INTRODUCTION
Hospital-acquired infection (HAI), also called nosocomial infection, is one of the most critical healthcare challenges, particularly in intensive care units (ICUs) (Ababneh, et al., 2022; Hu et al., 2015). It can lead to higher patient morbidity, mortality, and healthcare costs and prolong hospital stay (Ababneh et al., 2022). Contaminated environmental surfaces play an important role in the transmission of HAIs, specifically when patients come into direct contact with them or when they are handled by healthcare professionals’ hands or gloves (Boyce, 2007). Studies have shown the significance of contaminated environmental surfaces in HAI transmission as they found a reduction in the percentage of illnesses when suitable cleaning and disinfection measures for these surfaces were used (Quinn et al., 2015; Rutala et al., 2012; Rutala and Weber, 2016).
Moreover, previous studies reported that patients who were admitted to the rooms that were occupied by previously contaminated patients with vancomycin-resistant Enterococcus (VRE), methicillin-resistant Staphylococcus aureus (MRSA), Clostridium difficile, or Acinetobacter baumannii infection had a higher risk of getting these pathogens from contaminated environmental surfaces of up to threefold (Carling and Bartley, 2010; Otter et al., 2013; Rutala and Weber, 2016).
The severity and volatility of clinical conditions for patients in ICUs expose them to many invasive medical procedures such as intubation, mechanical ventilation, intravenous lines, central venous lines, and urine catheterization, which can all contribute to this high percentage of HAIs (Brusselaers et al., 2011; de Oliveira and Damasceno, 2010). In addition, ICUs are considered the most crowded areas in the hospitals with equipment and apparatuses for monitoring and support of critically ill patients, which leads to difficulties in their cleaning and disinfection (de Oliveira and Damasceno, 2010; Russotto et al., 2015). Panhotra et al., (2005) reported that the records in ICUs were more infected with pathogenic microorganisms than those in surgical units in research (85.2% vs. 25%, respectively) (Panhotra et al., 2005).
Environmental surfaces can be classified into two groups: those that are frequently touched by hands (e.g., beds, bed rails, and doorknob) and those that are rarely touched by hands (e.g., floors and walls) (Saka et al., 2016). The frequently touched surfaces and those closer to the infected or colonized patients have more infective pathogens, such as MRSA and VRE, which have been identified as the most pathogens found on contaminated surfaces in ICUs (de Oliveira and Damasceno, 2010; Quinn et al., 2015).
Infective bacteria, particularly those that are multiresistant to antibiotics, are more likely to infect the environment’s surfaces (Montero et al., 2015). Pathogens such as MRSA, VRE, and C. difficile can stay alive for long periods on hospital surfaces and medical devices (i.e., hours, days, or months) (Kramer et al., 2006; Rutala and Weber, 2013). Consequently, regular monitoring of these surfaces could help in increasing the infection control team and healthcare leadership awareness to design policy and intervention measures that may improve healthcare workers’ (HCWs) practices, disinfection procedures, and HAI prevention strategies (Tajeddin et al., 2016; Yusuf et al., 2017). Carling et al. (2010) emphasized the importance of environmental monitoring systems in giving quantitative and objective data to healthcare organizations to help them offer a clean and healthy environment for patients and healthcare providers (Carling and Bartley, 2010). In Yemen, no previous research has attempted to describe the prevalence of bacteria on surfaces in ICUs. As a result, this study was designed to investigate the frequency and antimicrobial pattern of the most common bacteria in ICUs at hospitals in Hodeida, Yemen.
METHODOLOGY
Design and setting
A cross-sectional descriptive study was conducted at six hospitals (Al-Thawrah, Al-Olafi, Al-Amal, Al-Aqsa, Al-Rasheed, and Al-Hodeida) in Hodeida City, Yemen, between March and June 2019. Hodeida City is considered one of the most populated cities after Sana’a, the capital of Yemen (Abdul-Ghani et al., 2021).
Sampling and data collection
Samples were collected from the environmental surfaces in ICU units of the six hospitals. The environmental surfaces include mechanical ventilators, oxygen masks, suctions, cardiac monitors, walls, bed rails, door handles, floors, medical tables, medical records, trolleys, electrocardiogram leads, and intravenous holders. The samples were taken with a sterile swab humidified in sterile normal saline and rolled along the surfaces several times before being deposited in a 1 ml tube.
After being properly capped and labeled, they were transported to the Microbiology Department at Al-Amal Hospital Laboratories for analysis. Blood and MacConkey agar were used to culture the swab samples, which were incubated aerobically at 35°C–37°C for 18–24 hours (Yusuf et al., 2017). Following aerobic incubation, Gram staining was used to assess the morphology of the bacteria, followed by routine biochemical assays to determine the bacteria’s species (Tajeddin et al., 2016).
Antimicrobial susceptibility testing
The Clinical and Laboratory Standard Institute recommended using the disk diffusion technique in the Mueller-Hinton agar to detect antibiotic susceptibility (Akhtar, 2010). Antibiotic discs such as piperacillin/tazobactam, co-amoxiclav (AMC), amikacin, gentamycin, erythromycin, ciprofloxacin, lomefloxacin, cephalexin, cefuroxime, ceftazidime, cefepime, and imipenem (IMP) were used for the antibiotic susceptibility test. In particular, data such as the hospital’s name, sampling date, surface type, and equipment or device name were collected for each sample.
Ethical approval
The study was permitted by the Ethics Committee in the Faculty of Medicine and Health Sciences, Hodeida University, under Ethical Approval no. 272-2019. A written approval was introduced to the head managers of the involved hospitals before the conduction of this study.
Statistical analysis
The data was analyzed using Statistical Package for the Social Sciences version 21, and the values were displayed in frequency and percentages.
RESULTS
A total of 240 samples collected from the 6 hospitals mentioned earlier were cultured and tested. Of them, 142 (59.2%) samples yielded positive bacterial growth. The results exhibited that the highest contamination rate was recorded with door handles (76.5%), followed by IV holders (71.4%), medical tables (66.7%), walls (65.0%), and suctions (64.3%). However, the lowest rate was recorded with heart monitors (33.3%) (Table 1).
From the overall swab samples’ positive growth, S. aureus had the highest frequency rate (37.2%), whereas Proteus spp. showed the lowest frequency rate (2.8%). Furthermore, S. aureus was the most predominant isolate across the surfaces, except for walls and IV holders, which were majorly contaminated with coagulase-negative Staphylococci (CoNS.) and Klebsiella pneumoniae, respectively. On the other hand, various species of bacteria were found on room surfaces and medical equipment, bringing the total number of isolates to 180 (Table 1).
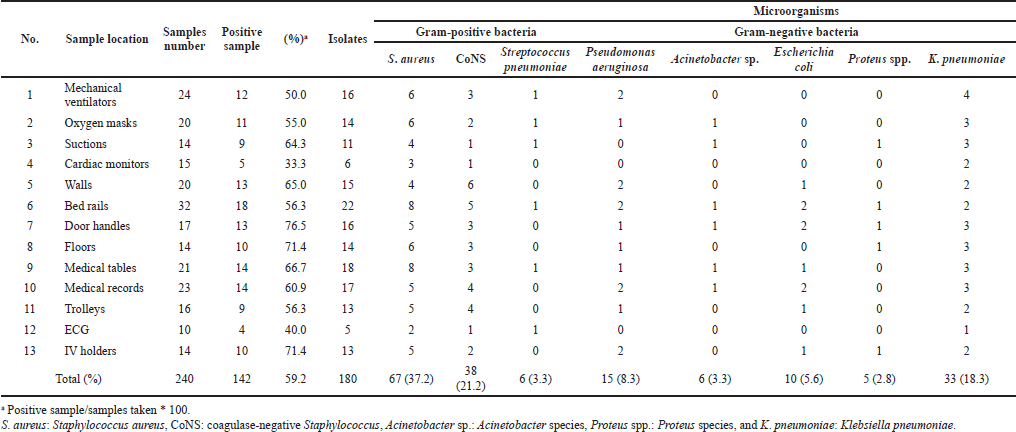 | Table 1. Type of isolates and their frequency according to the sample location. [Click here to view] |
In antibiotic susceptibility testing, S. aureus had the highest rate of resistance to piperacillin associated with tazobactam (89.6%), followed by amoxiclav (67.2%) and cefepime and cephalexin (65.7%), while it had the lowest resistance rate to amikacin (14.9%). The isolated CoNS had the highest ciprofloxacin resistance rate (89.5%) and the lowest gentamycin resistance rate (26.3%). On the other hand, Gram-negative isolates showed higher resistance compared with Gram-positive ones. Escherichia coli isolates were found to be multidrug resistant to the majority of antibiotics tested: piperacillin/tazobactam (80%), AMC, gentamycin, lomefloxacin, and cephalexin (70%), ceftazidime and amikacin (60%), and ciprofloxacin and cefepime (50%). Moreover, the results of this study revealed that P. aeruginosa isolates were resistant to piperacillin/tazobactam (80.0%), ciprofloxacin (73.3%), cefepime (73.3%), and gentamycin (66.7%) (Table 2).
Acinetobacter species showed higher resistance to AMC, whereas the lowest resistance rate was for cefepime (83%) and IMP (33%). From the findings, it was clarified that E. coli were mostly resistant to piperacillin/tazobactam (80%) and less resistant to IMP (40%). In addition, Proteus species were most resistant against AMC, ciprofloxacin, and ceftazidime (80%), while amikacin, cefuroxime, and IMP were observed to be of the lowest resistance (40%). AMC had the highest resistance impact against K. pneumoniae (78.9%), while IMP had the lowest resistant effect (36.4%).
DISCUSSION
The hospital ambient surfaces in ICUs play a crucial role in HAI because they are deemed a major reservoir of microbes, such as multidrug-resistant bacteria, which adversely affect patients and hospitals (Bitew et al., 2021). Continuous screening and understanding of the prevalence of the bacteria in the environment of the ICUs are highly advised, and they represent one of the most important techniques for increasing HCWs’ awareness thereof. The present study intended to disclose the prevalence of bacteria on the environmental surfaces in ICUs and their antimicrobial susceptibility among six different hospitals in Hodeida City, Yemen.
In this study, the average of the positive samples revealed bacterial contamination of the environmental surfaces in ICUs was 59.2%. This rate is considered dangerous and alarming, and it requires immediate attention and action to resolve the problem and reduce it to a very low level. A prior study in Wille et al. (2018), Austria, revealed a contamination rate of 10%, describing it as “extensive” and “requiring more attention,” which is deemed relatively low when compared to the contamination rate in our study. Alternatively, earlier investigations have found contamination rates as high as the current study (62.8% and 57%) (Ekrami et al., 2011; Yusuf J, 2017) or even higher as in Brazil and Saudi Arabia (94.7% and 85.2%, respectively) (Rodrigues et al., 2019).
The presence of a high contamination rate in the current study could be attributed to many factors such as the transmission of contamination by the hands of HCWs, patients, and visitors, improper cleaning and disinfectant practices, inappropriate cleaning and disinfection methods, and inadequate adherence to HCWs to hand hygiene (Faires et al., 2013). Furthermore, our country’s economic position has a direct impact on the infrastructure and equipment of institutions, including hospitals. As a result, the ICUs in the hospitals under investigation were consistently overloaded with patients, resulting in easy pathogen shedding, difficulty cleaning and disinfecting, and HCWs overload.
This was one of the reasons mentioned in a previous study that documented a significant relationship between HAI development and nurses’ workload or overload due to understaffing in ICUs (Aycan et al., 2015). The findings of this study showed more contamination on surfaces that are frequently touched by the HCWs, patients, or visitors. The most contaminated surfaces in our samples were the door handles (76.5%), IV holders (71.4%), and floors (71.4%), followed by medical tables, suctions, and medical documents. This could be because the majority of these surfaces are routinely touched by HCWs’ hands.
In the current study, all the intended units are not singular, which means they are not subject to appropriate disinfection. In addition, contamination of patients’ personal objects, bed linens, and bedsides may be related to the normal flora because almost 106 skin squamous contains viable microorganisms are shed every day from human’s skin. (Tajeddin et al., 2016). Failure to strictly adhere to the local infection control guidelines or the production of biofilms could lead to inappropriate surface cleaning, which eventually results in high contamination (Carling et al., 2010; Wille et al., 2018; Zuberi and Ptashnick, 2011). These findings highlight the necessity of implementing a plan for disinfecting and cleaning all surfaces in ICUs, as well as providing HCWs with ongoing education and training courses to improve their health hygiene awareness.
This study revealed that Gram-positive organisms are more prevalent than the Gram-negative ones; however, previous literature produced contradicting findings (Lemmen et al., 2004; Tan et al., 2014; Wille et al., 2018). Staphylococcus aureus was found to be the most isolated organism in the current study (58.4%). This could be due to the high frequency of S. aureus in the human body as normal flora, as well as their resistance to drying and heating, which is consistent with earlier research (Saka et al., 2016; Wille et al., 2018). Conversely, S. aureus was detected in low-frequency levels compared with other screened bacteria (Huang et al., 2006; Tajeddin et al., 2016; Tan et al., 2014; Zuberi and Ptashnick, 2011). This study found a high resistance pattern to routinely used antibiotics such as piperacillin/tazobactam, AMC, cefepime, ciprofloxacin, and cephalexin, which is consistent with previous findings in the literature (Akhtar, 2010; Tajeddin et al., 2016; Yusuf et al., 2017). In addition, worrying rates of medication resistance in microorganisms inhabiting various surfaces of the ICUs evaluated were discovered. These locations appear to be potential sources of common HAIs resistant to antibiotics employed in these hospitals.
Finally, infection control committees in hospitals should strictly follow the guidelines and maintain strong, effective infection control programs that focus on preventing and reducing bacterial contamination by implementing preventive techniques on the ambient surfaces in ICUs and restricting the random use of antibiotics to minimize antibiotic resistance.
 | Table 2. Pattern of antibiotic resistance among pathogenic bacteria isolated from ICUs environment (%). [Click here to view] |
CONCLUSION
According to the findings of the current study, the prevalence of environmental bacteria in ICUs was extremely high and alarming. Gram-positive bacteria were found to be more common than Gram-negative bacteria, with S. aureus being the most common isolated organism in the current investigation. Environmental surfaces contributed to more than 50% of the contamination. Antibiotic resistance was found to be extremely high, which is a concerning finding. Thus, there is an urgent need to develop and implement surveillance programs in these hospitals to tackle the origin and emerging pathways of resistant pathogens.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The study was permitted by the Ethics Committee in the Faculty of Medicine and Health Sciences, Hodeida University, under Ethical Approval no. 272-2019
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Ababneh Q, Abulaila S, Jaradat Z. Isolation of extensively drug resistant Acinetobacter baumannii from environmental surfaces inside intensive care units. Am J Infect Control, 2022; 50(2):159–65. CrossRef
Abdul-Ghani R, Mahdy MAK, Alkubati S, Al-Mikhlafy AA, Alhariri A, Das M, Dave K, Gil-Cuesta J. Malaria and dengue in Hodeidah city, Yemen: high proportion of febrile outpatients with dengue or malaria, but low proportion co-infected. PLoS One, 2021; 16(6):e0253556; doi:10.1371/journal.pone.0253556 PONE-D-21-03805 [pii] CrossRef
Akhtar N. Hospital acquired infections in a medical intensive care unit. J Coll Physicians Surg Pak, 2010; 20(6):386–90.
Aycan IO, Celen MK, Yilmaz A, Almaz MS, Dal T, Celik Y, Bolat E. Bacterial colonization due to increased nurse workload in an intensive care unit. Braz J Anesthesiol, 2015; 65(3):180–5. CrossRef
Bitew K, Gidebo DD, Ali MM. Bacterial contamination rates and drug susceptibility patterns of bacteria recovered from medical equipment, inanimate surfaces, and indoor air of a neonatal intensive care unit and pediatric ward at Hawassa University Comprehensive Specialized Hospital, Ethiopia. IJID Regions, 2021; 1:27–33. CrossRef
Boyce JM. Environmental contamination makes an important contribution to hospital infection. J Hosp Infect, 2007; 65:50–4. CrossRef
Brusselaers N, Vogelaers D, Blot S. The rising problem of antimicrobial resistance in the intensive care unit. Ann Intensive Care, 2011; 1(1):1–7. CrossRef
Carling PC, Bartley JM. Evaluating hygienic cleaning in health care settings: what you do not know can harm your patients. Am J Infect Control, 2010; 38(5):S41–50. CrossRef
Carling PC, Parry MF, Bruno-Murtha LA, Dick B. Improving environmental hygiene in 27 intensive care units to decrease multidrug-resistant bacterial transmission. Crit Care Med, 2010; 38(4):1054–9. CrossRef
de Oliveira AC, Damasceno QS. Surfaces of the hospital environment as possible deposits of resistant bacteria: a review. Rev Esc Enferm USP, 2010; 44(4):1112–7. CrossRef
Ekrami A, Kayedani A, Jahangir M, Kalantar E, Jalali M. Isolation of common aerobic bacterial pathogens from the environment of seven hospitals, Ahvaz, Iran. Jundishapur J Microbiol, 2011; 4(2):75–82.
Faires MC, Pearl DL, Berke O, Reid-Smith RJ, Weese JS. The identification and epidemiology of meticillin-resistant Staphylococcus aureus and Clostridium difficile in patient rooms and the ward environment. BMC Infect Dis, 2013; 13(1):1–13. CrossRef
Hu H, Johani K, Gosbell IB, Jacombs AS, Almatroudi A, Whiteley GS, Deva AK, Jensen S, Vickery K. Intensive care unit environmental surfaces are contaminated by multidrug-resistant bacteria in biofilms: combined results of conventional culture, pyrosequencing, scanning electron microscopy, and confocal laser microscopy. J Hosp Infect, 2015; 91(1):35–44; doi:10.1016/j.jhin.2015.05.016 S0195-6701(15)00258-3 [pii] CrossRef
Huang SS, Datta R, Platt R. Risk of acquiring antibiotic-resistant bacteria from prior room occupants. Arch Intern Med, 2006; 166(18):1945–51. CrossRef
Kramer A, Schwebke I, Kampf G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis, 2006; 6(1):1–8. CrossRef
Lemmen S, Häfner H, Zolldann D, Stanzel S, Lütticken R. Distribution of multi-resistant Gram-negative versus Gram-positive bacteria in the hospital inanimate environment. J Hosp Infect, 2004; 56(3):191–7. CrossRef
Montero JG, Lerma FÁ, Galleymore PR, Martínez MP, Rocha LÁ, Gaite FB, Rodríguez JÁ, González MC, Moreno IF, Baño JR, Campos J, Andrés JM, Varela YA, Gay CR, García MS, Scientific Expert Committee for Zero Resistance Project. Combatting resistance in intensive care: the multimodal approach of the Spanish ICU “Zero Resistance” program. Crit Care, 2015; 19(1):114; doi:10.1186/s13054-015-0800-510.1186/s13054-015-0800-5 [pii] CrossRef
Otter JA, Yezli S, Salkeld JA, French GL. Evidence that contaminated surfaces contribute to the transmission of hospital pathogens and an overview of strategies to address contaminated surfaces in hospital settings. Am J Infect Control, 2013; 41:S6–11. CrossRef
Panhotra BR, Saxena AK, Al-Mulhim AS. Contamination of patients’ files in intensive care units: an indication of strict handwashing after entering case notes. Am J Infect Control, 2005; 33(7):398–401. CrossRef
Quinn MM, Henneberger PK; National Institute for Occupational Safety and Health (NIOSH), National Occupational Research Agenda (NORA) Cleaning and Disinfecting in Healthcare Working Group, Braun B, Delclos GL, Fagan K, Huang V, Knaack JL, Kusek L, Lee SJ, Le Moual N, Maher KA, McCrone SH, Mitchell AH, Pechter E, Rosenman K, Sehulster L, Stephens AC, Wilburn S, Zock JP. Cleaning and disinfecting environmental surfaces in health care: toward an integrated framework for infection and occupational illness prevention. Am J Infect Control, 2015; 43(5):424–34. CrossRef
Rodrigues, D. O., Peixoto, L. da P., Barros, E. T. M., Guimaraes, J. R., Gontijo, B. C., Almeida, J. L., Azevedo, L. G. de, Lima, J. C. O. e, & Camara, D. S. (2020). Epidemiology of Bacterial Contamination of Inert Hospital Surfaces and Equipment in Critical and Non-critical Care Units: A Brazilian Study. Microbiology Research Journal International, 30(7), 31-43. https://doi.org/10.9734/mrji/2020/v30i730237 CrossRef
Russotto V, Cortegiani A, Raineri SM, Giarratano A. Bacterial contamination of inanimate surfaces and equipment in the intensive care unit. J Intensive Care, 2015; 3(1):1–8. CrossRef
Rutala W, Weber D. Role of the hospital environment in disease transmission, with a focus on Clostridium difficile. Healthcare Infect, 2013; 18:14–22. CrossRef
Rutala WA, Gergen MF, Weber DJ. Efficacy of different cleaning and disinfection methods against Clostridium difficile spores: importance of physical removal versus sporicidal inactivation. Infect Control Hosp Epidemiol, 2012; 33(12):1255–8. CrossRef
Rutala WA, Weber DJ. Monitoring and improving the effectiveness of surface cleaning and disinfection. Am J Infect Control, 2016; 44(5):e69–76. CrossRef
Saka KH, Akanbi II AA, Obasa TO, Raheem RA, Oshodi AJ, Kalgo ZM. Pathogenic aerobic bacterial contaminants on non-critical hospital surfaces within paediatric ward of a nigerian hospital. J Med Microb Diagn, 2016; 5(241):2161–0703.1000241.
Tajeddin E, Rashidan M, Razaghi M, Javadi SS, Sherafat SJ, Alebouyeh M, Sarbazi MR, Mansouri N, Zali MR. The role of the intensive care unit environment and health-care workers in the transmission of bacteria associated with hospital acquired infections. J Infect Public Health, 2016; 9(1):13–23. CrossRef
Tan R, Liu J, Li M, Huang J, Sun J, Qu H. Epidemiology and antimicrobial resistance among commonly encountered bacteria associated with infections and colonization in intensive care units in a university-affiliated hospital in Shanghai. J Microbiol Immunol Infect, 2014; 47(2):87–94. CrossRef
Wille I, Mayr A, Kreidl P, Brühwasser C, Hinterberger G, Fritz A, Posch W, Fuchs S, Obwegeser A, Orth-Höller D, Lass-Flörl C. Cross-sectional point prevalence survey to study the environmental contamination of nosocomial pathogens in intensive care units under real-life conditions. J Hosp Infect, 2018; 98(1):90–5. CrossRef
Yusuf JB, Okwong OK, Mohammed A, Abubakar KS, Babayo A, Barma MM, Ibrahim S, Sulaiman AI, Hafiz H, Bello ZS. Bacterial contamination of intensive care units at a tertiary hospital in Bauchi, Northeastern Nigeria. Am J Intern Med, 2017; 5(3):46–51. CrossRef
Zuberi DM, Ptashnick MB. The deleterious consequences of privatization and outsourcing for hospital support work: the experiences of contracted-out hospital cleaners and dietary aids in Vancouver, Canada. Soc Sci Med, 2011; 72(6):907–11. CrossRef