INTRODUCTION
Piperine, also known as International Union of Pure and Applied Chemistry 1-(5-[1,3-benzodioxol-5-yl]-1-oxo-2,4-pentadienyl), is the primary alkaloid component of white pepper. The Ayurvedic system of medicine has traditionally used black pepper as an antipain, amenorrhea, antiasthmatic, and cough, and cold medicine. Piperine compounds have several pharmacological effects, including antioxidant, hepatoprotective, antimetastatic (Koul and Kapil, 1993), antidepressant (Li et al., 2007), analgesic, anti-inflammatory, antipyretic (Mujumdar et al., 1990), antithyroid, immunomodulatory, antitumor, inhibit P-glycoprotein expression (Ee et al., 2010; Han et al., 2008; Srinivasan, 2007), and increase the bioavailability of other drugs through cell membranes by increasing the vasodilation of gastrointestinal tract (GIT) membrane (Khajuria et al., 2002). Although piperine compounds have many therapeutic benefits, they have several disadvantages: photosensitive, unstable in the ultraviolet (UV) light, oxidative stress, and low solubility in water (Kotte et al., 2014). The results of low solubility (22.34 mg/l at 25°C) and low dissolution rate lead to low bioavailability of orally administered drugs (Wu et al., 2012). Thus, piperine compounds have been identified as Biopharmaceutics Classification System (BCS) class II.
Many researchers have attempted to increase the dissolution rate, solubility, and oral bioavailability of piperine. In general, to increase the bioavailability of Active Pharmaceutical Ingredients (APIs) in BCS class II, one needs to increase the dissolution rate of APIs that are poorly soluble in water. Several piperine formulations have been proposed, including solid dispersions (Thenmozhi and Yoo, 2017), nanoparticles (Bhalekar et al., 2017), microemulsions (Etman et al., 2018), hot-melt extrusion technology (Ashour et al., 2016), liposomes (Dutta and Bhattacharjee, 2017; Pentak, 2016; Priprem et al., 2011), complex with α- and γ-cyclodextrin (Ezawa et al., 2016, 2018), piperine encapsulation with polyethylene glycol polylactide-co-glycolide (Pachauri et al., 2015), piperine encapsulation with soy-lecithin multilamellar vesicles (Aukunuru and Bonepally, 2017), and a lipid-based self-emulsifying drug delivery system (Shao et al., 2015).
Liquid self-nanoemulsifying drug delivery system (L-SNEDDS) is a lipid-based formulation consisting of a combination of anhydrous isotropic, active substances, liquid/solid surfactants, cosurfactants, and natural/synthetic oils/lipids. It can spontaneously form an oil in water emulsion (o/w) by gentle agitation in GIT fluids. This droplet emulsion is advantageous for drug absorption due to the large interfacial surface area. Therefore, it can improve the bioavailability of APIs with low solubility in water (Kim et al., 2014; Rashid et al., 2015). However, L-SNEDDS has several limitations, such as stability during handling and storage, interactions between L-SNEDDS and soft/hard capsule shells, and production problems that often hinder the L-SNEDDS application in the pharmaceutical industry (Ahmad et al., 2014; Cole et al., 2008).
Thus, along with technological developments, the solid- self-nanoemulsifying drug delivery systems (S-SNEDDS) formulation has been extensively explored to overcome the shortcomings of the L-SNEDDS formulation (Kang et al., 2012; Kim et al., 2017; Mustapha et al., 2017). The S-SNEDDS formulation was developed by converting L-SNEDDS into powder form through spray drying, freeze-drying (Kang et al., 2012; Seo et al., 2013), spray cooling (Cavallari et al., 2005; Passerini et al., 2006), melt granulation, melt extrusion/extrusion spheronization (Tang et al., 2008), or solid carrier adsorption (Ameeduzzafar et al., 2019). The S-SNEDDS dosage form provides many benefits, such as high stability, ease of scale-up, uniformity of content, accurate doses, and increase in patient’s compliance (Bari et al., 2015; Hu et al., 2012). The S-SNEDDS formulation provides a promising drug delivery system for active substances that are poorly soluble in water, photosensitive, and unstable in liquid preparations. S-SNEDDS provides advantages by combining the L-SNEDDS advantages (enhanced solubility and bioavailability) with solid dosage forms (high stability during handling and storage in a large selection of dosage forms) (Nazzal and Khan, 2006; Wang et al., 2009).
This study was aimed at solidifying L-SNEDDS containing isolated piperine through a mesoporous mannitol carrier and spray-dried to produce S-SNEDDS powder. It was to overcome the solubility and stability of piperine in liquid preparations. Miglyol 812 N, polyethylene glycol 400 (PEG400), and Cremophor RH40 at a ratio of 2.25:2.60:5.15 were chosen as the optimum formula for L-SNEDDS isolated piperine. The three ingredients are proven safe for oral formulations. Furthermore, the surfactant Cremophor RH40 has been successfully used in formulating SNEDDS products, including Neural® (Novartis) product containing cyclosporine A (Badran et al., 2014; Krsti? et al., 2018; Mohd et al., 2015). Mesoporous mannitol made of D-mannitol and templating agent ammonium bicarbonate in a ratio of 1:1 w/w% was selected as a high porous adsorbent for the solidification process of L-SNEDDS by spray drying technique.
MATERIAL AND METHODS
Isolated piperine (purity 95%) was obtained by isolating white pepper (Piper nigrum L) (Sorowako, South Sulawesi, Indonesia), Cremophor RH40 (Clariant, Indonesia), Miglyol 812 N (IOI Oleochemical, Germany), PEG 400 (Brataco, Indonesia), Tween 80 (Brataco, Indonesia), olive oil (Brataco, Indonesia), virgin coconut oil (VCO), soybean oil, isopropyl myristate (Brataco, Indonesia), propylene glycol (Brataco, Indonesia), water for injection (IKA Pharmindo, Indonesia), methanol for high performance liquid chromatography (HPLC) (Merck, Germany), D-mannitol (Merck, Germany), and ammonium bicarbonate (Merck, Germany).
Instrumentation
The instruments used in this study included stirrer (Stuart Hotplate Stirrer CB162, UK), analytical balance (Ohaus PA 323, China), semi-micro-analytical balance (Shimadzu AP135W, Japan), vortex (D-Lab MX-S, USA), centrifuge (Hettich, EBA 8, Germany), HPLC Hitachi L-2420 UV-Vis detector with Luna® 5 μm C18 100 Å LC Column 250 × 4.6 mm Phenomenex (Hitachi, Japan), UV-Vis spectrophotometer (Genesys 10S, United States Pharmacopeia), spray dryer (Buchi Mini Spray Dryer B-290, Swiss), scanning electron microscopy (SEM) (JSM-6510LA, Japan), Quadrasorb Evo surface area analyzer (SAA) (Boynton Beach FL, USA), Differential scanning calorimetry (DSC) (DSC-60 Plus Shimadzu, Japan), Fourier transform infrared (FTIR) (Thermo Scientific Nicolet iS10, USA), Malvern Zetasizer (Malvern Instruments, UK), and Dissolution Tester RC-1 (Hanchen Instrument).
Extraction and purification of piperine compounds
White pepper fruit powder was extracted using a proanalytical n-hexane solvent using the Soxhlet method. The extracted filtrate was then evaporated at room temperature of 25°C up to half portion and stored in the refrigerator for 2 days. The crystals formed were filtered and dried at room temperature of 25°C. The formed yellow crystals were washed using cyclohexane. Then the insoluble crystals were dried at room temperature of 25°C. The results obtained were isolated piperine with a purity of ≥95% and had been previously published by Kusumorini et al. (2021b).
Analytical method
Determination of isolated piperine was measured by a validated method using the HPLC system (Hitachi L-2420, Tokyo, Japan) with column conditions C18 Phenomenex®; size 4.6 × 250 mm; particle size 5 μm; mobile phase methanol:water with a ratio of 75:25% v/v; flow rate 1 ml/minute; sample injection volume 20 μl; and temperature of 25°C. The wavelength of the UV detector is 340 nm. The validation result of this method has been published in Kusumorini et al. (2021a).
Preparation of S-SNEDDS formulations
The S-SNEDDS formulation was prepared in two steps. First, L-SNEDDS was made from the ratio of oil:Smix (ratio of surfactant and cosurfactant) selected from the optimization results using D-Optimal design, Design-Expert software (DX ver.10, State Ease Inc, Minnesota). Next, the S-SNEDDS formulation was prepared using a mesoporous mannitol hydrophilic carrier with spray drying technology.
Solubility studies of isolated piperine
Solubility tests of isolated piperine in various oils (VCO, olive oil, canola oil, and Miglyol 812 N), surfactants (Cremophor RH40 and Tween 80), and cosurfactants (PEG400 and propylene glycol) were carried out by dissolving 10.0 mg of isolated piperine in 10.0 ml of the carrier. The mixture was vortexed for 10 minutes and stirred for 2 hours at 37°C ± 1°C. Furthermore, the insoluble active substance was separated through centrifugation at a speed of 3,000 rpm for 30 minutes. A precipitate, the rest of the active substance, was then separated from the supernatant. Furthermore, the supernatant dissolved in methanol and the levels were determined using HPLC in a validated method. The carriers with the highest solubility (oils, surfactants, and cosurfactants) were selected and used for SNEDDS liquid.
Construction of the pseudoternary phase diagrams
The pseudoternary phase diagram of nanoemulsion was made by mixing surfactants, cosurfactants, and oil titrated with water until they were homogeneous. The surfactant and cosurfactant (Smix) mixture was prepared at different volume ratios (1:1, 1:2, 2:1, and 1:3). Next, the oil phase was added to the Smix at the volume ratio of 1:9, 2:8, 3:7, 4:6, 5:5, 6:4, 7:3, 8:2, and 9:1, respectively. The monophasic mixture was slowly titrated with water and stirred using a magnetic stirrer. After achieving a homogeneous equilibrium mixture until the mixture was translucent in color, it was titrated with water until it became cloudy or turbid. The tendency to emulsify spontaneously and the development of emulsion droplets were visually observed (Khan et al., 2015). The ternary phase diagram was plotted using the Triplot version 4.1.2 software (Todd A. Thompson, LA).
Preparation of isolated piperine loaded in L-SNEDDS
SNEDDS containing isolated piperine was made by mixing isolated piperine with Cremophor RH40, PEG400, and Miglyol 812N. The SNEDDS was stirred for 30 minutes and then stored at room temperature at 30°C for 48 hours (Khan et al., 2015). The number of the three components was determined based on the optimization results of the three components using the D-Optimal design and Design-Expert software (DX version 10, State Ease Inc., MN). The results have been previously published in Kusumorini et al. (2021a). The optimization range of the three components was obtained from the results of the emulsion region of the ternary phase diagram. The optimum formula L-SNEDDS obtained consisted of Miglyol 812 N (oil), PEG400 (cosurfactant), and Cremophor RH40 (surfactant) at a ratio of 2.25:2.60:5.15, respectively (Kusumorini et al., 2021a).
Determination of the amount of isolated piperine loaded in L-SNEDDS formulation
The maximum amount of isolated piperine contained in the L-SNEDDS formulation was determined by a validated method using the HPLC system (Hitachi L-2420, Tokyo, Japan), as described in the “Analytical method.” A total of 50, 100, 150, 200, and 250 mg of isolated piperine were mixed with 10 g of L-SNEDDS optimum formula, homogenized using vortex for 5 minutes, and stirred for 30 minutes. Furthermore, the SNEDDS mixture was centrifuged at 3,000 rpm for 15 minutes. The supernatant was taken and filtered through a 0.45 μl cellulose membrane. The filtered solution was diluted with methanol and measured by the validated HPLC method (Vu et al., 2020).
Characterization of L-SNEDDS isolated piperine
Determination of percent transmittance
The transmittance percentage of the L-SNEDDS isolated piperine formulation was measured by dissolving 100 μl of L-SNEDDS isolated piperine in 10 ml of distilled water, homogenized with a stirrer. Afterward, the clarity of the isotropic system was read using a Shimadzu UV spectrophotometer (Genesys 10S, USA) at a wavelength of 650 nm (Khan et al., 2015).
Determination of emulsification time
The emulsification time of L-SNEDDS isolated piperine was measured by dropping 1 ml of L-SNEDDS isolated piperine into 500 ml of distilled water at 37°C ± 1°C, stirred using a magnetic stirrer at a speed of 120 rpm. The emulsification process was plotted against the time required to form a homogeneous SNEDDS droplet mixture in the medium (Khan et al., 2015).
Determination of droplet size, polydispersity index, and zeta potential
Particle size, polydisperse index, and zeta potential were analyzed by diluting 1 ml of L-SNEDDS isolated piperine in 100 ml of distilled water. The dilution was homogenized using a magnetic stirrer. A particle size analyzer (Malvern Instruments, UK) was used to monitor light scattering at 25°C and an angle of 172° (Salem et al., 2019).
Preparation of mesoporous mannitol
Mesoporous mannitol powder was the mixture of 50 g of D-mannitol and 50 g ammonium bicarbonate dissolved with 1 l of distilled water at 30°C and stirred for 10 minutes (Song et al., 2018). Then, the solution was dried using a spray dryer Buchi B-290 with an inlet and outlet temperature of 120°C and 80°C, an aspirator of 90%, a pump on a scale of 25%, and a pressure of 50 mBar. As a result, mesoporous mannitol powder obtained characterized attributes including specific surface area, pore size, pore volume, and surface morphological shape.
Characterization of mesoporous mannitol
Scanning electron microscopy
The spray drying of the mixture of D-mannitol with ammonium bicarbonate and pure D-mannitol was tested for their morphological form using SEM. Samples were prepared by coating with platinum for 130 seconds. Furthermore, the sample was observed using SEM with several magnifications to obtain a suitable morphological image (Kuncahyo et al., 2019).
Surface area analyzer
The spray drying of a mixture of D-mannitol and ammonium bicarbonate and pure D-mannitol was tested for surface area, pore size, and pore volume using a Quadrasorb Evo SAA (Boynton Beach, FL). The sample was vacuumed for 16 hours before the measurement process. The measurements used nitrogen gas sorption and desorption techniques. The specific area, pore size, and pore volume were based on the Brunauer-Emmet-Teller (BET) analysis (Kuncahyo et al., 2019).
Preparation of S-SNEDDS using spray drying technique
The spray drying technique was for the preparation of S-SNEDDS. Solidification of S-SNEDDS was done by dissolving the mesoporous mannitol as a hydrophilic carrier (5 g) in 200 ml of distilled water using a stirrer to produce a clear solution. Next, L-SNEDDS isolated piperine (5 ml) was added to the hydrophilic solution and stirred using a stirrer at 100 rpm to achieve a homogeneous solution. The solution was dried with a Buchi B-290 spray dryer at 120°C inlet and 80°C outlet temperatures, an aspirator of 90%, a pump on a scale of 25%, and a pressure of 50 mBar (Kuncahyo et al., 2019).
Characterization of S-SNEDDS
Measurement of globule size
The formation of S-SNEDDS was determined by measuring the globule size. A total of 50.0 mg of S-SNEDDS was weighted and added to 100.0 ml of distilled water at 37°C ± 2°C and rotated at 100 rpm until nanoemulsion disperse was obtained. Afterward, the globule size was measured using a Malvern Zetasizer globule size measuring device SZ-100 (Malvern Instruments, UK).
Fourier transform infrared
Functional group analysis of isolated piperine samples, mesoporous mannitol, and S-SNEDDS used attenuated total reflectance-FTIR with ZnSe crystals equipped with a deuterated triGlycine sulfate detector. Samples were read as absorbance at the wavelength of 400–4,000 cm−1 and the resolution of 8 with 32 iterations (Kuncahyo et al., 2019).
Scanning electron microscopy
Morphology was conducted on isolated piperine, mesoporous mannitol, and S-SNEDDS samples. Samples were prepared by coating with platinum for 130 seconds. Samples were observed using SEM at an acceleration voltage of 5 kV with several magnifications to obtain a suitable morphological image of mesoporous mannitol (Kuncahyo et al., 2019).
Differential scanning calorimetry
Thermal analysis of isolated piperine samples, mesoporous mannitol, and S-SNEDDS were done using DSC. A total of 5.0 mg of the specimen was placed in an aluminum pan and heated at a rate of 10°C/minute with a nitrogen rate of 30 ml/minute and measured at a temperature of 25°C–30°C. The temperature and enthalpy of the DSC tool were calibrated with iodine standard (Kuncahyo et al., 2019).
Powder X-ray diffraction (PXRD)
The crystallinity pattern characterization used PXRD with Cu-kα radiation (wavelength of 1.5406 Å and a voltage of 40 kV). The samples analyzed included isolated piperine, mesoporous mannitol, and S-SNEDDS. Readings were at a rate of 3°C/minute in the range of 2? (diffraction angle) of 3°C–40°C with a step size of 0.02° (Kuncahyo et al., 2019).
Test of the content of S-SNEDDS isolated piperine
100.0 mg of S-SNEDDS was dissolved in 10.0 ml of distilled water using a stirrer until it became clear. Observation of isolated piperine content in S-SNEDDS was determined using HPLC with a previously validated method.
In vitro dissolution study
The in vitro dissolution test of isolated piperine, L-SNEDDS isolated piperine, and S-SNEDDS isolated piperine (containing 50.0 mg of isolated piperine) used simulated gastric fluids (SGF) at pH 1.2 and simulated intestinal fluids (SIF) at pH 6.8 at 37°C ± 0.5°C of 900 ml. The dissolution tool used USP type II (paddle type) at 50 rpm. Isolated piperine, L-SNEDDS isolated piperine, and S-SNEDDS isolated piperine were put into a hard-shell capsule size of 0. After that, a 5 ml sample was taken using a volume pipette at intervals of 5, 10, 20, 30, 45, 60, 90, and 120 minutes. At each interval time, the medium was replaced with the same volume of fresh medium to preserve the sample volume. The specimen obtained was filtered using a membrane filter; then, the levels were determined using HPLC with a validated method after filtering through a 0.45 μm Millipore filter. Data are presented as mean ± standard deviation (SD) of triplicate samples. To assess the comparative rate of increased dissolution of the L-SNEDDS and S-SNEDDS formulations, the mean dissolution time or mean dissolution time (MDT) (minute) and dissolution efficiency (DE) were calculated with Microsoft Excel version 10.0 (Microsoft, USA).
Data analysis and statistics
Each experiment was carried out with three replications. The results were expressed as mean ± SD. The computerized data were described statistically using Microsoft Excel v.10.0 (Microsoft, USA). The results of the L-SNEDDS and S-SNEDDS particle sizes were analyzed using a paired t-test, Statistical Package for the Social Sciences (SPSS) v.24 software (IBM, AS).
RESULTS AND DISCUSSION
Screening of components
The solubility of isolated piperine in various excipients includes oils (VCO, olive oil, canola oil, and Miglyol 812 N), surfactants (Cremophor RH40 and Tween 80), and cosurfactants (PEG400 and propylene glycol), as shown in Figure 1. The components used in the SNEDDS formulation must be capable of maximally dissolving the isolated piperine and have a larger self-emulsification area in the ternary phase diagram (Marasini et al., 2012). The vehicle was selected on considerations of safety, compatibility, and dissolving capacity. Among the tested oils, Miglyol 812 N showed the highest solubility of isolated piperine (3.93 ± 0.37 mg/ml) and was selected as the oil phase for further investigation. Isolated piperine dissolved highly in semisynthetic oil derivatives compared to natural oils. It may be associated with the polarity of poorly soluble drugs that prefer to dissolve in oil volume with small/medium molar, such as medium-chain monoglycerides, diglycerides, or triglycerides (Lawrence and Rees, 2000). Miglyol 812 N was a medium-chain triglyceride (MCT) oil, a triglyceride ester from saturated coconut/palm kernel oil derived from caprylic, capric, and glycerol-derived fatty acids. MCT oil has a greater dissolving capacity than long-chain triglyceride (LCT), does not undergo oxidation, and can increase the ability to emulsify (Pouton and Porter, 2008).
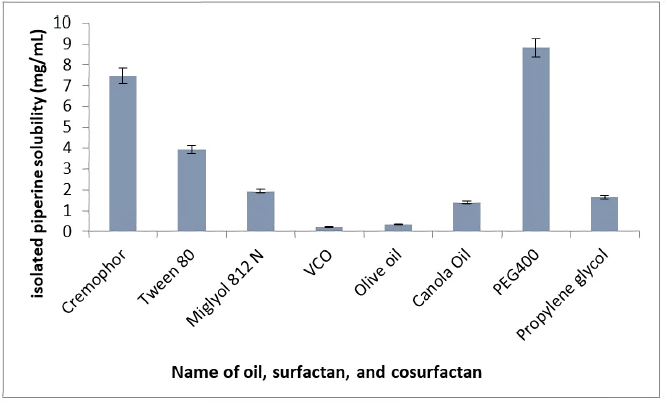 | Figure 1. Solubility study of isolated piperine. Each value represents the means ± SD (n = 3). [Click here to view] |
Screening of components in the manufacture of L-SNEDDS was not only based on the highest solubility of isolated piperine in oil but also with surfactants and cosurfactants. Among the tested surfactants and cosurfactants, Cremophor RH40 (7.48 ± 0.09 mg/ml) and PEG400 (8.82 ± 0.43 mg/ml) showed the highest solubility of isolated piperine. Therefore, both were selected for further research. Cremophor was a nonionic surfactant that was generally considered the safest to take orally and most widely used for lipid-based delivery systems (Pouton and Porter, 2008). Based on the results of previous research, the usage of Cremophor with MCT oil dissolved more active substances than LCT oil (Thomas et al., 2012). PEG400 was the most commonly used cosolvent for the manufacture of L-SNEDDS. Cosolvents serve to help increase the solubility capacity of drugs that dissolve in cosolvents.
The use of these three components in the L-SNEDDS isolated piperine formulation for oral administrations was safe. Moreover, the surfactant Cremophor RH40 (polyoxyl 40 hydrogenated castor oil) has been successfully used to formulate SNEDDS products on the market, namely, the Neural® product (Novartis). It contains cyclosporine A and is given 2–4 times a day, so a patient consumes approximately 2–3 g daily (Badran et al., 2014; Krsti? et al., 2018; Mohd et al., 2015).
Construction of pseudoternary phase diagrams
The relationship between the phase behavior and the L-SNEDDS mixture’s composition was studied using a pseudoternary phase diagram. A pseudoternary phase diagram was constructed without the active substance to identify the self-emulsification region and optimize the percentages of oil, surfactant, and cosurfactant for the L-SNEDDS formulation. The pseudoternary phase diagram of the system containing Miglyol 812 N as oil, Cremophor RH40 as a surfactant, and PEG400 as a cosurfactant is shown in Figure 2. The results show that Miglyol 812N, PEG400, and Cremophor RH40 have high nanoemulsion region capacity. Large emulsion regions were formed with the proportion of Miglyol 812 N in the range of 10%–65%, PEG400 in the span of 5%–65%, and Cremophor RH40 in the span of 5%–78%. The ternary phase diagram was constructed from the three constituent components of L-SNEDDS. The nanoemulsion region was determined based on the emulsion formed after adding a certain amount of water to the mixture of the three components in the ternary phase diagram. Based on the range of emulsion formation in the ternary phase diagram, the mixture of oil, surfactant, and cosurfactant was further optimized using the D-Optimal design, Design-Expert software (DX v.10, State Ease Inc., MN). Based on the optimization results published in Kusumorini et al., (2021a), the ratio of oil (Miglyol 812N), cosurfactant (PEG 400), and surfactant (Cremophor RH40) chosen was 2.25:2.60:5.15. Therefore, it was used as the constituent of L-SNEDDS isolated piperine.
Determination of the amount of isolated piperine loaded in L-SNEDDS formulation
Determining the maximum amount of isolated piperine contained in L-SNEDDS is needed to determine the weight of isolated piperine used for the L-SNEDDS formulation. The results of the study using isolated piperine weight series 50.0, 100.0, 200.0, and 250.0 mg were 5.03 ± 0.01, 9.48 ± 0.27, 19.66 ± 0.35, and 19.62 mg/ml, each. Isolated piperine with a 20 mg/ml weight was chosen as the weight of the active substance used for the L-SNEDDS formulation.
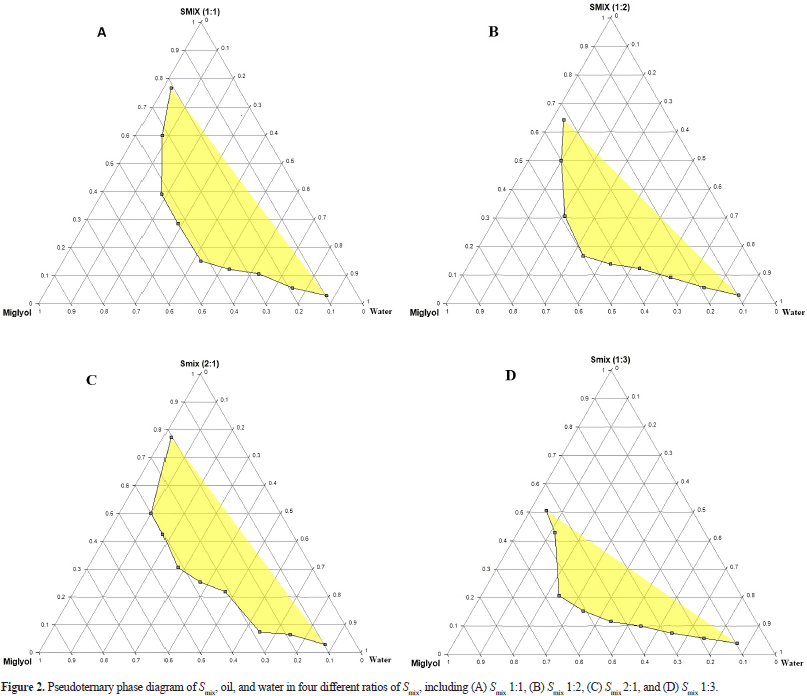 | Figure 2. Pseudoternary phase diagram of Smix, oil, and water in four different ratios of Smix, including (A) Smix 1:1, (B) Smix 1:2, (C) Smix 2:1, and (D) Smix 1:3. [Click here to view] |
Formulation and characterization of L-SNEDDS isolated piperine
L-SNEDDS isolated piperine formula in the ratio of oil (Miglyol 812N), cosurfactant (PEG 400), and surfactant (Cremophor RH40), 2.25:2.60:5.15, produced a particle size of 33.35 ± 1.97 nm, zeta potential of −22.87 ± 3.31 mV, percent transmittance of 96.34% ± 0.33%, and emulsification time of 14.91 ± 0.10 seconds. In the SNEDDS formulation, droplet size was the most crucial factor affecting the determination of the rate of drug release and absorption (Parmar et al., 2011), followed by the zeta potential value, which played a role in the stability of the colloid disperse. The increase in electrostatic repulsion forces between globules could prevent coalescence/fusion between droplets, while the decrease in electrostatic repulsion could cause phase separation (Khan et al., 2015). The zeta potential value limit for SNEDDS ranged from ±30 mV (Cherniakov et al., 2015).
The results of particle size and zeta potential showed that the characteristics of L-SNEDDS in this study met the requirements for the characteristics of nanoemulsions (<200 nm). The reduction of particle size in the L-SNEDDS preparation allowed isolated piperine to be easily permeated. In addition, negatively charged L-SNEDDS was also permeated faster in mucus than positively charged L-SNEDDS. Thus, both characteristics enhance biological transport through mucus as a barrier to oral absorption (Griesser et al., 2018).
Mesoporous mannitol
In the solidification of L-SNEDDS isolated piperine, solid excipients played a significant role in the L-SNEDDS drug’s successful loading. The carrier with high porosity and surface area allows numerous L-SNEDDS to be contained in the pore. Mesoporous silica is an attractive excipient for L-SNEDDS solidification because of its high surface area and large pore volume; however, mesoporous silica is expensive and has low water solubility (Millqvist-Fureby et al., 2014). Mannitol is a porous adsorbent with high water solubility and can potentially be used as a carrier for controlled drug release in drugs with low water solubility. However, mannitol has a much lower specific surface area and a smaller pore volume than mesoporous silica. The solidification process produces highly porous mannitol with a high surface area of mesoporous mannitol by mixing D-mannitol with ammonium bicarbonate as a sublimation agent using a spray drying process to maximize D-mannitol porosity. Ammonium bicarbonate, which sublimes above approximately 36°C by breaking down into ammonia, carbon dioxide, and water, was used to induce pore formation during the drying process (Song et al., 2018).
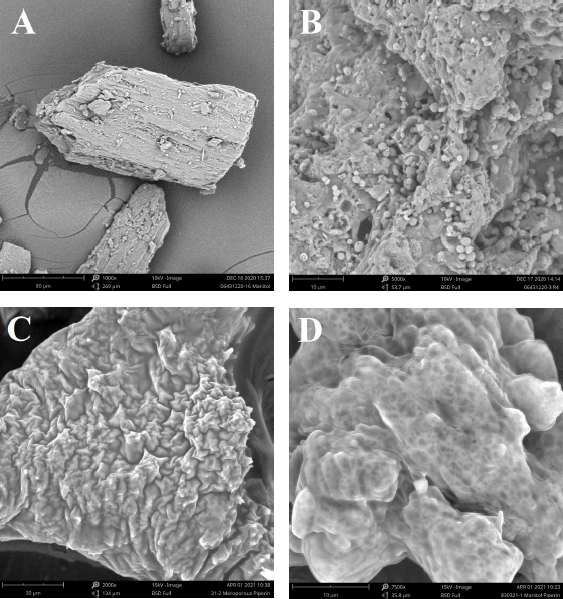 | Figure 3. Scanning electron microscopy imaging of (A) D-mannitol, (B) mesoporous mannitol, (C) S-SNEDDS isolated piperine, and (D) mannitol loaded L-SNEDDS isolated piperine. [Click here to view] |
Scanning electron microscopy
SEM images of isolated piperine, D-mannitol, mesoporous mannitol, and S-SNEDDS can be seen in Figure 3. The surface morphology of isolated piperine and D-mannitol powder was most likely a characteristic description of the crystalline particles, irregular in shape with an uneven surface. The pore surface of the mixture of D-mannitol with ammonium bicarbonate resulted in the number of open pores compared to without the addition. Sublimation and decomposition of ammonium bicarbonate during the spray drying process resulted in different pore sizes. The larger pore size was due to the space filled by ammonium bicarbonate before sublimation and ultimately changed the pore shape of D-mannitol (Song et al., 2018).
Surface area analyzer
As shown in Table 1, the specific surface area of mesoporous mannitol (4.192 m2/g) was two times greater than that of D-mannitol (2.096 m2/g). The SEM test results in morphological studies show that mesoporous mannitol has a larger pore volume than D-mannitol. The larger specific surface area of mesoporous mannitol was due to a change in pore size. It was larger because the space of D-mannitol was filled with ammonium bicarbonate before the sublimation process (Song et al., 2018). The larger the pore surface area of the carrier, the greater the number of L-SNEDDS contained in the pore carrier (Chavan et al., 2015; Peng et al., 2017). Based on these considerations, mesoporous mannitol adsorbent was used for the solidification process of L-SNEDDS isolated piperine in the hope that mesoporous mannitol was able to contain large amounts of L-SNEDDS isolated piperine.
 | Table 1. Surface area analysis of D-mannitol and mesoporous mannitol by the BET method. [Click here to view] |
 | Table 2. The result of globule size, zeta potential, and statistical analysis S-SNEDDS in water, SGF pH 1.2, and SIF pH 6.8 compared with L-SNEDDS (n = 3). [Click here to view] |
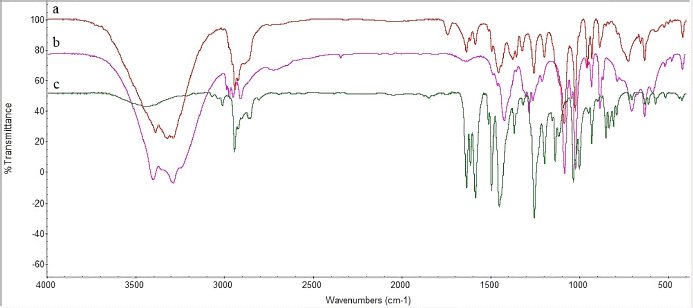 | Figure 4. FTIR analysis of (A) isolated piperine, (B) mesoporous mannitol, and (C) S-SNEDDS isolated piperine. [Click here to view] |
Previous studies have reported the use of ammonium bicarbonate to increase the surface area of D-mannitol using the spray drying technique (Kuncahyo et al., 2019; Peng et al., 2017). Ammonium bicarbonate decomposes during the spray drying process, so it can act as a pore-forming agent. Peng et al. (2017) proved that the results of spray drying have no N atoms detected. Two factors must be considered in making porous mannitol: the outlet temperature during the spray drying process and the ratio of the mixture of D-mannitol and ammonium bicarbonate. The higher the outlet temperature used, the more the porous mannitol produced cracks, and the less ammonium bicarbonate used, the smaller the pore size formed. In addition, the more ammonium bicarbonate is used, the more the small pore size is produced (Peng et al., 2017).
Formulation and characterization of S-SNEDDS
Reconstitution studies
The reconstitution rate is the time required for the S-SNEDDS to be uniformly dispersed in the aqueous medium. From the research, the reconstitution rate of S-SNEDDS isolated piperine was 40.03 ± 3.13 seconds. The S-SNEDDS isolated piperine reconstitution studies were dispersed in simulated gastric and intestinal fluids. Since S-SNEDDS was intended for oral drugs administration, it provided an overview of the particle size and zeta potential formed in gastric and intestinal fluids. The result analysis using paired t-test on SPSS v.24 software (IBM, USA) shows that the S-SNEDDS form did not change the particle size of L-SNEDDS in water, SGF pH 1.2, and SIF pH 6.8 (Table 2). Conversion of L-SNEDDS to solid dosage form did not significantly change the particle size of L-SNEDDS.
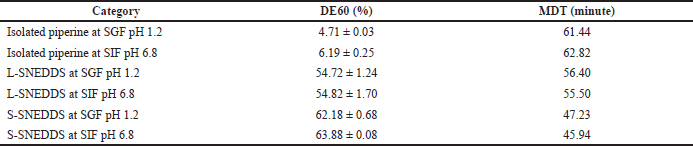 | Table 3. Dissolution efficiency 60 minutes (DE60) and MDT of isolated piperine, L-SNEDDS, and S-SNEDDS at SGF pH 1.2 and SIF pH 6.8. [Click here to view] |
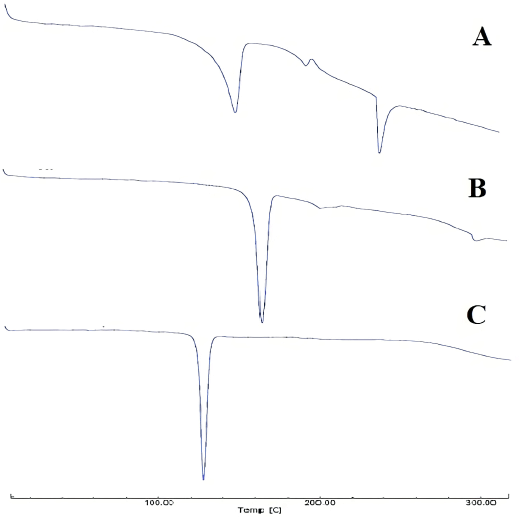 | Figure 5. Thermal DSC profiles of (A) S-SNEDDS isolated piperine, (B) mesoporous mannitol, and (C) isolated piperine. [Click here to view] |
Fourier transform infrared
The FTIR spectrum serves to explain the interaction between L-SNEDDS and porous carriers. The vibrational spectra of isolated piperine and S-SNEDDS can be seen in Figure 4. The FTIR spectrum of the mesoporous mannitol shows that the host (Fig. 4) was determined by the specific peak vibrations, namely, the OH hydrogen bonds and the OH vibrations occurring at wavenumbers of 3,286 and 3,463 cm−1, respectively. The peaks at 1,022 and 1,084 cm−1 vibrated from the C–O stretching. The alkyl vibrations of the mesoporous mannitol were increased from 1,450–1,300 cm−1. In addition, the peak vibrations of isolated piperine (Fig. 4) were determined by the presence of the functional group’s CH, C–H2, O=CN, C=C, =COC, and COC at the peaks of 2,800–3,000, 1,635, 1,495–1,589, 1,030–1,257, and 1,134 cm−1, respectively.
The vibration spectrum showed that S-SNEDDS had a similar pattern with the spectrum pattern of isolated piperine and mesoporous mannitol, although the peak vibration of mesoporous mannitol was the most dominant. Thus, the specific peak vibration of the isolated piperine was not well observed. The FTIR spectrum results revealed no observed vibrational interactions in the incorporation of L-SNEDDS isolated piperine with pore carriers. Further characterization was carried out to see the interaction between the two.
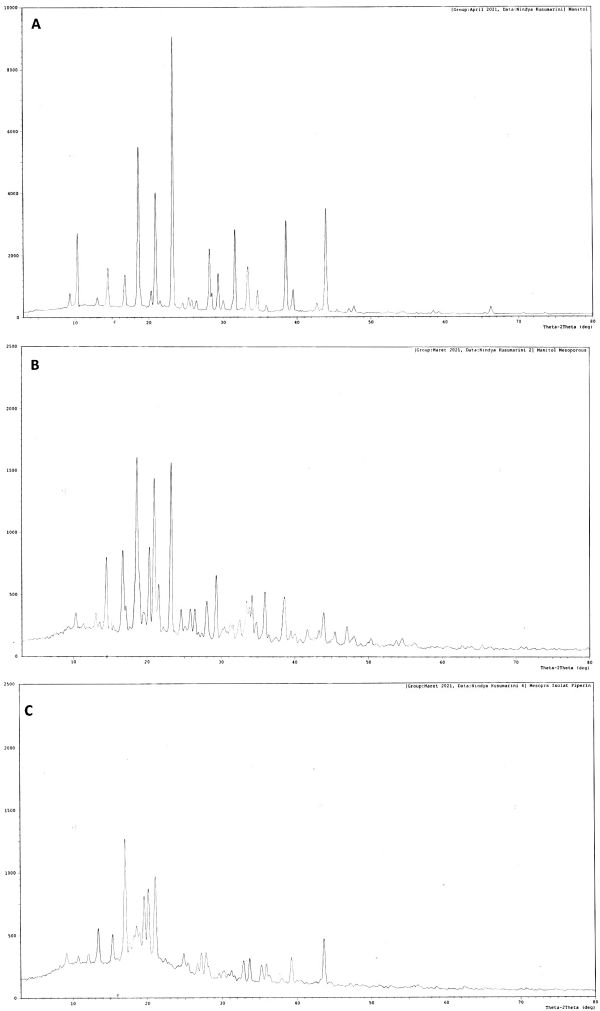 | Figure 6. XRD diffraction patterns of (A) D-mannitol, (B) mesoporous mannitol, and (C) S-SNEDDS isolated piperine. [Click here to view] |
Scanning electron microscopy
Figure 3 presents D-mannitol, mesoporous mannitol, L-SNEDDS isolated piperine, and S-SNEDDS to understand their morphological characteristics. The morphology of mesoporous mannitol shows a rough, irregular surface shape, and it has holes in the carrier core space. It is different from the form of the D-mannitol structure that is irregular with a flat crystal surface. The morphology of S-SNEDDS shows that L-SNEDDS was contained in the empty space of the mesoporous mannitol pore. It produced larger particles. Meanwhile, the morphological form of directly mixed L-SNEDDS with D-mannitol resulted in small irregular particles. So that fewer L-SNEDDS was contained in the pores.
Differential scanning calorimetry
The thermal DSC profiles of isolated piperine, mesoporous mannitol, and S-SNEDDS can be seen in Figure 5. The thermal profile of the S-SNEDDS samples showed a shift in the endothermic peak of mesoporous mannitol at a temperature of 166.62°C, the melting point of mannitol, and shifted to a temperature of 154.07°C. Meanwhile, isolated piperine that melted at a temperature of 126.37°C–139.27°C did not appear in S-SNEDDS. It indicated a change in the physical state to nonamorphous crystals or irregular forms of molecular dispersions. In the thermal profile of the S-SNEDDS sample, there was a new endothermic peak with a melting point of 234.69°C, which was most likely a component of the SNEDDS constituent. The melting point of D-mannitol in this study was similar to previous studies on the melting point of D-mannitol, where the spray drying process did not change the melting point of D-mannitol (Burger et al., 2000; Hulse et al., 2009).
Powder X-ray diffraction
PXRD is the fastest and easiest method for determining basic information about the structure of a crystalline material (Brittain, 2001). Through X-ray diffraction, it could determine the presence of amorphous properties, polymorphisms, and crystal forms. Thus, this method was used to evaluate the polymorph content of each sample. The XRD reference pattern of each polymorph refers to research from Hulse et al. (2009). Based on his research, mannitol had three polymorphic forms, namely, α, β, and δ. The α-polymorph form had a peak position at 2θ 9.57 and 13.79, β-polymorph at 10.56, a relatively intense peak at 14.71, δ-polymorph with a very sharp peak at 9.74, and no peak until 14.66.
Based on the XRD results of the mesoporous mannitol, D-mannitol, and S-SNEDDS samples (Fig. 6), the D-mannitol sample contained two forms of polymorphism, namely, α-polymorph and β-polymorph. The spray drying process of a mixture of D-mannitol and ammonium bicarbonate of mesoporous mannitol samples produced one polymorph form, namely, β-polymorph, since the intensity was relatively reduced compared to the mannitol sample. In addition, it showed that samples containing α-polymorphs underwent a polymorphic transition into β-polymorphs during the spray drying process. The finding is similar to Hulse et al. (2009). Finally, the XRD results of S-SNEDDS showed one polymorphic form, namely, β-polymorph, with an intensity lower than mesoporous mannitol. The not-sharp intensity in the S-SNEDDS may be because the drug crystals were converted to an amorphous state.
Test the content of isolated piperine in S-SNEDDS
The isolated piperine content determination in S-SNEDDS was carried out by dissolving 100.0 mg of S-SNEDDS into 10.0 ml of distilled water. Furthermore, the solution obtained was determined using HPLC with a validated method, using the regression equation y = 148.11x + 8,208.5 (r = 0.9999). This resulted in 9.53 ± 0.54 mg/ml of isolated piperine content in 100.0 mg S-SNEDDS. Then, the results were used to equalize the dose of S-SNEDDS in the dissolution test.
DISSOLUTION STUDY
Dissolution profiles of piperine, L-SNEDDS isolated piperine, and S-SNEDDS isolated piperine (containing 50.0 mg isolated piperine) in SGF pH 1.2 and SIF pH 6.8 are shown in Figure 7, and the DE60 and MDT values are shown in Table 3. The dissolution profile shows that S-SNEDDS and L-SNEDDS at pH 1.2 and 6.8 dissolution were faster than pure isolated piperine at pH 1.2 and 6.8. S-SNEDDS at pH 1.2 and 6.8 showed faster drug release than L-SNEDDS at pH 1.2 and 6.8. It is possible that L-SNEDDS has emulsified longer when in the dissolution medium than S-SNEDDS. The presence of mesoporous mannitol with high porosity is possible to accelerate the dissolution and emulsification between the dissolution medium and the SNEDDS carrier (Inugala et al., 2015).
In the dissolution medium, S-SNEDDS disintegrates first and forms a fine emulsion in the GIT with mild agitation due to the normal mobility of the GIT. Once disintegrated, the fate of S-SNEDDS in the body will be the same as that of L-SNEDDS. The droplet size of the emulsion generated by S-SNEDDS should be equal to the resulting emulsion droplet size of L-SNEDDS. Based on the paired t-test analysis using SPSS v.24 software (IBM, USA), as shown in Table 2, the droplet size of S-SNEDDS emulsion was the same as L-SNEDDS at both pH 1.2 and 6.8. This shows that the change from liquid to solid did not change the droplet size of the emulsion, and the emulsion did not bind to the mesoporous mannitol pore carrier.
A previous study on the pharmacokinetics of SNEDDS preparations shows that linearly the SNEDDS preparations, which were able to increase dissolution in vitro when tested for pharmacokinetics, also showed an increase in peak maximum plasma concentrations (Cmax) and area under the curve (Shao et al., 2015). The pharmacokinetic profile of most SNEDDS preparations will have a double peak phenomenon. This is due to the presence of hepato-enteric circulation caused by bile elimination (Shao et al., 2015). Piperine is rapidly absorbed through the GIT and can be detected in plasma 15 minutes after administration to rats (Bajad et al., 2003). This is in line with Shao et al. (2015) on the study of intestinal absorption in situ. Suresh and Srinivasan (2010) stated that piperine does not undergo metabolic changes during the absorption process in the intestine but is metabolized rapidly in the liver and other tissues. In the body of piperine, piperine undergoes four metabolisms through glucuronidation and sulfation into pipecolinic acid, piperonyl alcohol, piperonal, and vanillic acid, which are found in the free form of urine at 0 to 97 hours (Bhat and Chandrasekhara, 1987).
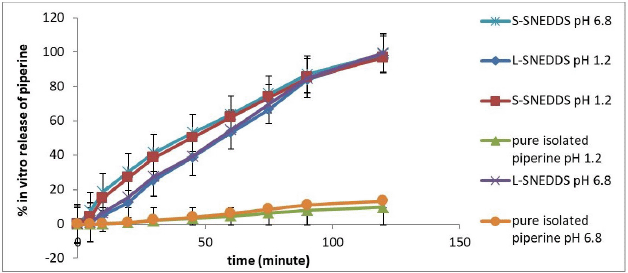 | Figure 7. Dissolution study of pure isolated piperine, L-SNEDDS isolated piperine, and S-SNEDDS isolated piperine at pH 1.2 and 6.8. [Click here to view] |
 | Figure 8. Fishbone diagram of the S-SNEDDS formulation of isolated piperine. [Click here to view] |
In addition, the change in the liquid dosage form of L-SNEDDS to S-SNEDDS shows that S-SNEDDS did not undergo physicochemical changes in the in vivo study and did not experience differences in pharmacokinetic parameters with L-SNEDDS (Garg et al., 2016). Even so, many studies of pharmacokinetic testing of S-SNEDDS preparations have resulted in differences in pharmacokinetic parameters with L-SNEDDS (Chatterjee et al., 2016). The S-SNEDDS form has been shown to produce maximum time (Tmax) and a longer half-life than liquid preparations (Cheng et al., 2015). Several reports describe the comparative evaluation of in vivo parameters between L-SNEDDS and S-SNEDDS, showing variations in pharmacokinetic parameters (Chatterjee et al., 2016).
According to our results, L-SNEDDS containing isolated piperine spray-dried with mesoporous mannitol produced the drug in an amorphous state in the S-SNEDDS formulation. In S-SNEDDS, L-SNEDDS isolated piperine is physically absorbed in the mesoporous mannitol pores and adsorbed onto the surface. When S-SNEDDS underwent reconstitution in water, L-SNEDDS isolated piperine came out of the solid carrier, made contact with water, and spontaneously formed nanoemulsions in water. S-SNEDDS is rapidly dissolved and dispersed in the dissolution medium. Thus, this S-SNEDDS succeeded in increasing the solubility of isolated piperine that is difficult to dissolve in water (Fig. 8).
In our perspective, obtaining high drug potency in S-SNEDDS can be increased by increasing the loading of L-SNEDDS isolated piperine into the porous carrier. However, it can affect physical properties such as nanodroplet formation, compatibility, and flowability. It is all due to the interaction of L-SNEDDS isolated piperine with porous carriers (Kim et al., 2014; Oh et al., 2011; Seo et al., 2015). Physical modification of D-mannitol has succeeded in increasing the surface area and pore characteristics. Thus, the higher the surface area, the more likely the L-SNEDDS is loaded into the porous carrier.
CONCLUSION
In conclusion, the solid formation of L-SNEDDS isolated piperine succeeded in increasing the dissolution of piperine and protecting piperine compounds from photosensitivity. The change in the form of L-SNEDDS to S-SNEDDS did not change the size of SNEDDS particles in SGF pH 1.2 and SIF pH 6.8. S-SNEDDS release of isolated piperine was slightly faster observed than L-SNEDDS at both pH 1.2 and 6.8. This may be the effect of increasing the specific surface area provided by the pore carrier. Mesoporous mannitol pore carriers have succeeded in forming good S-SNEDDS, which are amorphous and do not show interaction with L-SNEDDS constituent components as evidenced by compatibility assessment by FTIR, XRD, and DSC. The surface morphology of S-SNEDDS revealed that L-SNEDDS was successfully adsorbed on highly porous amorphous carriers. S-SNEDDS features a slightly rough surface indicating that the drug is loading efficiently. Thus, the mesoporous mannitol pore carrier can be used for the development of S-SNEDDS.
ACKNOWLEDGMENT
The authors would like to acknowledge the Ministry of Research, Technology, and Higher Education of the Republic of Indonesia for providing financial support during this research via the Master Degree to Doctoral Program for Excellent Graduate Student (PMDSU) scheme year 2018–2020.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
ETHICAL APPROVAL
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Ahmad J, Mir SR, Kohli K, Chuttani K, Mishra AK, Panda AK, Amin S. Solid-nanoemulsion preconcentrate for oral delivery of paclitaxel: formulation design, biodistribution, and γ scintigraphy imaging. BioMed Res Int, 2014; 2014:1–12. CrossRef
Ameeduzzafar EI, Alruwaili NK, Elkomy MH, Ahmad J, Afzal M, Ahmad N, Elmowafy M, Alharbi KS, Md SA. Development of novel dapagliflozin loaded solid self-nanoemulsifying oral delivery system: physiochemical characterization and in vivo antidiabetic activity. J Drug Deliv Sci Technol, 2019; 54:101279. CrossRef
Ashour EA, Majumdar S, Alsheteli A, Alshehri S, Alsulays B, Feng X, Gryczke A, Kolter K, Langley N, Repka MA. Hot melt extrusion as an approach to improve solubility, permeability and oral absorption of a psychoactive natural product, piperine. J Pharm Pharmacol, 2016; 68(8):989–98. CrossRef
Aukunuru JV, Bonepally CR. Optimization of encapsulation efficiency of piperine in soya- lecithin multilamellar vesicles. Int JChemTech Res, 2017; 7:723–9.
Badran MM, Taha EI, Tayel MM, Al-Suwayeh SA. Ultra-fine self nanoemulsifying drug delivery system for transdermal delivery of meloxicam: dependency on the type of surfactants. J Mol Liq, 2014; 190:16–22. CrossRef
Bajad S, Khajuria RK, Suri OP, Bedi KL. Characterisation of a new minor urinary metabolite of piperine, an omnipresent food component, by LC-MS/MS. J Sep Sci, 2003; 26(9–10):943–6. CrossRef
Bari A, Chella N, Sanka K, Shastri NR, Diwan PV. Improved anti-diabetic activity of glibenclamide using oral self nano emulsifying powder. J Microencapsulation, 2015; 32(1):54–60. CrossRef
Bhalekar MR, Madgulkar AR, Desale PS, Marium, G. Formulation of piperine solid lipid nanoparticles (SLN) for treatment of rheumatoid arthritis. Drug Dev Indus Pharm, 2017; 43(6):1003–10. CrossRef
Bhat BG, Chandrasekhara N. Metabolic disposition of piperine in the rat. Toxicology, 1987; 44(1):99–106. CrossRef
Brittain HG. X-ray diffraction III: pharmaceutical applications. Spectroscopy, 2001; 16(14):5.
Burger A, Henck JO, Hetz S, Rollinger JM, Weissnicht AA, Stöttner H. Energy/temperature diagram and compression behavior of the polymorphs of D-mannitol. J Pharm Sci, 2000; 89(4):457–68. CrossRef
Cavallari C, Rodriguez L, Albertini B, Passerini N, Rosetti F, Fini A. Thermal and fractal analysis of diclofenac/Gelucire 50/13 microparticles obtained by ultrasound-assisted atomization. J Pharm Sci, 2005; 94(5):1124–34. CrossRef
Chatterjee B, Hamed AS, Ahmed MDA, Mandal UK, Sengupta P. Controversies with self-emulsifying drug delivery system from pharmacokinetic point of view. Drug Deliv, 2016; 23(9):3639–52. CrossRef
Chavan RB, Modi SR, Bansal AK. Role of solid carriers in pharmaceutical performance of solid supersaturable SEDDS of celecoxib. Int J Pharm, 2015; 495(1):374–84. CrossRef
Cheng G, Hu R, Ye L. Preparation and in vitro/in vivo evaluation of puerarin solid self-microemulsifying drug delivery system by spherical crystallization technique. AAPS PharmSciTech, 2015; 1–11:1336–46. CrossRef
Cherniakov I, Domb AJ, Hoffman A. Self-nano-emulsifying drug delivery systems: an update of the biopharmaceutical aspects. Exp Opin Drug Deliv, 2015; 12(7):1121–33. CrossRef
Cole ET, Cadé D, Benameur H. Challenges and opportunities in the encapsulation of liquid and semi-solid formulations into capsules for oral administration. Adv Drug Deliv Rev, 2008; 60(6):747–56. CrossRef
Dutta S, Bhattacharjee P. Nanoliposomal encapsulates of piperine-rich black pepper extract obtained by enzyme-assisted supercritical carbon dioxide extraction. J Food Eng, 2017; 201:49–56. CrossRef
Ee GCL, Lim CM, Rahmani M, Shaari K, Bong CFJ. Pellitorine, a potential anti-cancer lead compound against HL6 and MCT-7 cell lines and microbial transformation of piperine from Piper Nigrum. Molecules (Basel, Switzerland), 2010; 15(4):2398–404. CrossRef
Etman SM, Elnaggar YSR, Abdelmonsif DA, Abdallah OY. Oral brain-targeted microemulsion for enhanced piperine delivery in Alzheimer’s disease therapy: in vitro appraisal, in vivo activity, and nanotoxicity. AAPS PharmSciTech, 2018; 19(8):3698–711. CrossRef
Ezawa T, Inoue Y, Murata I, Takao K, Sugita Y, Kanamoto I. Characterization of the dissolution behavior of piperine/cyclodextrins inclusion complexes. AAPS PharmSciTech, 2018; 19(2):923–33. CrossRef
Ezawa T, Inoue Y, Tunvichien S, Suzuki R, Kanamoto I. Changes in the physicochemical properties of piperine/β-cyclodextrin due to the formation of inclusion complexes. Int J Med Chem, 2016:1–9. CrossRef
Garg B, Katare OP, Beg S, Lohan S, Singh B. Systematic development of solid self-nanoemulsifying oily formulations (S-SNEOFs) for enhancing the oral bioavailability and intestinal lymphatic uptake of lopinavir. Colloids Surf B, 2016; 141:611–22. CrossRef
Griesser J, Hetényi G, Kadas H, Demarne F, Jannin V, Bernkop-Schnürch A. Self-emulsifying peptide drug delivery systems: how to make them highly mucus permeating. Int J Pharm, 2018; 538(1):159–66. CrossRef
Han Y, Chin TTM, Lim LY. In vitro and in vivo evaluation of the effects of piperine on P-gp function and expression. Toxicol Appl Pharmacol, 2008; 230(3):283–9. CrossRef
Hu X, Lin C, Chen D, Zhang J, Liu Z, Wu W, Song H. Sirolimus solid self-microemulsifying pellets: formulation development, characterization and bioavailability evaluation. Int J Pharm, 2012; 438(1–2):123–33. CrossRef
Hulse WL, Forbes RT, Bonner MC, Getrost M. The characterization and comparison of spray-dried mannitol samples. Drug Dev Indus Pharm, 2009; 35(6):712–8. CrossRef
Inugala S, Eedara BB, Sunkavalli S, Dhurke R, Kandadi P, Jukanti R, Bandari S. Solid self-nanoemulsifying drug delivery system (S-SNEDDS) of darunavir for improved dissolution and oral bioavailability: in vitro and in vivo evaluation. Eur J Pharm Sci, 2015; 74:1–10. CrossRef
Kang JH, Oh DH, Oh YK, Yong CS, Choi HG. Effects of solid carriers on the crystalline properties, dissolution and bioavailability of flurbiprofen in solid self-nanoemulsifying drug delivery system (solid SNEDDS). Eur J Pharm Biopharm, 2012; 80(2):289–97. CrossRef
Khajuria A, Thusu N, Zutshi U. Piperine modulates permeability characteristics of intestine by inducing alterations in membrane dynamics: influence on brush border membrane fluidity, ultrastructure and enzyme kinetics. Phytomedicine, 2002; 9(3):224–31. CrossRef
Khan AW, Kotta S, Ansari SH, Sharma RK, Ali J. Self-nanoemulsifying drug delivery system (SNEDDS) of the poorly water-soluble grapefruit flavonoid Naringenin: design, characterization, in vitro and in vivo evaluation. Drug Deliv, 2015; 22(4):552–61. CrossRef
Kim DW, Kwon MS, Yousaf AM, Balakrishnan P, Park JH, Kim DS, Lee BJ, Park YJ, Yong CS, Kim JO, Choi HG. Comparison of a solid SMEDDS and solid dispersion for enhanced stability and bioavailability of clopidogrel napadisilate. Carbohydr Polym, 2014; 114:365–74. CrossRef
Kim KS, Yang ES, Kim DS, Kim DW, Yoo HH, Yong CS, Youn YS, Oh KT, Jee JP, Kim JO, Jin SG, Choi HG. A novel solid self-nanoemulsifying drug delivery system (S-SNEDDS) for improved stability and oral bioavailability of an oily drug, 1-palmitoyl-2-linoleoyl-3-acetyl-rac-glycerol. Drug Deliv, 2017; 24(1):1018–25. CrossRef
Kotte SCB, Dubey PK, Murali PM. Identification and characterization of stress degradation products of piperine and profiling of a black pepper (Piper nigrum L.) extract using LC/Q-TOF-dual ESI-MS. Anal Methods, 2014; 6(19):8022–9. CrossRef
Koul IB, Kapil A. Evaluation of the liver protective potential of piperine, an active principle of black and long peppers. Planta Med, 1993; 59(5):413–7. CrossRef
Krsti? M, Medarevi? ?, ?uriš J, Ibri? S. Chapter 12—Self-nanoemulsifying drug delivery systems (SNEDDS) and self-microemulsifying drug delivery systems (SMEDDS) as lipid nanocarriers for improving dissolution rate and bioavailability of poorly soluble drugs. In: Grumezescu AM (ed.). Lipid nanocarriers for drug targeting. William Andrew Publishing, New York, NY, pp 473–508, . CrossRef
Kuncahyo I, Choiri S, Fudholi A, Rohman A, Martien R. Understanding the effect of lipid formulation loading and ethanol as a diluent on solidification of pitavastatin super-saturable SNEDDS using factorial design approach. Res Pharm Sci, 2019; 14(5):378–90. CrossRef
Kusumorini N, Nugroho AK, Pramono S, Martien R. Application of D-Optimal design for the optimization of isolated piperine from Piper nigrum L-loaded self-nanoemulsifying drug delivery systems (SNEDDS). Acta Poloniae Pharm Drug Res, 2021a; 78(3):415–23. CrossRef
Kusumorini N, Nugroho AK, Pramono S, Martien R. Development of new isolation and quantification method of Piperine from white pepper seeds (Piper nigrum L) using a validated HPLC. Indones J Pharm, 2021b; 32(2):158–65. CrossRef
Lawrence MJ, Rees GD. Microemulsion-based media as novel drug delivery systems. Adv Drug Deliv Rev, 2000; 45(1):89–121. CrossRef
Li S, Wang C, Wang M, Li W, Matsumoto K, Tang Y. Antidepressant like effects of piperine in chronic mild stress treated mice and its possible mechanisms. Life Sci, 2007; 80(15):1373–81. CrossRef
Marasini N, Yan YD, Poudel BK, Choi HG, Yong CS, Kim JO. Development and optimization of self-nanoemulsifying drug delivery system with enhanced bioavailability by Box-Behnken design and desirability function. J Pharm Sci, 2012; 101(12):4584–96. CrossRef
Millqvist-Fureby A, Larsson A, Jarn M, Speets E, Ahniyaz A, Macakova L. Chapter 15. Mesoporous solid carrier particles in controlled delivery and releas. In: Gaonkar AG, Vasisht N, Khare AR, Sobel R (eds.). Microencapsulation in the food industry, Academic Press, San Diego, CA, pp 299–319, 2014 CrossRef
Mohd AB, Sanka K, Bandi S, Diwan PV, Shastri N. Solid self-nanoemulsifying drug delivery system (S-SNEDDS) for oral delivery of glimepiride: development and antidiabetic activity in albino rabbits. Drug Deliv, 2015; 22(4):499–508. CrossRef
Mujumdar AM, Dhuley JN, Deshmukh VK, Raman PH, Naik SR. Anti-inflammatory activity of piperine. Jpn J Med Sci Biol, 1990; 43(3):95–100. CrossRef
Mustapha O, Kim KS, Shafique S, Kim DS, Jin SG, Seo YG, Youn YS, Oh KT, Lee BJ, Park YJ, Yong CS, Kim JO, Choi HG. Development of novel cilostazol-loaded solid SNEDDS using a SPG membrane emulsification technique: physicochemical characterization and in vivo evaluation. Colloids Surf B, 2017; 150:216–22. CrossRef
Nazzal S, Khan MA. Controlled release of a self-emulsifying formulation from a tablet dosage form: stability assessment and optimization of some processing parameters. Int J Pharm, 2006; 315(1–2):110–21. CrossRef
Oh DH, Kang JH, Kim DW, Lee BJ, Kim, JO, Yong CS, Choi HG. Comparison of solid self-microemulsifying drug delivery system (solid SMEDDS) prepared with hydrophilic and hydrophobic solid carrier. Int J Pharm, 2011; 420(2):412–8. CrossRef
Pachauri M, Gupta ED, Ghosh PC. Piperine loaded PEG-PLGA nanoparticles: preparation, characterization and targeted delivery for adjuvant breast cancer chemotherapy. J Drug Deliv Sci Technol, 2015; 29:269–82. CrossRef
Parmar N, Singla N, Amin S, Kohli K. Study of cosurfactant effect on nanoemulsifying area and development of lercanidipine loaded (SNEDDS) self nanoemulsifying drug delivery system. Colloids Surf B, 2011; 86(2):327–38. CrossRef
Passerini N, Albertini B, Perissutti B, Rodriguez L. Evaluation of melt granulation and ultrasonic spray congealing as techniques to enhance the dissolution of praziquantel. Int J Pharm, 2006; 318(1–2):92–102. CrossRef
Peng T, Zhang X, Huang Y, Zhao Z, Liao Q, Xu J, Huang Z, Zhang J, Wu C, Pan X, Wu C. Nanoporous mannitol carrier prepared by non-organic solvent spray drying technique to enhance the aerosolization performance for dry powder inhalation. Sci Rep, 2017; 7(1):46517. CrossRef
Pentak D. In vitro spectroscopic study of piperine-encapsulated nanosize liposomes. Eur Biophys J, 2016; 45(2):175–86. CrossRef
Pouton CW, Porter CJH. Formulation of lipid-based delivery systems for oral administration: materials, methods and strategies. Adv Drug Deliv Rev, 2008; 60(6):625–37. CrossRef
Priprem A, Chonpathompikunlert P, Sutthiparinyanont S, Wattanathorn J. Antidepressant and cognitive activities of intranasal piperine-encapsulated liposomes. Adv Biosci Biotechnol, 2011; 02(02):108–16. CrossRef
Rashid R, Kim DW, Din FU, Mustapha O, Yousaf AM, Park JH, Kim JO, Yong CS, Choi, HG. Effect of hydroxypropylcellulose and Tween 80 on physicochemical properties and bioavailability of ezetimibe-loaded solid dispersion. Carbohydr Poly, 2015; 130:26–31. CrossRef
Salem HF, Kharshoum RM, Sayed OM, Abdel HLF. Formulation development of self-nanoemulsifying drug delivery system of celecoxib for the management of oral cavity inflammation. J Liposome Res, 2019; 29(2):195–205. CrossRef
Seo YG, Kim DH, Ramasamy T, Kim JH, Marasini N, Oh YK, Kim DW, Kim JK, Yong CS, Kim JO, Choi HG. Development of docetaxel-loaded solid self-nanoemulsifying drug delivery system (SNEDDS) for enhanced chemotherapeutic effect. Int J Pharm, 2013; 452(1–2):412–20. CrossRef
Seo YG, Kim DW, Yousaf AM, Park JH, Chang PS, Baek HH, Lim SJ, Kim JO, Yong CS, Choi HG. Solid self-nanoemulsifying drug delivery system (SNEDDS) for enhanced oral bioavailability of poorly water-soluble tacrolimus: physicochemical characterisation and pharmacokinetics. J Microencapsulation, 2015; 32(5):503–10. CrossRef
Shao B, Cui C, Ji H, Tang J, Wang Z, Liu H, Qin M, L X, Wu L. Enhanced oral bioavailability of piperine by self-emulsifying drug delivery systems: in vitro, in vivo and in situ intestinal permeability studies. Drug Deliv, 2015; 22(6):740–7. CrossRef
Song H, Moon C, Lee BJ, Oh E. Mesoporous pravastatin solid dispersion granules incorporable into orally disintegrating tablets. J Pharm Sci, 2018; 107(7):1886–95. CrossRef
Srinivasan K. Black pepper and its pungent principle-piperine: a review of diverse physiological effects. Crit Rev Food Sci Nutr, 2007; 47(8):735–48. CrossRef
Suresh D, Srinivasan K. Tissue distribution & elimination of capsaicin, piperine & curcumin following oral intake in rats. Indian J Med Res, 2010; 131:682–91.
Tang B, Cheng G, Gu JC, Xu CH. Development of solid self-emulsifying drug delivery systems: preparation techniques and dosage forms. Drug Discovery Today, 2008; 13(13–14):606–12. CrossRef
Thenmozhi K, Yoo YJ. Enhanced solubility of piperine using hydrophilic carrier-based potent solid dispersion systems. Drug Dev Indus Pharm, 2017; 43(9):1501–9. CrossRef
Thomas N, Müllertz A, Graf A, Rades T. Influence of lipid composition and drug load on the In Vitro performance of self-nanoemulsifying drug delivery systems. J Pharm Sci, 2012; 101(5):1721–31. CrossRef
Vu GTT, Phan NT, Nguyen HT, Nguyen HC, Tran T, Pham TB, Nguyen LT, Nguyen HD. Application of the artificial neural network to optimize the formulation of self-nanoemulsifying drug delivery system containing rosuvastatin. J Appl Pharm Sci, 2020; 10(09):001–11.
Wang L, Dong J, Chen J, Eastoe J, Li X. Design and optimization of a new self-nanoemulsifying drug delivery system. J Colloid Interface Sc, 2009; 330(2):443–8. CrossRef
Wu Z, Xia X, Huang X. Determination of equilibrium solubility and apparent oil/water partition coefficient of piperine. J Jinan Univ, 2012; 33:473–6.