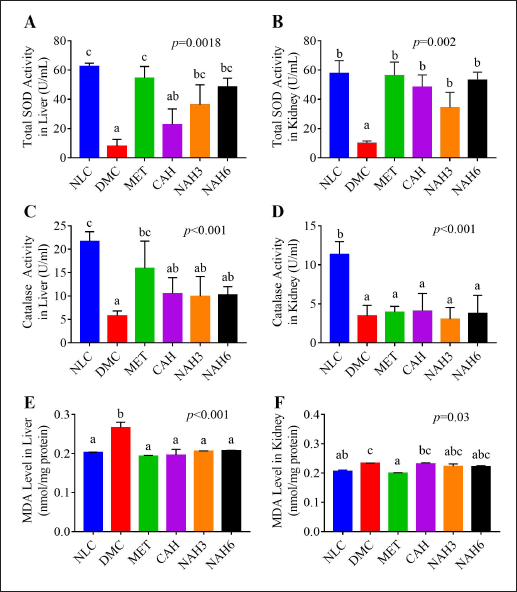 | Figure 2. Total SOD (A–B), Cat activity (C–D), and MDA levels (E–F) in the liver and kidney of rats. NLC, normal control; DMC, diabetic control; MET, diabetes + metformin 88 mg/kg BW; CAH, diabetes + crude extract 300 mg/kg BW; NAH3, diabetes + nanoextract at 30 mg/kg BW; NAH6, diabetes + nanoextract at 60 mg/kg BW. Data are expressed as mean ± SEM (n = 4). The same letters (a and b) are not significantly different from Duncan’s test results (α = 0.05). [Click here to view] |
In the MET, CAH, NAH3, and NAH6 groups, there was an increase in the Cu, Zn-SOD content in renal tubular tissue compared to the DMC group (Table 2), as shown by the number of renal tubules in these groups where a strong positive reaction was higher than in the DMC group. Also, it was supported that the number of renal tubules with weak positive and negative reactions in these groups was lower than in the DMC group. Cu, Zn-SOD content in the CAH, NAH3, and NAH6 groups was significantly higher than in the MET group. It was shown that the number of renal tubules with strong positive reactions in these groups was higher than in the MET group. The results showed that the extract and nanoextracts of A. hispida increased Cu, Zn-SOD content in diabetic rats as well as that in normal rats.
DISCUSSION
The study showed that diabetic rats (DMC) lost weight during the treatments, as demonstrated by a negative growth rate (−0.0034%) (Table 1). This was caused by a diabetic condition induced by STZ so that the carbohydrate metabolism of diabetic rats was impaired. STZ enters pancreatic cells via glucose transporter 2, which transfers its methyl group to DNA, causing DNA fragmentation and a lack of insulin production (Al-Awar et al., 2016). In this study, metformin and nanoextract 60 mg/kg BW increased the BW of diabetic rats, as shown by a positive growth rate (0.0019% and 0.0013%, respectively). Metformin works through the activation of activated protein kinase, which increases insulin sensitivity and decreases cyclic adenosine monophosphate (Rena et al., 2017). Previous research found that alloxan- and STZ-induced diabetic rats lost weight and gained weight after Swietenia mahagoni seed extract treatment for 28 days (Wresdiyati et al., 2015b).
The treatment of metformin and the extract and nanoextract in diabetic rats maintained liver function as well as that in normal rats (Fig. 1A and B), as shown by ALT and AST levels that were not significantly different from the NLC group (90.60 ± 23.68 and 142.62 ± 42.61 U/l, respectively). ALT and AST are enzymes that function to digest protein in the body and have been used as biomarkers for hepatic injury. High levels of ALT and AST in the blood are indicators of impaired liver function (Pal’chikova et al., 2018). Previous research found that ALT and AST enzymes under normal physiology are 67.3 ± 17.3 and 155.1 ± 46.9 U/l, respectively (Mohammad et al., 2019; Napierala et al., 2019). The histomorphological analysis supported these results (Fig. 3C), showing that the extract and nanoextract (60 mg/kg BW) had the same cytoplasmic intensity as the normal group. Diabetic rats exhibited a more alkaline liver tissue cytoplasmic condition, which was indicated by an increase in the liver tissue cytoplasmic intensity through increasing absorption of the excess (eosinophilic) eosin dye (Fig. 3C). Eosinophilia in liver tissue was caused by the denaturation of proteins in the cytoplasm (Alfarisi et al., 2020b).
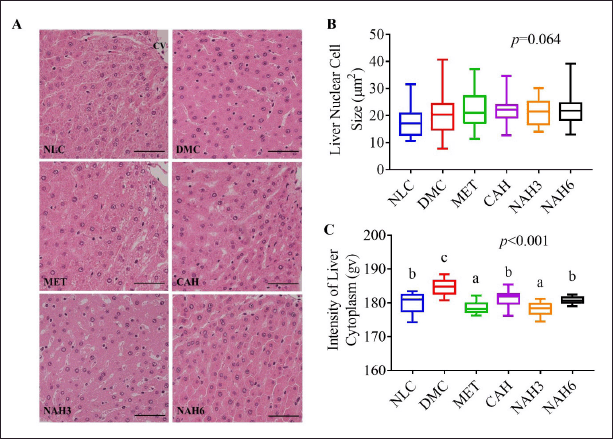 | Figure 3. The extract and nanoextract of A. hispida suppressed liver damage in diabetic rats. (A) Photomicrograph of the liver with HE staining; (B) the nuclear cell size of the liver; and (C) the cytoplasm intensity of the liver. NLC, normal control; DMC, diabetic control; MET, diabetes + metformin 88 mg/kg BW; CAH, diabetes + crude extract 300 mg/kg BW; NAH3, diabetes + nanoextract at 30 mg/kg BW; NAH6, diabetes + nanoextract at 60 mg/kg BW. Data are expressed as mean ± SEM (n = 4). CV, central vein. The same letters (a and b) are not significantly different from Duncan’s test results (α = 0.05). Bar = 50 μm. [Click here to view] |
The treatments in diabetic rats did not significantly affect urea and creatinine levels in the blood (Fig. 1C and D), as shown by both levels that were not significantly different from the NLC group. However, diabetic rats exhibited increased urea and creatinine levels in the blood when compared to normal rats (DMC 60.62 ± 25 U/l vs. NLC 51.05 ± 5.38 U/l; DMC 1.38 ± 0.63 U/l vs. NLC 0.61 ± 0.10 U/l, respectively). Urea and creatinine levels are used as indicators of kidney function (Nosrati et al., 2021). Previous research showed that the urea and creatinine levels of normal rats are 17.71 ± 0.64 and 0.62 ± 0.02 U/l, respectively (Dubey et al., 2020). However, diabetic rats (DMC) were found to have expanded Bowman’s capsule space, decreased glomerular cell density, increased necrosis, and severe tubular dilatation (Fig. 4B–D and F). Previous studies have reported that diabetic rats exhibited Bowman’s capsule space dilatation, tubular necrosis, and tubular dilatation (Al-Za’abi et al., 2021). This research found that metformin and the extract and nanoextract suppressed microstructure damage of kidneys in diabetic rats, indicated by increasing G/B ratio and glomerular cell density and decreasing necrosis and TDI.
Metformin and the extract and nanoextract of A. hispida in diabetic rats reduced oxidative stress, as shown by decreasing MDA levels in the liver and kidney (Fig. 2E and F). In addition, the extract and nanoextract increased antioxidant defense through increasing total SOD and Cat activity and Cu, Zn-SOD content in the liver and kidney (Figs. 2A–D and 5A and B). SOD and Cat are the first defense in overcoming free radicals in cells (Pang et al., 2020). In addition, the role of Cat is relatively insignificant at low H2O2 concentrations but becomes a significant role at high H2O2 concentrations (Gandhi and Sasikumar, 2012). Improving Cu, Zn-SOD can neutralize O2•− excess before reacting with •NO to form peroxynitrite (ONOO−), a strong oxidant that can damage the cells by several mechanisms (Forman and Zhang, 2021; Soliman et al., 2021). In similar research, Wresdiyati et al. (2010) found that Momordica charantia L. powder increased Cu, Zn-SOD content of the liver and kidney in oxidative stress in diabetic rats. Previous research also reported that the treatment of Rheum ribes decreased oxidative stress and increased total SOD and Cat levels in the liver and kidney (Yildirim et al., 2021).
The capability of the nanoextract of A. hispida leaves of increasing Cu, Zn-SOD content in the liver and kidney was associated with bioactive contents. Our previous research showed that the bioactive contents of the nanoextract of A. hispida leaves were gallic acid 11.2 mg/g and catechin 78.2 mg/g dry nanoextract (Alfarisi et al., 2022). In addition to the H-donating antioxidant process, catechin directly or indirectly stimulates the expression of the enzymatic antioxidants, such as SOD and Cat (Simos et al., 2012). Gallic acid can stimulate nuclear factor-erythroid 2-related factor 2 (NrF2) signaling to mediate its antioxidant activity (Yu et al., 2012). There are mechanisms to stimulate Cu, Zn-SOD, including pathways involving AKT, ERK1/2 activation, NrF2 protein, and muscarinic M1 receptor (Mondola et al., 2016). When the cells are activated, NrF2 is released from Keap1 and translocates to the nucleus, then binds to antioxidant responsive elements to transcribe antioxidant enzymes, including Cu, Zn-SOD (Lin et al., 2020).
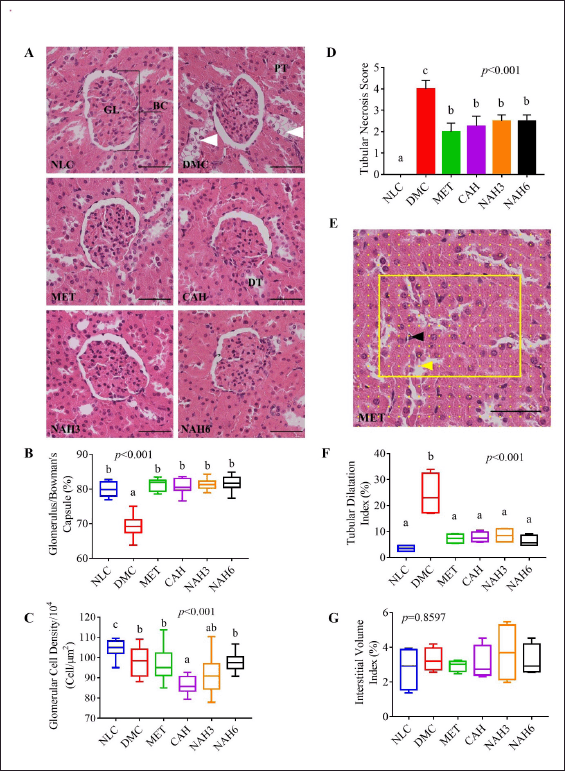 | Figure 4. The nanoextract of A. hispida suppressed progressivity in kidney damage. (A) Photomicrograph of kidney rats with HE staining; (B) glomerulus/Bowman’s capsule ratio; (C) glomerular cell density; (D) tubular necrosis score; (E) grid containing 117 (13 × 9) sampling points on an image; (F) TDI; and (G) IVI. NLC, normal control; DMC, diabetic control; MET, diabetes + metformin 88 mg/kg BW; CAH, diabetes + crude extract 300 mg/kg BW; NAH3, diabetes + nanoextract at 30 mg/kg BW; and NAH6, diabetes + nanoextract at 60 mg/kg BW. GL, glomerulus; BC, Bowman’s capsule; DT, distal tubule; and TP, proximal tubule. White arrowhead: tubular necrosis; yellow arrowhead: grid point in tubular lumen; black arrowhead: grid point in interstitial space. Data are expressed as mean ± SEM (n = 4). The same letters (a and b) are not significantly different from Duncan’s test results (α = 0.05). Bar = 50 μm. [Click here to view] |
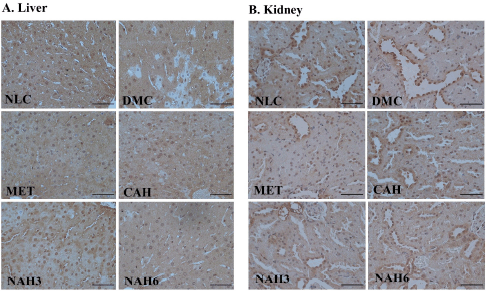 | Figure 5. The photomicrograph of Cu, Zn-SOD localization in the liver (A) and kidney (B) of diabetic rats. NLC, normal control; DMC, diabetic control; MET, diabetes + metformin 88 mg/kg BW; CAH, diabetes + crude extract 300 mg/kg BW; NAH3, diabetes + nanoextract at 30 mg/kg BW; and NAH6, diabetes + nanoextract at 60 mg/kg BW. Bar = 50 μm. [Click here to view] |
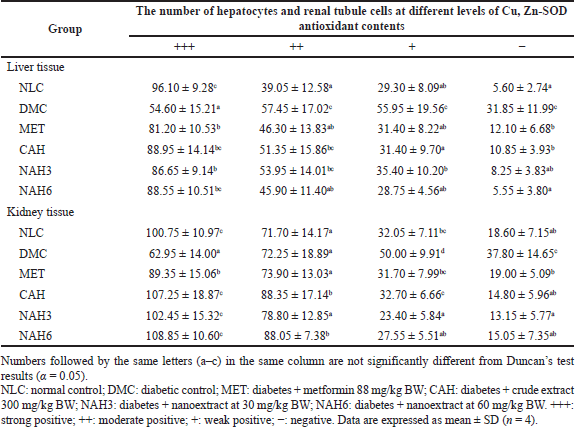 | Table 2. The number of hepatocytes and renal tubule cells at different levels of antioxidant Cu, Zn-SOD content in liver and kidney tissue. [Click here to view] |
Interestingly, the nanoextract at a dose of 60 mg/kg BW had the same efficacy as the crude extract at 300 mg/kg BW and was even better in certain parameters. However, the nanoextract dose was five-fold lower than the crude extract. This may be caused by the size of nanoextract particles (512 nm), which is five-fold smaller than crude extract (1,271 nm) in our previous report (Alfarisi et al., 2022). This effect is associated with the smaller particle size, making it easier to enter the cell through phagocytosis and pinocytosis (Danaei et al., 2018; Zafari et al., 2020). Previous research revealed that Posidonia oceanica nanoparticles in the nanosize range 242–591 nm increased antidiabetic activity compared to the crude extract of P. oceanica at the same dose at 100 mg/kg BW (Ammar et al., 2021).
CONCLUSION
The nanoextract of A. hispida leaves at a dose of 60 mg/kg BW and metformin reduced oxidative stress (decreasing MDA level) in the liver and kidney through increasing the enzymatic antioxidant defenses (total SOD and Cat activity and Cu, Zn-SOD content). The nanoextract (60 mg/kg BW) with a dose five times smaller than the crude extract (300 mg/kg BW) had better efficacy. The nanoextract and metformin suppressed microstructure damage in the liver (mean cell nucleus area and cytoplasmic intensity) and kidney (B/Gs capsule area, glomerular cell density, TDI, and tubular necrosis) of diabetic rats.
CONFLICTS OF INTEREST
All authors declare that they have no conflicts of interest.
FUNDING
The authors thank the Deputy of Strengthening Research and Development, Ministry of Research and Technology - National Research and Innovation Agency, The Republic of Indonesia for funding research through the PMDSU research grant scheme year 2021 (Number: 1/E1/KP.PTNBH/2021).
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
ETHICAL APPROVALS
Ethical approval was approved from the Animal Ethics Committee at the Faculty of Veterinary Medicine, IPB University (No. 143/KEH/SKE/VI/2019).
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Al-Awar A, Kupai K, Veszelka M, Szucs G, Attieh Z, Murlasits Z, Török S, Pósa A, Varga, C. Experimental diabetes mellitus in different animal models. J Diabetes Res, 2016; 2016:1–12. CrossRef
Al-Za’abi M, Ali BH, Al Suleimani Y, Adham SA, Ali H, Manoj P, Ashique M, Nemmar A. The effect of metformin in diabetic and non-diabetic rats with experimentally-induced chronic kidney disease. Biomolecules, 2021; 11:1–18. CrossRef
Alfarisi H, Sa’diah S, Wresdiyati T. Polyphenol profile, antioxidant and hypoglycemic activity of Acalypha hispida leaf extract. Indian J Pharm Sci, 2020a; 82:291–9. CrossRef
Alfarisi H, Sadiah S, Juliandi B, Wresdiyati T. Preparation and characterization of nanopowder of Acalypha hispida leaves extract using planetary ball milling. Molekul, 2022; 17(1):68–75. CrossRef
Alfarisi H, Subangkit M, Sa’diah S, Wresdiyati T. Acute toxicity of ethanolic extract of Acalypha hispida leaves in female rats: a physiological and histological study. J Kedokt Hewan, 2020b; 14:48–53. CrossRef
Ammar NM, Hassan HA, Mohammed MA, Serag A, Abd El-Alim SH, Elmotasem H, El Raey M, El Gendy AN, Sobeh M, Abdel-Hamid AZ. Metabolomic profiling to reveal the therapeutic potency of Posidonia oceanica nanoparticles in diabetic rats. RSC Adv, 2021; 11:8398–410. CrossRef
Arifin WN, Zahiruddin WM. Sample size calculation in animal studies using resource equation approach. Malaysian J Med Sci, 2017; 24:101–5. CrossRef
Asmat U, Abad K, Ismail K. Diabetes mellitus and oxidative stress—a concise review. Saudi Pharm J, 2016; 24:547–53. CrossRef
Bussmann AR, Filho MAM, Módolo MP, Módolo RP, Amado P, Domingues MAC, Castiglia YMM, Módolo NSP. Effect of allopurinol on the kidney function, histology and injury biomarker (NGAL, IL-18) levels in uninephrectomised rats subjected to ischaemia-reperfusion injury. Acta Cir Bras, 2014; 29:515–21. CrossRef
Caro-Ordieres T, Marín-Royo G, Opazo-Ríos L, Jiménez-Castilla L, Moreno JA, Gómez-Guerrero C, Egido J. The coming age of flavonoids in the treatment of diabetic complications. J Clin Med, 2020; 9:346. CrossRef
Danaei M, Dehghankhold M, Ataei S, Hasanzadeh Davarani F, Javanmard R, Dokhani A, Khorasani S, Mozafari MR. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics, 2018; 10:1–17. CrossRef
Dubey S, Yadav C, Bajpeyee A, Singh MP. Effect of Pleurotus fossulatus aqueous extract on biochemical properties of liver and kidney in streptozotocin-induced diabetic rat. Diabetes Metab Syndr Obes, 2020; 13:3035–46. CrossRef
Fattah I Omar A, Badawi MHAL, Mohamed MH, Hameed ASA. Autologous-versus allogeneic-bone marrow cell grafting in prevention of obstructive nephropathy in rats. Egypt J Histol, 2019; 42:276–84. CrossRef
Federer W. Experimental design: theory and application. IBH Publishing Company, Oxford, UK, 1967.
Forman HJ, Zhang H. Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat Rev Drug Discov, 2021; 20:689–709. CrossRef
Gandhi GR, Sasikumar P. Antidiabetic effect of Merremia emarginata Burm. F. in streptozotocin induced diabetic rats. Asian Pac J Trop Biomed, 2012; 2:281–6. CrossRef
Gandhi S, Abramov AY. Mechanism of oxidative stress in neurodegeneration. Oxid Med Cell Longev, 2012; 2012:428010. CrossRef
Gelen V, Kükürt A, ?engül E, Ba?er ÖF, Karapehlivan M. Can polyphenols be used as anti-inflammatory agents against COVID-19 (SARS-CoV-2)-induced inflammation? In: Badria F (ed.). Phenolic compdounds—chemistry, synthesis, diversity, non-conventional industrial, pharmaceutical and therapeutic applications, IntechOpen, London, UK, pp 1–21, 2021. CrossRef
Hadwan MH. Simple spectrophotometric assay for measuring catalase activity in biological tissues. BMC Biochem, 2018; 19:1–8. CrossRef
International Diabetes Federation (IDF). IDF Diabetes Atlas. 9th edition, 2019. Available via https://diabetesatlas.org/atlas/ninth-edition/ (Accessed 08 December 2021).
Kükürt A, Gelen V, Ba?er Ö, Deveci H, Karapehlivan M. Thiols: role in oxidative stress-related disorders. In: Atukeren P (ed.). Accent. Lipid peroxidation, IntechOpen, London, UK, pp 1–21, 2022. CrossRef
Kurutas EB. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr J, 2016; 15:1–22. CrossRef
Lin Y, Luo T, Weng A, Huang X, Yao Y, Fu Z, Li Y, Liu A, Li X, Chen D, Pan H. Gallic acid alleviates gouty arthritis by inhibiting NLRP3 inflammasome activation and pyroptosis through enhancing Nrf2 signaling. Front Immunol, 2020; 11:1–13. CrossRef
Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem, 1972; 247:3170–5. CrossRef
Mohammad P, Esfandiar KZ, Abbas S, Ahoora R. Effects of moderate-intensity continuous training and high-intensity interval training on serum levels of resistin, chemerin and liver enzymes in streptozotocin-nicotinamide induced type-2 diabetic rats. J Diabetes Metab Disord, 2019; 18:379–87. CrossRef
Mondola P, Damiano S, Sasso A, Santillo M. The Cu, Zn superoxide dismutase: not only a dismutase enzyme. Front Physiol, 2016; 7:1–8. CrossRef
Napierala M, Olszewski J, Miechowicz I, Jablecka A, Czarnywojtek A, Malinger S, Florek E. The influence of tobacco smoke exposure on selected markers of oxidative stress, kidneys and liver function in the serum of rats with streptozotocin-induced diabetes. Pharmacol Rep, 2019; 71:1293–8. CrossRef
Nosrati H, Hamzepoor M, Sohrabi M, Saidijam M, Assari MJ, Shabab N, Gholami Mahmoudian Z, Alizadeh Z. The potential renal toxicity of silver nanoparticles after repeated oral exposure and its underlying mechanisms. BMC Nephrol, 2021; 22:1–12. CrossRef
Pal’chikova NA, Selyatitskaya VG, Kuz’minova OI, Pasechnaya KV. Effects mifepristone on aminotransferase activities in the liver in rats with streptozotocin-induced diabetes mellitus. Bull Exp Biol Med, 2018; 165:474–7. CrossRef
Pang L, Lian X, Liu H, Zhang Y, Li Q, Cai Y, Ma H, Yu X. Understanding diabetic neuropathy: focus on oxidative stress. Oxid Med Cell Longev, 2020; 2020:1–13. CrossRef
Ragheb SR, El Wakeel LM, Nasr MS, Sabri NA. Impact of rutin and vitamin C combination on oxidative stress and glycemic control in patients with type 2 diabetes. Clin Nutr ESPEN, 2020; 35:128–35. CrossRef
Ratheesh G, Xiao Y, Ezhilarasu H, Sadiq A, Devassy G, Tian L, Venugopal JR. Nanodrug delivery system using medicinal plants. In: Sharma CP (ed.). Drug delivery nanosystems for biomedical applications, Elsevier Inc., Oxford, UK, pp 357–75, 2018. CrossRef
Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia, 2017; 60:1577–85. CrossRef
Simos YV, Verginadis II, Toliopoulos IK, Velalopoulou AP, Karagounis IV, Karkabounas SC, Evangelou AM. Effects of catechin and epicatechin on superoxide dismutase and glutathione peroxidase activity, in vivo. Redox Rep, 2012; 17:181–6. CrossRef
Soliman E, Shewaikh SM, Fahmy A, Elshazly S. Entacapone scavenges peroxynitrite and protects against kidney and liver injuries induced by renal ischemia/reperfusion in rats. Int Urol Nephrol, 2021; 53:1713–21. CrossRef
Stephenie S, Chang YP, Gnanasekaran A, Esa NM, Gnanaraj C. An insight on superoxide dismutase (SOD) from plants for mammalian health enhancement. J Funct Foods, 2020; 68:103917. CrossRef
Ulhusna FA, Winarto A, Wresdiyati T. The profile of superoxida dismutase and malondialdeyde level in the liver tissue of hypercholesterolemic rats treated with Holothuria nobilis polysaccharide. J Kedokt Hewan, 2019; 13:37–44. CrossRef
Wresdiyati T, Karmila A, Astawan M, Karnila R. Sea cucumber increased antioxidant superoxide dismutase in the pancreatic tissue of diabetic rats. J Vet, 2015a; 16:145–51.
Wresdiyati T, Sa’diah S, Winarto A, Febriyani V. Alpha-glucosidase inhibition and hypoglycemic activities of Sweitenia mahagoni seed extract. HAYATI J Biosci, 2015b; 22:73–8. CrossRef
Wresdiyati T, Sinulingga TS, Zulfanedi Y. Effect of Mamordica charantia L. powder on antioxidant superoxide dismutase in liver and kidney of diabetic rats. HAYATI J Biosci, 2010; 17:53–7. CrossRef
Yildirim M, Degirmenci U, Akkapulu M, Comelekoglu U, Balli E, Metin Ozcan T, Berköz M, Yalin AE, Yalin S. The effect of Rheum ribes L. On oxidative stress in diabetic rats. J Basic Clin Physiol Pharmacol, 2021; 32:1–10. CrossRef
Yu J, Zhao Y, Li B, Sun L, Huo H. 17β-Estradiol regulates the expression of antioxidant enzymes in myocardial cells by increasing Nrf2 translocation. J Biochem Mol Toxicol, 2012; 26:264–9. CrossRef
Zafari M, Aghajani S, Mansouri Boroujeni M, Nosrati H. Vancomycin-loaded electrospun polycaprolactone/nano-hydroxyapatite membrane for the treatment of blood infections. Med Hypotheses, 2020; 144:109992. CrossRef