INTRODUCTION
In India, in vitro diagnostic (IVD) medical devices are regulated under the Medical Devices Rules 2017 by CDSCO, Ministry of Health and Family Welfare, Government of India, under the arrangements of the Drugs and Cosmetics Act 1940. These rules came into power on 1 January 2018 to control the production, import, and marketing of medical devices and IVD medical devices in the nation (Medical Devices Rules, 2017). As per the provision of Medical Devices Rules 2017 under normal circumstances for the grant of license to import or manufacture for sale or distribution of any new IVDs, the applicant has to first obtain a test license in MD-13 (domestic manufacture)/MD-17 (import). Then, the applicant has to obtain permission for clinical performance evaluation in form MD-25 (for protocol approval in consultation with the IVD experts’ committee). After carrying out clinical performance evaluation, data on such clinical study need to be submitted by applying in form MD-28 for approval in consultation with IVD experts’ committee and if the same is found satisfactory, then permission to import or manufacturer of new IVDs is granted in form MD-29. Once the permission in MD-29 is issued, the applicant has to obtain a license to import (MD-15)/domestic manufacture (MD9/10) for sale or distribution (New Medical Device Rules, 2017). This paper provides a comprehensive overview and assessment of the steps and approaches for the regulatory approval process made available by the regulatory authority to stakeholders in India and covers an overview of the current regulatory approval process for COVID-19 IVD in the USA, EU, Canada, Singapore, and Japan.
METHODS
Every study has certain trends and paths to achieve its goal. The approach to be followed, therefore, plays an important role in deciding the output and the effect of the analysis. This research was conducted to study in detail the COVID-19 IVD guidelines by comparing India’s COVID-19 IVD guidelines with other guidelines, and the need for industries to switch into harmonization and collaboration in the regulatory framework. Concentrating on the recent updates and changes in the COVID-19 IVD guide, we analyzed the necessity for India to become efficient in manufacturing SARS-CoV-2 IVDs. The study was conducted with data gathered by evaluating the following guidelines: introduction and detailed description about the COVID-19 IVD guidelines; review on the current regulatory approval process for COVID-19 IVDs in USA, EU, Canada, Singapore, and Japan; and brief study on recent updates and changes on the COVID-19 IVD guide. The current regulatory approval process for COVID-19 IVDs in the USA, EU, Canada, Singapore, and Japan is shown in Table 1. The approval authorities responsible for the device are shown in Figure 1.
Internet using web page content
Various search engines were used to compile the literature. The primary sources are official websites of regulatory agencies and also magazines, newsletters, and regulatory events, etc. Secondary sources are Scopus, ResearchGate, PubMed, Web of Science, Google scholar, etc.
Regulatory pathway for COVID-19 IVDs under MDR 2017
In India, import, manufacturing, clinical validation, sale, and distribution of in-IVD kit/reagents intended for the novel coronavirus (2019-nCoV) are regulated under the provisions of the Drugs and Cosmetic Act and Medical Devices Rules 2017 (MHRA, 2020).
Firstly, applicants, such as importers (authorized agents)/domestic manufacturers, are required to obtain login credentials and password from CDSCO for online submission of application on the CDSCO MD Portal (Rapid Antigen Test Kits for COVID-19, 2021), as shown in Figure 2.
Regulatory pathway for import of PCR or enzyme-linked immunoassay (ELISA) or Rapid IVDs for COVID-19
A small quantity of the subject product may be imported for testing/evaluation at ICMR designated laboratory under the import test license in MD-17 obtained by applying in form MD-16 along with a $100 fee for each IVD and document as per the checklist available on the MD portal. The product shall be imported for sale or distribution under the import license in MD-15 obtained by applying in form MD-14 along with a $3,000 fee for site and $500 fee per product and document as per checklist available on the MD portal (where products shall be regulated as Class-C under MDR 2017). The importer shall obtain permission to import new IVD medical devices in MD-29 by applying in form MD-28 along with an INR 25,000 fee and documents as per the checklist available on the MD portal. The above-mentioned applications (i.e., MD-16, MD-14, and MD-28) may be submitted simultaneously and processed parallelly and the licenses shall be issued by the CDSCO. For information on testing requirements, applicants may approach ICMR (HQ), New Delhi, and to the concerned testing laboratories for validation of kits, a request should be made through the online portal (https://cvtestkit.icmr.org.in). For information on obtaining a wholesale license, applicants may approach the concerned state’s drug controllers/licensing authority (Kits Validation & Batching Testing).
 | Table 1. Current regulatory approval process for COVID-19 IVD. [Click here to view] |
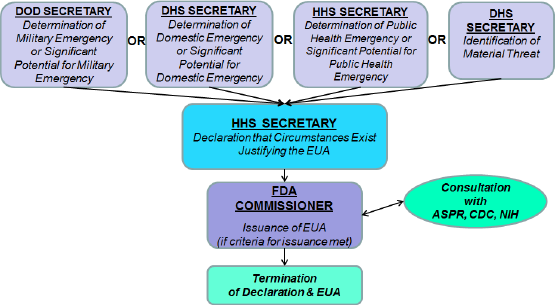 | Figure 1. Authorities responsible for the device’s approval. [Click here to view] |
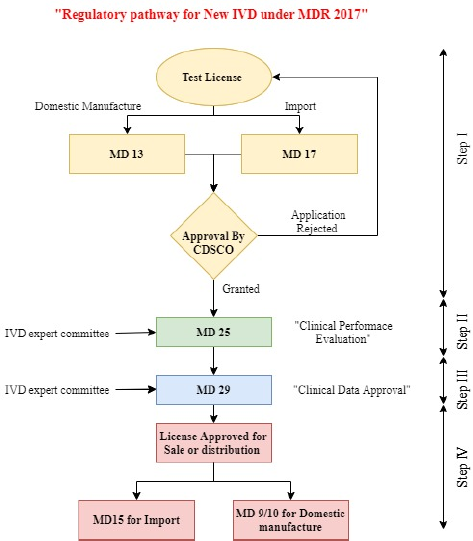 | Figure 2. Regulatory pathway for the new IVD under MDR 2017. [Click here to view] |
Regulatory pathway for domestic manufacturing of PCR/ELISA/Rapid IVDs for COVID-19
A small quantity of the subject product may be manufactured for in-house testing/evaluation at ICMR designated laboratory under manufacturing test license in MD-13 obtained by applying in form MD-12 along with an INR 500 fee for each IVD and document as per the checklist available on the MD portal. The product shall be manufactured for sale or distribution under manufacturing license in MD-9 or MD-10 (loan license) obtained by applying in form MD-7 or MD-8 (loan license) along with an INR 50,000 fee for the site and INR 1,000 per product and document as per the checklist available on the MD portal. (The product shall be regulated as Class-C under MDR 2017.) The manufacturer shall obtain permission to manufacture a new IVD medical device in MD-29 by applying in form MD-28 along with an INR 25,000 fee and documents as per the checklist available on the MD portal. The abovementioned applications (i.e., MD-12, MD-7/8, and MD-28) may be submitted simultaneously and processed parallelly. Applications were received, reviewed, audited (where applicable), and recommended to LA by the concerned CDSCO zonal/subzonal office in coordination with the CDSCO (HQ). For information on testing requirements, applicants may approach ICMR (HQ), New Delhi, and to the concerned testing laboratories for validation of kits, a request should be made through the online portal (https://cvtestkit.icmr.org.in) (Validation Kit Portal).
Regulatory pathway for imports of antigen/antibodies-coated uncut sheet/ELISA plate for further manufacturing finished COVID-19 IVDs
A small quantity of the subject product may be imported for testing/evaluation at the ICMR designated laboratory under the import test license in MD-17 obtained by applying in form MD-16 along with a $100 fee for each IVD and document as per the checklist available on the MD portal. The product shall be imported for further manufacturing under the import license in MD-15 obtained by applying in form MD-14 along with a $3,000 fee for site and $500 per product and document as per checklist available on the MD portal. (The product shall be regulated as Class-C under MDR 2017.) The abovementioned applications (i.e., MD-16 and MD-14) may be submitted simultaneously and processed parallelly. For information on the testing requirements app, the license may approach ICMR (HQ), New Delhi, and to the concerned testing laboratories for validation of kits, a request should be made through the online portal (https://cvtestkit.icmr.org.in) (Validation Kit Portal).
Regulatory pathway for domestic manufacturing of finished COVID-19 IVDs
A small quantity of the subject product may be manufactured for in-house testing/evaluation at the ICMR designated laboratory under the manufacturing test license in MD-13 obtained by applying in form MD-12 along with an INR 500 fee for each IVD and document as per the checklist available on the MD portal. The product shall be manufactured for sale or distribution under the manufacturing license in MD-9 or MD-10 (loan license) obtained by applying in form MD-7 or MD-8 (loan license) along with an INR 50,000 fee for site and INR 1,000 per product and document as per the checklist available on the MD portal. (The product shall be regulated as Class-C under MDR 2017.) The manufacturer shall obtain permission to manufacture new an IVD medical device in MD-29 by applying in form MD-28 along with an INR 25,000 fee and documents as per the checklist available on the MD portal. The abovementioned applications (i.e., MD-12, MD-7/8, and MD-28) may be submitted simultaneously and processed parallelly. Applications were received, reviewed, audited (where applicable), and recommended to LA by the concerned CDSCO zonal/subzonal office in coordination with CDSCO (HQ). For information on testing requirements, applicants may approach ICMR (HQ), New Delhi, and to the concerned testing laboratories for validation of kits, a request should be made through the online portal (https://cvtestkit.icmr.org.in) (In-vitro Diagnostics, Central Drugs Standard Control Organization).
Regulatory pathway for import of viral transport kit for IVD
A small quantity of the subject product may be imported for testing/evaluation at the ICMR designated laboratory under the import test license in MD-17 obtained by applying in form MD-16 along with a $100 fee for each IVD and document as per the checklist available on the MD portal. The product shall be imported for sale or distribution under the import license in MD-15 obtained by applying in form MD-14 along with a $1,000 fee for site and $10 per product and document as per checklist available on the MD portal. (The product shall be regulated as Class-A under MDR 2017.) The abovementioned applications (i.e., MD-16 and MD-14) may be submitted simultaneously and processed parallelly and the license is issued by the CDSCO. For information on testing requirements, applicants may approach ICMR (HQ), New Delhi, and to the concerned testing laboratories for validation of kits, a request should be made through the online portal (https://cvtestkit.icmr.org.in) (In-vitro Diagnostics. Central Drugs Standard Control Organization).
Regulatory pathway for domestic manufacturing of viral transport kit for IVD
A small quantity of the subject product may be manufactured for in-house testing/evaluation at the ICMR designated laboratory under the manufacturing test license in MD-13 obtained by applying in form MD-12 along with an INR 500 fee for each IVD and document as per the checklist available on the MD portal. The product shall be manufactured for sale or distribution under manufacturing license in MD-5 or MD-6 (loan license) obtained by applying in form MD-3 or MD-4 (loan license) along with an INR 5,000 fee for site and INR 500 per product and document as per the checklist available on the MD portal. The product shall be regulated as Class-A under MDR 2017 and the license shall be issued by the concerned state licensing authority. No audit of the manufacturing site shall be necessary before the grant of license. The required audit of such manufacturing site by the registered/notified body shall be carried out within 120 days from the date on which the license was granted by the State Licensing Authority (Matheeussen et al., 2020). The abovementioned applications (i.e., MD-12 and MD-5/6) may be submitted simultaneously and processed parallelly. For information on testing requirements, applicants may approach ICMR (HQ), New Delhi, and to the concerned testing laboratories for validation of kits, a request should be made through the online portal (https://cvtestkit.icmr.org.in) (Central Drugs Standard Control Organization).
Regulatory pathway for import of the MTM/ RNA/DNA extraction kit
A small quantity of the subject product may be imported for testing/evaluation at the ICMR designated laboratory import test license in MD-17 obtained by applying in form MD-16 along with a $100 fee for each IVD and document as per the checklist available on the MD portal. The product shall be imported for sale or distribution under the import license in MD-15 obtained by applying in form MD-14 along with a $3,000 fee for site and $500 per product and document as per checklist available on the MD portal (The product shall be regulated as Class-C under MDR 2017). The abovementioned applications (i.e., MD-16 and MD-14) may be submitted simultaneously and processed parallelly. For information on testing requirements, applicants may approach ICMR (HQ), New Delhi, and to the concerned testing laboratories for validation of kits, a request should be made through the online portal (https://cvtestkit.icmr.org.in) (Health Canada’s regulatory response to COVID-19).
Regulatory pathway for domestic manufacturing of MTM/RNA/DNA extraction kit
A small quantity of the subject product may be manufactured for in-house testing/evaluation at the ICMR designated laboratory under the manufacturing test license in MD-13 obtained by applying in form MD-12 along with an INR 500 fee for each IVD and document as per the checklist available on the MD portal. The product shall be manufactured for sale or distribution under the manufacturing license in MD-9 or MD-10 (loan license) obtained by applying in form MD-7 or MD-8 (loan license) along with an INR 50,000 fee for site and INR 1,000 per product and document as per checklist available on the MD portal. (The product shall be regulated as Class-C under MDR 2017.) The abovementioned applications (i.e., MD-12 and MD-7/8) may be submitted simultaneously, processed parallelly, and issued by the CDSCO. Applications were received, reviewed, audited (where applicable), and recommended to LA by the concerned CDSCO zonal/subzonal office in coordination with CDSCO (HQ). For information on testing requirements, applicants may approach ICMR (HQ), New Delhi, and the concerned testing laboratories, i.e., NIV, Pune, etc., (Applications for medical devices under the Interim Order for use about COVID-19). The requirements of COVID-19 IVDs are shown in Table 2 and Figures 4–7.
Timeline for fast-track approval
The timeline for fast tract approval is shown in Table 3. Note that for all the complete applications, import/manufacturing licenses were issued within 15–30 days, including site inspection in case of domestic manufacturers (medical devices for uses related to COVID-19). Acceptance criteria for the COVID-19 kits and reagents (medical devices for uses related to COVID-19) are shown in Figure 3.
RESULT AND DISCUSSION
In India, IVD kits intended for the novel coronavirus (2019-nCoV) are regulated as new IVD kits, and getting market authorization through import or manufacturing license for such new IVDs under standard regulatory pathway requires four stages, which include test license, permission for clinical performance evaluation for protocol approval in consultation with the IVD experts committee, permission to import or manufacturer new IVDs after verification of clinical data in consultation with the IVD experts committee, and market authorization to complete the abovementioned stages will take a minimum of 17 months. It may also extend based on the time taken by the applicant for replying and submitting details/data requested by the regulator. To provide a certain exemption to accelerate the regulatory approval for timely availability and use of IVD used for COVID-19 needed during public health emergencies, waive off the requirements of conducting clinical performance evaluation under MDR 2017 as per Rule 59(4) and abbreviate the clinical data requirements for issuing permission to import or manufacture new IVD medical device as per Rule 64 of MDR 2017 can be considered by the Central Licensing Authority (CDSCO). As the kits have been approved under emergencies during premarket approval, a very limited amount of clinical data about the performance of the product has been submitted. Thus, it is expected to collect the retrospective data on the performance of kits in the form of post-marketing surveillance data as much as possible by adopting the best possible mechanisms by using the licensee. Furthermore, it is also expected to report any deviation from the claimed performance of the kit so that corrective action could be initiated. The same is required under the provisions of MDR 2017. To achieve the rapid availability of quality diagnostic tests for COVID-19 in the country, specific tool for the regulatory pathway, exemption available, products’ standards, importance of postmarked surveillance for the availability of the quality COVID-19 IVDs are required, which is fulfilled through this article for the benefit of the Indian and global stakeholders. This regulatory tool has been made based on the risk and benefit of pathways, followed by stringent regulatory authorities like USA, EU, Canada, Singapore, and Japan without any additional legal requirements which speed up the availability of the good quality COVID-19 IVDs and weed out the falsified from the market in India (Validation of SARS-CoV-2 Diagnostic Commodities, 2020).
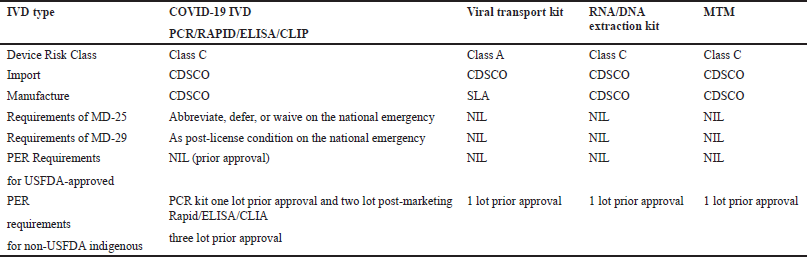 | Table 2. Requirements of COVID-19 IVDs (Applications for medical devices under the interim order for use in relation to COVID-19). [Click here to view] |
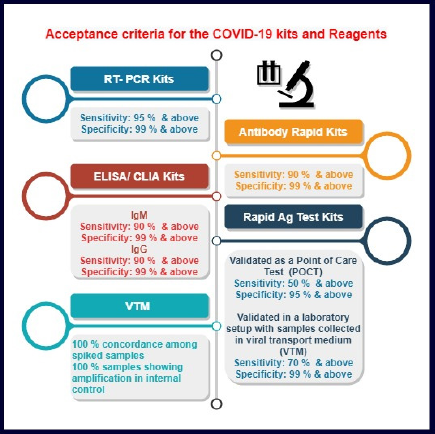 | Figure 3. Acceptance criteria for the COVID-19 kits and reagents. [Click here to view] |
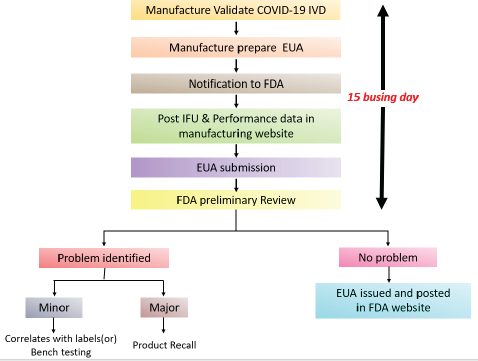 | Figure 4. USFDA COVID-19 IVD EUA approval pathway. [Click here to view] |
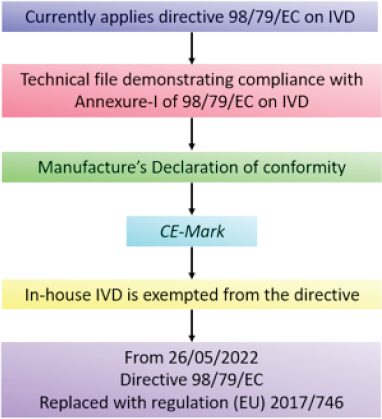 | Figure 5. EU COVID-19 approval pathway. [Click here to view] |
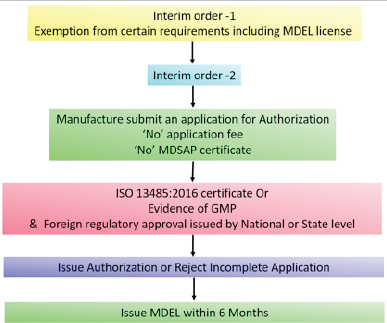 | Figure 6. Health Canada COVID-19 approval pathway. [Click here to view] |
Challenge and opportunity
When the pandemic had only just begun, India hardly had the infrastructure to develop COVID-19 testing solutions and was heavily dependent on imports. The government asked the indigenous IVD manufacturers to initiate steps and invested heavily in manufacturing a comprehensive range of COVID-19 solutions including RT-PCR tests, RNA extraction kits, viral transport media, total antibody ELISA test, ELISA IgG and COVID-19 antigen testing kits. This pandemic triggered an unprecedented demand for high-performance health technology products with extremely short turnaround times and unmatched precision. In March 2020, despite being the first and only one Indian company to get approval from the Indian FDA for COVID-19 test kits, CDSCO offered COVID-19 kits at one-fourth the cost borne by the government. The government is working with the importers, domestic manufacturers, state governments, ICMR, and NIV to ensure kits are available in the remotest parts of the country and to the friends of India.
Improved IVD supply chain
Performing a lab-based molecular assay typically requires around 20 different reagents, raw materials, consumables, and other pieces of equipment, and despite the importance of COVID-19 diagnostic assays, major shortages have been reported in RNA extraction kits and reagents, including enzymes and primers, which in this instance has led to RNA extraction kits being the bottleneck to higher diagnostic capacity. Due to the lockdown and restrictions in the different countries and within the country, the logistic support affects COVID-19 testing. These raw materials used for the finished IVDs are exempted from the regulatory controls, thereby the local manufacturers get these raw materials imported and cleared in the port of entry without any regulatory restriction. Licenses for the import and manufacturing of COVID-19 are processed under expiated review and a fast-track basis has improved the uninterrupted supply of the COVID-19 IVDs.
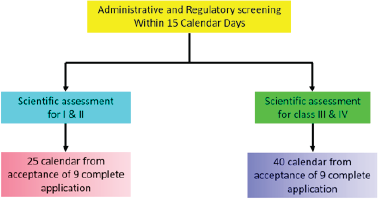 | Figure 7. Health Canada COVID-19 approval timeline. [Click here to view] |
Ensuring the quality of COVID-19 IVDs
Given the national emergency due to the COVID-19 pandemic, ICMR has taken up the task of validating diagnostic products related to COVID-19 and also setting up a minimum standard of the COVID-19 IVDs which are described in the guidance tool. So far, ICMR has validated a total of 515 RT-PCR kits that have been evaluated by the ICMR validation centers; 205 were found to be satisfactory based on the report CDSCO issued market authorizations, which included 93 indigenous and 157 imported. Till 13/01/2022, 13 rapid Ag-based home/self-test kits have been validated and 8 were found to be satisfactory by the CDSCO issued market authorizations, which included 7 indigenous and 2 imported. Till 21/01/2022, 149 Antigen-based rapid test kits have been validated (including 31 revalidations) and 55 were found to be satisfactory by the CDSCO issued market authorizations, which included 46 indigenous and 36 imported. Till 12/02/2021, 196 antibody-based rapid tests have been validated by ICMR centers and 30 were found to be satisfactory by the CDSCO issued market authorizations, which include 58 indigenous and 96 imported. Currently, ICMR has designated one center for the validation of COVID-19 diagnostic kits molecular diagnostics, two centers for the validation of COVID-19 diagnostic kits serology diagnostics, and 28 centers for the validation of COVID-19 diagnostic kits antigen diagnostics. The current global health emergency of the coronavirus disease (COVID-19) pandemic (caused by the SARS-CoV-2) has increased demand for diagnostics and reagents, related to COVID-19, creating an opportunity for ill-intended persons to distribute falsified IVDs. The WHO Medical Product Alert warns consumers, healthcare professionals, and health authorities against a growing number of falsified medical products that claim to prevent, detect, treat, or cure COVID-19. In India, the national drug regulatory authority (CDSCO) in collaboration with ICMR found two IVDs, SARS-CoV-2 antibody tests (lateral flow method) manufactured by Guangzhou Wondfo Biotech, China, and COVID-19 IgM/IgG antibody rapid test, manufactured by Zhuhai Livzon Diagnostics, China, faulty by testing at the ICMR laboratory. The marketing licenses to the distributors of these two companies have been cancelled by the regulatory authority of the Central Drugs Standard Control Organization (CDSCO).
Uniform and transparent approval of COVID-19 IVDs
Under MDR 2017, approval for the import of all COVID-19 IVDs is done only by the CDSCO at the headquarters in Delhi. However, approval for manufacturing of class A and class B COVID-19 IVDs are processed and issued by the concerned state licensing authority; class C and class D COVID-19 IVDs are processed by the concerned CDSCO zonal or subzonal office, under which the manufacturing premises are located. By adopting the regulatory tool, uniformity and transparency in the COVID-19 approval have been ensured.
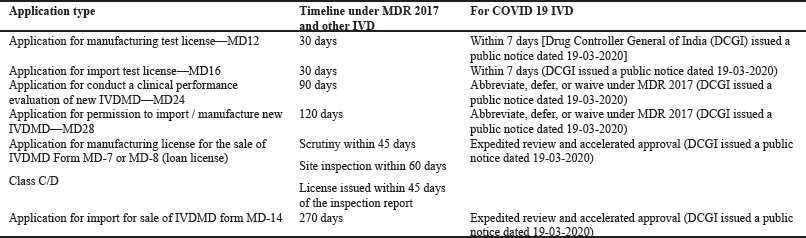 | Table 3. Timeline for fast track approval. [Click here to view] |
Collaboration to make newer COVID-19 technology
Various research institutes, like ICMR, IIT, DRDO, AIIMS, and CSIR, have developed various COVID-19 kits and have collaborated with potential manufacturers in the country and transferred the technology and, guided through the regulatory guidance with the support of CDSCO, brought the tests to the usage within the record timelines under the health emergency, which also enhanced the availability of COVID-19 testing kits and newer innovative testing.
CONCLUSION
The regulatory tools described in this article are manufactured based on the comparison with the data available on the procedure following the approval/authorization by the other major countries under the health emergency without compromising on the quality of the product within the existing provisions given in the Drugs and Cosmetic Act and Medical Devices Rules 2017. This is very useful to national and international stakeholders to market quality products in the domestic market and for export to other countries. To react efficiently to the COVID-19 outbreak, rapid diagnosis of cases and contacts is critical to control further spread and monitoring. In this regard, CDSCO and ICMR have taken various measures and issued guidance, public notices, designated various validation centers and also set up an acceptance criteria as the quality benchmark for COVID-19 IVDs and reagents to help and address urgent public health concerns by expanding the number and variety of quality IVD tests, promptly in India. Under the fast track approval process till 02.12.2020, the CDSCO has issued 168 market authorizations in the form of import/manufacturing licenses for COVID-19 PCR kits, which include 14 indigenous manufactured. 121 numbers of COVID-19 rapid / CLIA/ ELISA kits, which include 10 indigenous manufactured details, are regularly updated on the website of the CDSCO. Furthermore, ICMR has made an online portal for evaluating the COVID-19 kits and reagents to speed up the testing process through their 24 designated kit validation centers.
ABBREVIATIONS
ICMR: The Indian Council of Medical Research; EUA: Emergency Use Authorization; MDEL: medical device establishment license; IVD: in vitro diagnostics; MDR: Medical Devices Rules; FD&C: Federal Food, Drug, and Cosmetic Act; DRH: Devices and Radiological Health; MS: member states; MOA: Minister of Health; FDA: Food and Drugs Act; IO: interim order; HSA: Health Sciences Authority, QMS: Quality management system.
ACKNOWLEDGMENT
The authors would like to thank the Department of Science and Technology (fund) for the improvement of the science and technology infrastructure in universities and higher educational institutions (DST-FIST), New Delhi, for their infrastructure support to our department.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
SOURCE OF FUNDING
There is no funding support for this article.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Applications for medical devices under the Interim Order for use in relation to COVID-19: guidance document. Government of Canada, India. Available via https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/announcements/interim-order-importation-sale-medical-devices-covid-19/guidance-medical-device-applications.html
Bhave AC. Indian regulatory update during the COVID-19 pandemic. Perspect Clin Res, 2020; 11(3):132. CrossRef
Central Drugs Standard Control Organization. Government of India, India. Available via https://cdsco.gov.in/opencms/opencms/en/Notifications/Public-Notices/
Das P, Mondal S, Pal S, Roy S, Vidyadharan A, Dadwal R, Bhattacharya S, Mishra DK, Chandy M., 2021 COVID diagnostics by molecular methods: a systematic review of nucleic acid-based testing systems. Indian J Med Microbiol, 2021; 39(3):271–8. CrossRef
Health-Canada. Guidance document—applications for medical devices under the Interim Order for use in relation to COVID-19, Government of Canada, pp 1–25.
Health Sciences Authority (HSA). HSA Expedites approval of COVID-19 diagnostic tests in Singapore via provisional authorisation, Qual tech: medical device regulatory & CRO, Singapore, pp 1–7, 202.
Health Canada’s regulatory response to COVID-19: access to health products. Government of Canada, Canada. Available via https://www.canada.ca/en/health-canada/services/drugs-health-products/covid19-industry/regulatory-response-health-product-access.html
Indian Council of Medical Research (ICMR). Validation of SARS-COV2 diagnostic commodities: acceptance criteria, p 1, 202. Available via https://www.icmr.gov.in/pdf/covid/kits/Acceptance_Criteria_SARSCoV2_diagnostic/_commodities_10092020.pdf (Accessed 9 October 2020).
In-vitro Diagnostics. Central Drugs Standard Control Organization. Government of India, India. Available via https://cdsco.gov.in/opencms/opencms/en/Medical-Device-Diagnostics/InVitro-Diagnostics/
Kits Validation & Batching Testing. ICMR, New Delhi, India. Available via https://www.icmr.gov.in/ckitevaluation.html
Matheeussen V, Corman VM, Mantke OD, McCulloch E, Lammens C, Goossens H, Niemeyer D, Wallace PS, Klapper P, Niesters HG, Drosten C. International external quality assessment for SARS-CoV-2 molecular detection and survey on clinical laboratory preparedness during the COVID-19 pandemic, April/May 2020. Euro Surveilll, 2020; 25(27):2001223. CrossRef
Medicines & Healthcare Products Regulatory Agency (MHRA). Specification criteria for serology point of care tests and self-tests, pp 1–12, 2020.
Medical devices for uses related to COVID-19. Government of Canada, India. Available via https://www.canada.ca/en/health-canada/services/drugs-health-products/covid19industry/medicaldevices/about.html#_Expedited_authorization_path
New Medical Device Rules. Ministry of Health and Family Welfare in the Official Gazette of India, New Delhi, India, 2017.
Petrillo M, Querci M, Tkachenko O, Siska IR, Ben E, Angers-Loustau A, Bogni A, Brunetto A, Fabbri M, Garlant L, Lievens A. Munoz A, Paracchini V, Pietretti D, Puertas-Gallardo A, Raffael B, Sarno E, Tregoat V, Zaro F, Van den Eede G. The EU one-stop-shop collection of publicly available information on COVID-19 in vitro diagnostic medical devices. F1000 Res, 2020; 9:1296; doi:10.12688/f1000research.27308.1.. CrossRef
Rapid Antigen Test Kits for COVID-19 (Oropharyngeal / Nasopharyngeal swabs). ICMR, India. Available viahttps://www.icmr.gov.in/pdf/covid/kits/archive/List_of_rapid_antigen_kits_11022021.pdf (Accessed 2 November 2021)
US Department of Health and Human Services Food and Drug Administration, Center for Devices and Radiological Health. Policy for Coronavirus Disease-2019 Tests During the Public Health Emergency, Immediately in Effect Guidance for Clinical Laboratories, Commercial Manufacturers, and Food and Drug Administration Staff, pp 1–41, 2020.
US-FDA. Bio-Rad Reliance SARS-CoV-2/FluA/FluB RT-PCR assay kit, emergency use authorization. EUA202965,, pp 1–8, 2021.
Validation Kit Portal. ICMR, India. Available via https://cvtestkit.icmr.org.in/login.php