INTRODUCTION
Inflammation is a cellular event in response to infection and tissue damage. The response of inflammation in increasing vascular permeability and migration of immune cells at the damaged site is also characterized by the production of nitric oxide (NO) and cytokines, such as tumor necrosis factor (TNF-α) and interleukin-6 (IL-6) (Kim et al., 2015). Production of more proinflammatory molecules and nitric oxide (NO) may damage the structure, function, and integrity of lipids, proteins, and nucleic acids, leading to various chronic diseases (Ben-Baruch, 2006). These inflammatory mediators are widely involved in the pathogenesis of human diseases (Chen et al., 2018). Traditional medicine with a holistic concept that has been tested in clinical practice has a definite therapeutic effect. Compared to Western medicine, it is less toxic in treating various diseases, especially complex chronic diseases (Yuan et al., 2017). Ethnomedicine often uses blended ingredients of several plant extracts to exploit the biological activity of different phytochemical assemblages through synergistic interactions (Komape et al., 2017; Liu et al., 2016; Williamson, 2001).
Research on the interactions between synergistic phytomedicines plays a very important role because it explains the efficacy of low doses of active constituents in herbal products. The concept of synergistic interaction suggests that plant extracts offer more advantages than isolated single ingredients (Williamson, 2001). There is evidence supporting synergies in phytomedicines; for example, the use of a combination of extracts made of Strophanthus hispidus (root) and Aframomum melegueta (seeds) significantly provides a synergistic anti-inflammatory effect (John Kenneth et al., 2019). The combination of Ocimum bacilicum L. leaf extract and Syzygium polyanthum is more effective as an anti-inflammatory agent than each used separately (Sukmawati et al., 2018). There was also a similar report regarding the combination of Euphorbia hirta L. (herb) and Carica papaya L. (leaf) extracts (Dermiati et al., 2018).
Curcuma xanthorrhiza has long been used as a traditional medicine and has been confirmed to have pharmacological properties such as anti-inflammatory effects (Rahmat et al., 2021). Although C. xanthorrhiza has demonstrated anti-inflammatory properties, clinical trials have shown that curcumin exhibits poor bioavailability. An approach to solving this problem is to combine two or more phytochemicals to obtain a synergistic effect. Therefore, there is no need to increase the dose (Anand et al., 2007; Zhang et al., 2019). One way to increase its anti-inflammatory activity is to combine it with other plants such as Physalis angulata. This plant is easy to grow in the tropics and has been used in different countries in the treatment of various diseases such as inflammatory diseases, including dermatitis and arthritis, pain, malaria, leishmania, asthma, tuberculosis, fluid retention, and cancer (Saldago et al., 2012). The selection of the two plants was also based on taxonomic family differences. This will provide a diversity of phytoconstituents, and the interactions between phytochemicals will cause a synergistic effect.
This study aimed to investigate the combination of the two plant extracts in providing anti-inflammatory effects. The results of this study expectedly could provide information on strategies for treating inflammation. In this study, the anti-inflammatory activity of the extracts made of C. xanthorrhiza and P. angulata was studied using lipopolysaccharide- (LPS-) stimulated RAW 264.7 macrophages. LPS-stimulated macrophages have been widely used to study anti-inflammatory compounds in vitro (Dong et al., 2017). The synergistic effect of the combination of these extracts was calculated using the combination index (CI) equation (Chou, 2010). This study provides scientific evidence regarding the use of a combination of herbal medicines to enhance anti-inflammatory effects.
MATERIALS AND METHODS
Materials
Curcuma xanthorrhiza rhizomes were collected in March 2020 in Singaraja, while P. angulata herbs were collected in April in the same year in Gianyar. Both regencies are in Bali, Indonesia. The two plants were identified at the Bali Botanical Gardens, Indonesian Institute of Sciences, with plant identification numbers of B-198/IPH.7/AP/VII/2020 for C. xanthorrhiza and B-199/IPH.7/AP/VII/2020 for P. angulata. Curcumin standard was obtained from the isolation carried out by Prof. apt. Suwijiyo Pramono., DEA, with 88.65% purity. Quercetin (Sigma), Folin–Ciocalteu reagent, aluminum chloride, vanillin sulfuric acid reagent, potassium hydroxide, Liebermann–Burchard reagent, ferric chloride reagent, Dragendorff reagent, gallic acid, hydrochloric acid, Thin Layer Chromatography (TLC) plate of silica gel 60 F254, toluene, ethanol, methanol, chloroform, acetone, acetic acid, diethyl ether, n-hexane, and ethyl acetate were purchased from Merck.
The RAW 264.7 murine macrophages were obtained from the Cancer Chemoprevention Research Center, Faculty of Pharmacy, Universitas Gadjah Mada. Other materials used were Dulbecco’s modified Eagle’s media (DMEM) (Gibco), fetal bovine serum (FBS) (Sigma), phosphate-buffered saline (PBS) (Sigma), penicillin-streptomycin (Gibco), Trypsin-Ethylenediaminetetraacetic acid (EDTA) (Gibco), [3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl tetrazolium bromide] (MTT) (Bio Basic), LPS from Escherichia coli (Sigma), sodium dodecyl sulfate (SDS) (Merck), TNF-α and IL-6 enzyme-linked immunosorbent assay (ELISA) kit (Sigma), and dexamethasone (Kimia Farma).
Equipment
The equipment used consisted of TLC-Densitometry CAMAG TLC Scanner 3, CAMAG Linomat 5 Sample Applicator, Spectrophotometry Hitachi UH5300, Inverted Microscope Leica/DM IL LED, CO2 Incubator Thermo Science-341/8000DH, Cytotoxic Safety Cabinet LABCONCO Purifier Class II, Microplate Reader TECAN SPARK 20M, and ELISA Reader Bio-Rad 680XR, Centrifuge Mini Refrigerated Eppendorf 5424R.
Extraction
The C. xanthorrhiza rhizomes and P. angulata herbs were cleaned under running water and drained at room temperature separately. After the C. xanthorrhiza rhizomes were sliced thinly and P. angulata was cut, they were then oven-dried at 50°C for 2 days and ground to a fine powder. The dry powder of both plant materials was extracted using the maceration method. One kilogram of dry plant powder was macerated with 10 l of ethanol (70%) for 24 hours, and the mixture was stirred occasionally. This process was repeated three times. The collected macerate was then concentrated using a rotary evaporator and put into a desiccator (Kemenkes Republik Indonesia, 2017).
Characterization of the extracts
The characteristics of the ethanolic extract of C. xanthorrhiza (EECX), including the contents of curcumin and essential oil, were determined. The total phenolic and flavonoid contents of the ethanolic extract of P. angulata (EEPA) were determined. The extraction yield and moisture content of both extracts were also determined. All the extract characteristic parameters were assessed in accordance with the Indonesian Herbal Pharmacopoeia (Kemenkes Republik Indonesia, 2017). Preliminary screening for secondary metabolites such as steroids, phenols, alkaloids, anthraquinones, saponins, flavonoids, and monoterpenes was carried out according to Indonesian Herbal Pharmacopoeia and Harborne (1998). A test solution was prepared by dissolving 10 mg of the extract with 10 ml of chloroform–ethanol (1:1) v/v. The eluted TLC plate was then tested with various spray reagents. The color of the spots was observed under ultraviolet (UV 254 nm and UV 365 nm) and visible light (Wagner and Bladt, 2001).
Cell culture
RAW 264.7 cells were grown in DMEM with 10% FBS supplementation and a 2% penicillin-streptomycin solution. The cell cultures were incubated at 37°C in a humidified atmosphere of 5% CO2 until the cells were confluent.
Anti-inflammatory activity on LPS-activated cells
For TNF-α and IL-6 determinations, RAW 264.7 cells were seeded in a 24-well plate with a cell density of 1.5 × 105 cells per well. The cell line was incubated in a 37°C and 5% CO2 incubator. After 24 hours of incubation, the supernatant was discarded and the adherent cells were washed with PBS. RAW 264.7 cells were incubated with LPS from E. coli at 1 μg/ml. In addition, nontoxic concentrations of EECX and EEPA and controls (0.5% Dimethyl sulfoxide (DMSO) and 5 μg/ml dexamethasone) were added to the wells. After 24 hours, the cells or their supernatants were collected and analyzed.
Measurement of nitric oxide (NO) concentration
The cell supernatant was mixed with an equal volume of Griess reagent, 1% sulfanilamide (Sigma), and 0.1% N-(1-naphthyl)ethylenediamine dihydrochloride (Sigma) in 2.5% phosphoric acid (Merck) and incubated at room temperature for 15 minutes. Sodium nitrite (Sigma) was used as the reference standard. The nitrite concentration was then determined at a wavelength of 540 nm (Kim et al., 2015).
Measurement of proinflammatory cytokines TNF-α and IL-6
The TNF-α and IL-6 levels in the cell supernatants were quantified using sandwich-enzyme immunoassays, in accordance with the manufacturer’s protocol (Sigma) with catalog Nos. RAB0477-IKT for TNF-α and RAB308-IKT for IL-6.
Evaluation of cell viability
The cell viability was determined by the 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. The supernatant was taken for analysis, and then each well was washed using 500 μl PBS/well. After this, a 500 μl MTT solution was added to each well, which was then incubated for 4 hours in a 37°C CO2 incubator. Formazan crystal formation was observed under an inverted microscope. After formazan crystals were formed, a 500 μl/well stopper reagent was added (10% SDS in 0.1 N HCl) and incubated at room temperature in the dark overnight. Cell density was determined by reading the absorbance at a wavelength of 550 nm (Braga et al., 2019; Kim et al., 2015). The percentage of viable cells was calculated using the following formula:
Inhibitory activity of inflammatory mediators
The inhibitory concentration (IC50) value of all samples was calculated based on the linear regression equation y = bx + a, which was generated from the plot of sample concentration versus %inhibition. The percentage of inhibition of the formation of NO, TNF-α, and IL-6 in each sample was obtained using the following formula:
The value of An was obtained from the absorbance of the negative control (0.5% DMSO with 1 μg/ml LPS) minus the absorbance of the control (0.5% DMSO without LPS), while the value of As was obtained from the absorbance of the sample minus the absorbance of the control (0.5% DMSO without LPS).
Determination of anti-inflammatory synergism
The combined effect was determined based on the combination of the IC50 of every single extract. For this purpose, a series of solution concentrations was made, resulting in inhibition percentages of 12.5%, 25%, 37.5%, and 50%. The four concentrations were combined based on the checkerboard method to produce a total of 16 combinations. The combinations were then tested to obtain the percentage of the inhibition of each proinflammatory mediator parameter. The CI was used to determine whether a combination provided a synergistic effect. The CI was calculated using the following formula:
CI is the combination index, where CI = 1 indicates additive effect, CI < 1 synergism, and C > 1 antagonism. (D)1 and (D)2 are the concentration of each combined extract that produces the x effect, while (Dm)1 and (Dm)2 are the concentration of every single extract that produces the x effect.
RESULTS AND DISCUSSION
The chemical compositions of the extracts made of these two species varied in both quality and quantity due to environmental factors that affect plant metabolism (Rahmat et al., 2021; Sandeep et al., 2015; Zhang et al., 2016). The presence of secondary metabolites in both plants was determined, and the results are summarized in Table 1. Several secondary metabolites have been identified in the genus Physalis, such as alkaloids, flavonoids, saponins, glycosides, terpenoids, tannins, physalins, and withanolides (Ferreira et al., 2019). Ethanol organic extracts have shown the presence of more groups of compounds than aqueous extracts. The EECX mainly consisted of terpenoids, phenols, flavonoids, saponins, cardiac glycosides, alkaloids, and coumarins (Halim et al., 2012). The characteristics of the EECX and EEPA can be seen in Table 2. Based on the spectrophotometric analysis, the total phenol and flavonoid contents on EEPA were 8.172% ± 0.340% Gallic acid equivalents (GAE) and 0.513% ± 0.014% Quercetin equivalents (QE), respectively. Various biological activities attributed to Physalis are due to the different metabolic and structural compounds in this plant (Bhat et al., 2008). The levels of total phenolics, flavonoids, and the concentration of some of the individual flavonols were significantly correlated to antioxidant properties (Cobaleda-Velasco et al., 2017). The curcumin content in EECX was analyzed with thin-layer chromatography with curcumin as a standard; the curcumin content in the extract was measured with the winCATS software version 1.4.3.6336. The analyte concentration was determined from the intensity of the reflected light, which was indicated as the peak area. These results were then correlated with the analyte concentrations using the standard curcumin. The mobile phase used n-hexane: ethyl acetate (1:1 v/v). The Retardation factor (Rf) value of the curcumin compound was reported as 0.46 (Fig. 1). The curcumin content in EECX was 5.991% ± 0.0903%. The essential oil content was obtained by a distillation method, with the result of 6.429% ± 0.119% ml/g. Curcumin yellow pigment is a phenolic compound and the main phytochemical constituent of the Curcuma species (Pulido-Moran et al., 2016). The essential oil extracted from the C. xanthorrhiza rhizomes showed that xanthorrhizol was the main compound, followed by camphene pinene, thujene, and myrcene (Mary et al., 2012). Curcumin and xanthorrhizol potent natural bioactive compounds have protective health effects mainly through anti-inflammatory and antioxidant mechanisms (Oon et al., 2015; Pulido-Moran et al., 2016).
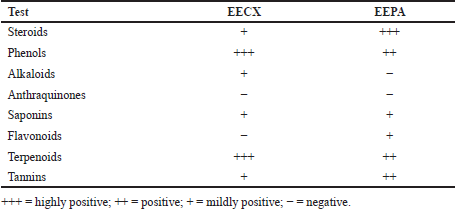 | Table 1. Preliminary screening of secondary metabolites from EECX and EEPA. [Click here to view] |
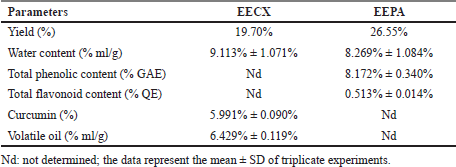 | Table 2. The characteristics of the EECX and EEPA. [Click here to view] |
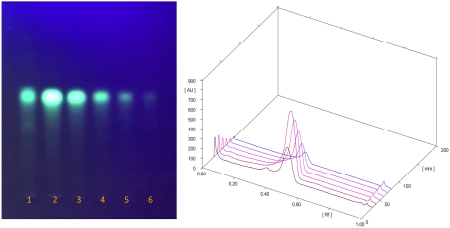 | Figure 1. TLC profile of EECX (Track 1) curcumin standard at different concentrations: 188, 94, 47, 23.5, and 11.75 μg/ml (Tracks 2–6) 3D view of densitogram at 425 nm. [Click here to view] |
In previous research, both plants were indicated to have a cytotoxic activity. The phytochemical content of P. angulata has cytotoxic activity in A549 (human non-small cell lung cancer cell lines), Hela (human cervical cancer cell lines), and p388 (human leukemia cell lines) (Meng et al., 2019). Preclinical studies have shown that curcumin has a cytotoxic activity against various types of cancer cells by modulating multiple molecular targets (Bimonte et al., 2016). Before testing the activity of EECX and EEPA in vitro cell assays, a cytotoxic analysis of both extracts was performed. As shown in Figure 2, the cell viability decreased as the concentration of the extract increased. The results showed that the concentration required for RAW 264.7 cells to have 80% viability for each of the EECX extracts was 24.411 ± 2.214 μg/ml and that of EEPA was 26.062 ± 2.062 μg/ml. For this reason, a concentration of 20 μg/ml was used to evaluate the anti-inflammatory activity of each extract. To evaluate the anti-inflammatory activity, the ability to inhibit the production of NO, TNF-α, and IL-6 using LPS-stimulated RAW 264.7 cells of both extracts was tested. LPS is a component of the outer cell membrane of Gram-negative bacteria that induce macrophages to produce proinflammatory mediators (Lee et al., 2017; Park et al., 2014).
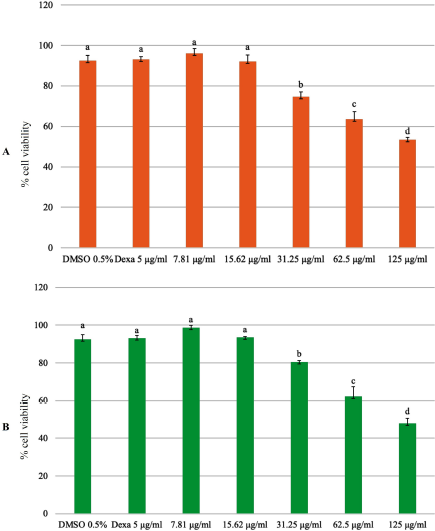 | Figure 2. Cell viability assay using the MTT assay; RAW 264.7 cells were treated with (A) EECX and (B) EEPA at different concentrations (7.81, 15.62, 31.25, 62.5, and 125 μg/ml). The control group was cultured in the presence of 0.5% of DMSO and dexamethasone 5 μg/ml (Dexa). Error bars represent the standard deviation (n = 3). Different letters (a–d) indicate significant differences in analysis of variance (ANOVA), followed by Tukey’s test (p < 0.05). [Click here to view] |
After being activated by endotoxins or cytokines, macrophages produce NO, which can help kill and inhibit the growth of microorganisms. However, the presence of excess NO may cause the development of cancer (McAdam et al., 2012). TNF-α is a cytokine mainly produced by monocytes, macrophages, and T cells and is triggered by endotoxins from other pathogens or substances. TNF-α is a strong inducer for IL-1, IL-2, and IL-6 (Kitaura et al., 2013). In addition, TNF-α is involved in various inflammatory and infectious diseases. At the cellular level, TNF-α strengthens body responses such as inflammation, immunoregulation, proliferation, apoptosis, and antiviral activity (Lam et al., 2000). When infection or tissue injury occurs, IL-6 is immediately produced by monocytes and macrophages. IL-6 contributes to the elimination of infectious agents and the restoration of damaged tissues through the activation of immune responses, but excessive and uncontrolled IL-6 production plays a pathological role in the development of various inflammatory diseases (Tanaka et al., 2016). The release of proinflammatory mediators is like a double-edged sword for the host. If not controlled, this may lead to the development of various chronic diseases.
Curcuma xanthorrhiza rhizomes have long been used in traditional medicine and to treat diseases. In this study, EECX can inhibit the production of proinflammatory mediators (Fig. 3). Research using extracts of C. xanthorrhiza and xanthorrhizol was carried out to prevent the recruitment of immune cells to adipose tissue through the downregulation of inflammatory cytokine genes (Kim et al., 2014). In another study, the methanol extract made of C. xanthorrhiza rhizomes has the activity of inhibiting the production of nitric oxide (NO) in LPS-induced RAW 264.7 cells where the active compounds are curcuminoids and xanthorrhizol (Park et al., 2014). Curcuminoids vary in their ability to suppress Nuclear Factor Kappa Beta (NF-κβ) activation. Curcumin has the highest activity of inhibiting TNF-α compared to demethoxycurcumin and bisdemethoxycurcumin (Sandur et al., 2007). Xanthorrhizol can reduce the production of TNF-α, IL-6, and IL-1β by turning off the activation of NF-κβ, thus providing an anti-inflammatory effect (Oon et al., 2015). The mechanism of curcumin in lowering the proinflammatory cytokine levels is by inhibiting NF-κβ activation while increasing anti-inflammatory cytokines. Curcumin drives antioxidant defense mechanisms by increasing the transcription and expression levels of antioxidant enzymes and by enhancing mitochondrial function (Gounden et al., 2017; Trujillo et al., 2014).
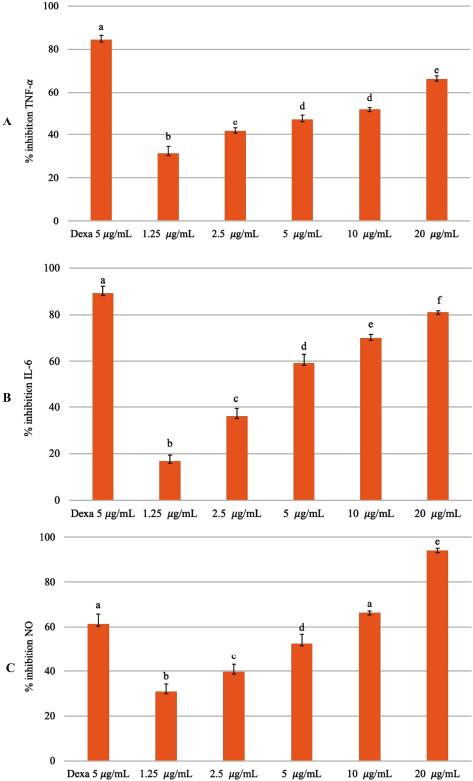 | Figure 3. Inhibitory percentage against (A) TNF-α, (B) IL-6, and (C) NO from LPS-stimulated RAW 264.7 cells treated with different concentrations of EECX (1.25, 2.5, 5, 10, and 20 μg/ml). The positive control group: dexamethasone 5 μg/ml (Dexa). Error bars represent the standard deviation (n = 3). Different letters (a–f) indicate significant differences in ANOVA, followed by Tukey’s test (p < 0.05). [Click here to view] |
The plant P. angulata produces a triterpenoid withanolide, which contains a steroid lactone with 28 carbon atoms (Huang et al., 2020; Tuan Anh et al., 2021). These compounds have antimicrobial, anticancer, anti-inflammatory, hepatoprotective, and immunomodulatory properties (Saldago et al., 2012; Sun et al., 2017b). The pharmacological activity of EEPA can reduce NO production because the aqueous extract of P. angulata is associated with inhibition of nitric oxide synthase (Bastos et al., 2008). The physalin E component of P. angulata can suppress TNF-α and IL-6 cytokines by inhibiting NF-κβ activation (Yang et al., 2017). Physalin B, F, G compounds contained in P. angulata may inhibit the production of TNF-α in vitro and in vivo. The protective effect of physalin not only inhibits the action of TNF-α but also suppresses other cytokines such as IL-6, IL-12, and NO (Soares et al., 2003). This is in accordance with previous studies, in which the EEPA can suppress the production of inflammatory mediators (Fig. 4). The ethanol extract of P. angulata can also inhibit COX-2 activity in MCF-7 cells (Sutrisna et al., 2012).
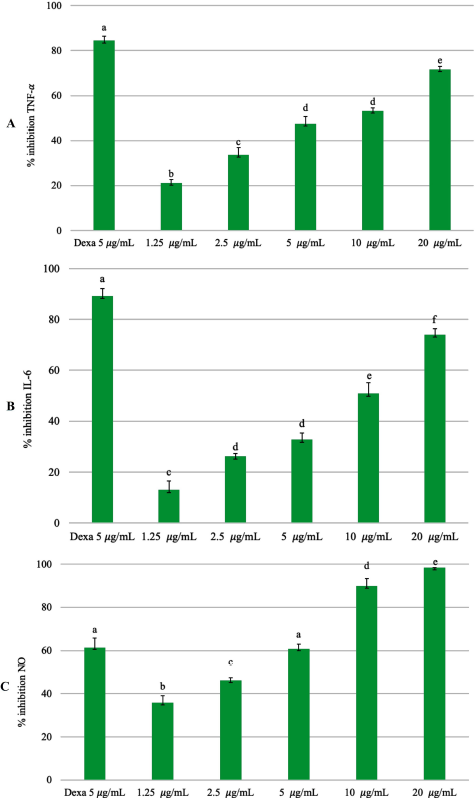 | Figure 4. Inhibitory percentage against (A) TNF-α, (B) IL-6, and (C) NO from LPS-stimulated RAW 264.7 cells treated with different concentrations of EEPA (1.25, 2.5, 5, 10, and 20 μg/ml). The positive control group: dexamethasone 5 μg/ml (Dexa). Error bars represent the standard deviation (n = 3). Different letters (a–f) indicate significant differences in ANOVA, followed by Tukey’s test (p < 0.05). [Click here to view] |
Both extracts were able to reduce the production of inflammatory mediators. The quantification of inflammatory mediators such as the levels of NO, IL-6, and TNF-α in the supernatant from LPS-stimulated RAW 264.7 cells can be seen in Table 3. The quantification of the inflammatory mediator levels for the positive control (dexamethasone 5 µg/ml with LPS 1 µg/ml) was as follows: TNF-α = 823.60 ± 244.221 pg/ml; IL-6 = 107.706 ± 5.463 ng/ml; and NO = 3.049 ± 0.181 µM. The quantification of the inflammatory mediator levels for the negative control (DMSO 0.5% with LPS 1 µg/ml) was as follows: TNF-α = 4,713.600 ± 424.276 pg/ml; IL-6 = 341.706 ± 50.634 ng/ml; and NO = 6.325 ± 0.133 µM. The quantification of the inflammatory mediator levels for the control (DMSO 0.5% without LPS 1 µg/ml) was as follows: TNF-α = 110.933 ± 59.003 pg/ml; IL-6 = 82.640 ± 12.931 ng/ml; and NO = 0.991 ± 0.314 µM.
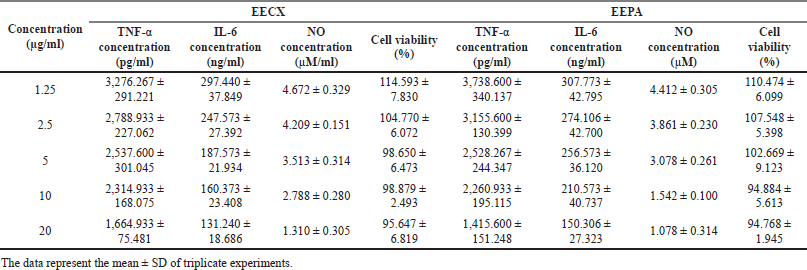 | Table 3. The viability and quantification of inflammatory mediators in supernatants from LPS-stimulated RAW 264.7 cells. [Click here to view] |
 | Table 4. The IC50 values of inflammatory mediator inhibition. [Click here to view] |
The inhibition activities against TNF-α, IL-6, and NO in EECX and EEPA were lower than that of the positive control (dexamethasone 5 µg/ml), and interestingly the inhibition against NO production in the extract (doses 10 µg/ml and 20 µg/ml) was greater than that of the positive control. This was possibly due to the presence of a group of phenolic compounds and flavonoids in the extract, which have a strong antioxidant activity (Fakhrudin et al., 2016). The presence of reactive oxygen species (ROS) has a fatal effect on oxygen toxicity and cellular dysfunction that exacerbate inflammation. Therefore, antioxidant activity is required to remove ROS (Lee et al., 2015). Curcuma xanthorrhiza rhizomes, containing curcumin and xanthorrhizol, and P. angulata, containing phenol glycoside, are potential for anti-inflammatory treatment because they can inhibit NO production (Park et al., 2014; Sun et al., 2017a). The results of the anti-inflammatory activity test of each extract determined the IC50 value (Table 4) for each inhibition against NO, TNF-α, and IL-6 production.
The combination of natural compounds present in EECX and EEPA was shown to increase the effectiveness of the anti-inflammatory effect due to the synergistic effect (CI < 1). The percentage of inhibition of inflammatory mediators also increased with increasing concentrations, but unfortunately, there was a decrease in cell viability, especially in the TNF-α and IL-6 assays (Table 5). The use of the combination of these two extracts at high doses can cause toxic effects to RAW 264.7 cells. The NO test showed cell viability above 80% because the dose used was relatively lower than the dose in the TNF-α and IL-6 tests. Interestingly, the combination of EECX and EEPA with cell viability above 80% had more synergistic than antagonistic effects.
The combination test of EECX and EEPA extracts showed a synergistic effect, along with an increase in the concentration ratio. The mechanism of why synergism occurs is probably due to antioxidant regeneration. The combination of curcumin and quercetin provides anti-inflammatory and antioxidant effects that also fight oxidative stress (Abdel-Diam et al., 2019). The role of oxidative stress is very important because it is responsible for chronic development and chronic disease (Miller et al., 2018). The synergistic effect may result from the increase in antioxidant capacity by protecting each other from oxidative agents and differences in the solubility of the phytochemical constituents (Becker et al., 2007).
Chemical compounds have very specific interactions with inflammatory markers and signaling pathways. The use of the combination reached the threshold level of signaling pathway activation, which cannot be reached by single components (Zhang et al., 2019). This combination also had an effect in regulating NF-κβ; high NF-κβ will affect the expression of proinflammatory cytokines (Güran et al., 2019). The NF-κβ pathway is responsible for the development of chronic inflammation as activated NF-κβ increases the production of cytokines, chemokines, and adhesion molecules as well as leukocyte recruitment. Therefore, weakening the NF-κβ pathway can be a treatment target (Lawrence, 2009).
A combination with a low concentration ratio did not show a synergistic effect. However, at a high concentration ratio, the NO inhibition test caused an antagonistic effect (CI > 1). Antagonistic effects were also found in this combination. This may be because of the presence of phenolic and flavonoid compounds in the combined extract, causing the antioxidant regeneration effect to decrease and causing the two to compete. One of the appropriate reasons is the interference with each other that may cause a reduction in individual activity. Complex stoichiometric factors should be investigated further because they can affect antioxidant activity (Peyrat-Maillard et al., 2003). Hydroxy groups are protected from steric effects that, in combination with curcumin, impair removal capacity and reduce activity (Naksuriya and Okonogi, 2015).
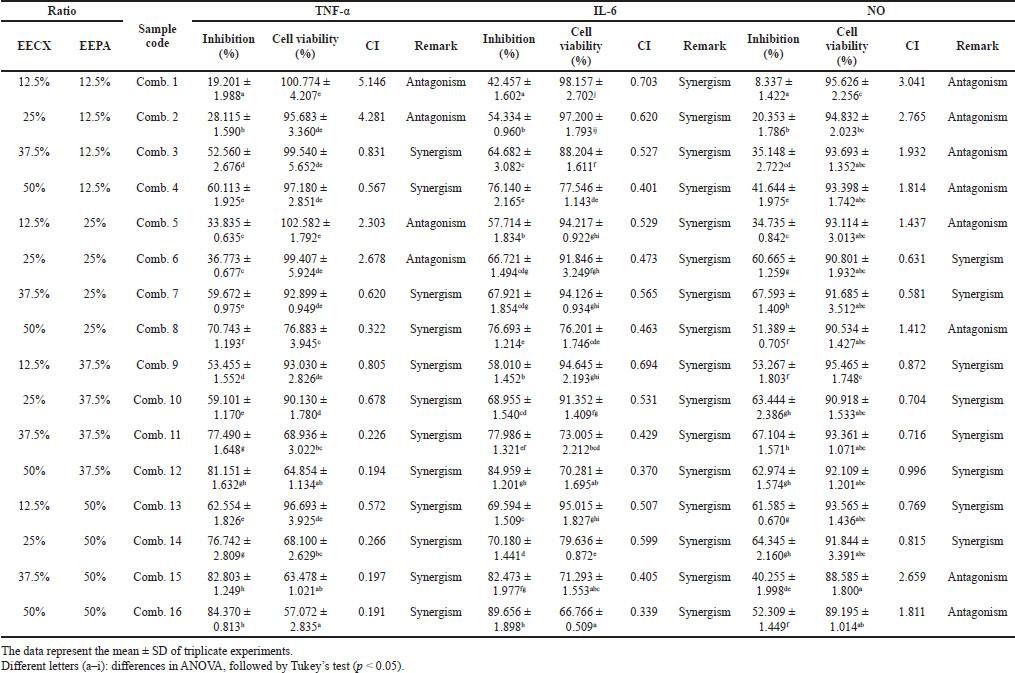 | Table 5. The inhibitory activity of inflammatory mediators and cell viability in the combination of EECX and EEPA. [Click here to view] |
CONCLUSION
Both extracts showed inhibitory effects against proinflammatory mediators such as TNF-α, IL-6, and NO in vitro tests. The combination of these extracts produced a synergistic effect in inhibiting inflammatory mediators. Further tests need to be conducted to see the effectiveness and toxic effects, so it can be used as an alternative treatment for chronic inflammatory diseases.
ACKNOWLEDGMENTS
The authors would like to acknowledge the funding support from UGM No. 2488/UN1.PIII/DIT-LIT/PT/2020.
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FUNDING
The authors would like to acknowledge the funding support from UGM No. 2488/UN1.PIII/DIT-LIT/PT/2020.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abdel-Diam MM, Samak DH, El-Sayed YS, Aleya L, Alarifi S, Alkahtani S. Curcumin and quercetin synergistically attenuate subacute diazinon-induced inflammation and oxidative neurohepatic damage, and acetylcholinesterase inhibition in albino rats. Environ Sci Pollut Res Int, 2019; 26(4):3659–65. CrossRef
Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm, 2007; 4(6):807–18. CrossRef
Bastos GNT, Silveira AJA, Salgado CG. Physalis angulata extract exerts anti-in?ammatory effects in rats by inhibiting different pathways. J Ethnopharmacol, 2008; 118:246–51. CrossRef
Becker EM, Ntouma G, Skibsted LH. Synergism and antagonism between quercetin and other chain-breaking antioxidants in lipid systems of increasing structural organisation. Food Chem, 2007; 103(4):1288–96. CrossRef
Ben-Baruch A. Inflammation-associated immune suppression in cancer: the roles played by cytokines, chemokines and additional mediators. Semin Cancer Biol, 2006; 16(1):38–52. CrossRef
Bhat N, Jeri L, Mipun P, Kumar Y. Systematic studies (micro-morphological, leaf architectural, anatomical and palynological) of genus Physalis L. (Solanaceae) in Northeast India. Plant Arch, 2008; 18(2):2229–38.
Bimonte S, Barbieri A, Leongito M, Piccirillo M, Giudice A, Pivonello C, de Angelis C, Granata V, Palaia R, Izzo F. Curcumin anticancer studies in pancreatic cancer. Nutrients, 2016; 8(7):433. CrossRef
Braga MA, Rodrigues R de O, Yaochite JNU. Pro-inflammatory activity of Astronium fraxinifolium Schott on lipopolysaccharide-stimulated RAW 264.7 cells. J App Pharm Sci, 2019; 9(12):30–6. CrossRef
Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, Li Y, Wang X, Zhao L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget, 2018; 9(6):7204–18. CrossRef
Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res, 2010; 70(2):440–6. CrossRef
Cobaleda-Velasco M, Alanis-Bañuelos R, Almaraz N, Rojas-López M, Gonzalez L, Ávila-Reyes J, Morales Rodrigo S. Phenolic profiles and antioxidant properties of Physalis angulata L. as quality indicators. J Pharm Pharmacogn Res, 2017; 5:114–28.
Dermiati CN, Tandi J. Uji Efek Antiinflamasi Dan Analgesik Kombinasi Ekstrak Etanol Herba Patikan Kebo (Euphorbia Hirta L) Dan Daun Pepaya (Carica papaya L) Pada Tikus Putih Jantan. Farmakol J Farm, 2018; 15(2):98–105.
Dong L, Zhang Y, Wang X, Dong YX, Zheng L, Li YJ, Ni JM. In vivo and in vitro anti-inflammatory effects of ethanol fraction from Periploca forrestii Schltr. Chin J Integr Med, 2017; 23(7):528–34. CrossRef
Fakhrudin N, Nisa K, Azzahra A, Ajiningtyas RJ. Study of radical scavenger activity, total phenol and flavonoid contents of Artocarpus altilis leaves extracts. Int J Pharm Clin Res, 2016; 8:352–6.
Ferreira L dos SL, do Vale A, de Souza A, Leite K, Sacramento C, Moreno MV, Araújo TH, Soares MB, Grassi MF. Anatomical and phytochemical characterization of Physalis angulata L.: a plant with therapeutic potential. Pharmacogn Res, 2019; 11(2):171–7. CrossRef
Gounden S, Chuturgoon A. Curcumin upregulates antioxidant defense, lon protease, and heat-shock protein 70 under hyperglycemic conditions in human hepatoma cells. J Med Food, 2017; 20(5):465–73. CrossRef
Güran M, ?anl?türk G, Kerküklü NR, Altunda? EM, SühaYalç?n A. Combined effects of quercetin and curcumin on anti-inflammatory and antimicrobial parameters in vitro. Eur J Pharmacol, 2019; 859(172486):1–6. CrossRef
Halim M, Muhammad Zabri Tan MS, Ismail S, Mahmud R. Standardization and phytochemical studies of Curcuma xanthorrhiza Roxb. Int J Pharm Pharm Sci, 2012; 4:606–10.
Harborne JB. Phytochemical methods a guide to modern techniques of plant analysis. Springer Science & Business Media, Berlin, Germany, 1998.
Huang M, He JX, Hu HX, Zhang K, Wang XN, Zhao BB, Lou HX, Ren DM, Shen T.. Withanolides from the genus Physalis?: a review on their phytochemical and pharmacological aspects. J Pharm Pharmacol, 2020; 72(5):649–69. CrossRef
John Kenneth M, Amina I, Yakubu J. Co-extract mixture from Strophanthus hispidus (roots) and Aframomum meleguta (seeds) show phytochemical synergy in its anti-inflammatory activity. Arch Pharm Pharm Sci, 2019; 3(1):089–100. CrossRef
Kemenkes Republik Indonesia. Farmakope herbal Indonesia. 2nd edition, Kementrian Kesehatan Republik Indonesia, Jakarta, Indonesia, 2017.
Kim MB, Kim C, Song Y, Hwang JK. Antihyperglycemic and anti-inflammatory effects of standardized Curcuma xanthorrhiza Roxb. extract and its active compound xanthorrhizol in high-fat diet-induced obese mice. Evid Based Complement Alternat Med, 2014; 2014:205915. CrossRef
Kim S, Ko H, Hyun CG, Baik J, Bu H, Lee N. Anti-inflammatory constituents from branches of Corylus hallaisanensis Nakai. J App Pharm Sci, 2015; 5(12):162–6. CrossRef
Kitaura H, Kimura K, Ishida M, Kohara H, Yoshimatsu M, Takano-Yamamoto T. Immunological reaction in TNF-α-mediated osteoclast formation and bone resorption in vitro and in vivo. Clin Dev Immunol, 2013; 2013:1–8. CrossRef
Komape NPM, Bagla VP, Kabongo-Kayoka P, Masoko P. Anti-mycobacteria potential and synergistic effects of combined crude extracts of selected medicinal plants used by Bapedi traditional healers to treat tuberculosis related symptoms in Limpopo Province, South Africa. BMC Complement Altern Med, 2017; 17(1):128. CrossRef
Lam J, Takeshita S, Barker JE, Kanagawa O, Ross FP, Teitelbaum SL. TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest, 2000; 106(12):1481–8. CrossRef
Lawrence T. The nuclear factor NF- B pathway in inflammation. Cold Spring Harbor Perspect Biol, 2009; 1(6):1–10. CrossRef
Lee HA, Koh EK, Sung JE, Kim JE, Song SH, Kim DS, Song SH, Kim DS, Son HJ, Lee CY, Lee HS, Bae CJ, Hwang DY. Ethyl acetate extract from Asparagus cochinchinensis exerts anti?inflammatory effects in LPS?stimulated RAW264.7 macrophage cells by regulating COX?2/iNOS, inflammatory cytokine expression, MAP kinase pathways, the cell cycle and anti-oxidant activity. Mol Med Rep, 2017; 15(4):1613–23. CrossRef
Lee KJ, Oh YC, Cho WK, Ma JY. Antioxidant and anti-inflammatory activity determination of one hundred kinds of pure chemical compounds using offline and online screening HPLC assay. Evid Based Complement Alternat Med, 2015; 2015:165457. CrossRef
Liu Z, Luo Z, Jia C, Wang D, Li D. Synergistic effects of Potentilla fruticosa L. leaves combined with green tea polyphenols in a variety of oxidation systems. J Food Sci, 2016; 81(5):1091–101. CrossRef
Mary H, Gomathy S, Jayasree S, Nizzy AM, Rajagopal B, Jeeva S. Phytochemical characterization and antimicrobial activity of Curcuma xanthorrhiza Roxb. Asian Pac J Trop Biomed, 2012; 2:637–40. CrossRef
McAdam E, Haboubi HN, Forrester G, Eltahir Z, Spencer-Harty S, Davies C, Griffiths AP, Baxter JN, Jenkins GJ. Inducible nitric oxide synthase (iNOS) and nitric oxide (NO) are important mediators of reflux-induced cell signalling in esophageal cells. Carcinogenesis, 2012; 33(11):2035–43. CrossRef
Meng Q, Fan J, Liu Z, Li X, Zhang F, Zhang Y, Sun Y, Li L, Liu X, Hua E. Cytotoxic Withanolides from the whole herb of Physalis angulata L. Molecules, 2019; 24(8):1608. CrossRef
Miller MW, Lin AP, Wolf EJ, Miller DR. Oxidative stress, inflammation, and neuroprogression in chronic PTSD. Harvard Rev Psychiatry, 2018; 26(2):57–69. CrossRef
Naksuriya O, Okonogi S. Comparison and combination effects on antioxidant power of curcumin with gallic acid, ascorbic acid, and xanthone. Drug Discov Ther, 2015; 9(2):136–41. CrossRef
Oon SF, Nallappan M, Tee TT, Shohaimi S, Kassim NK, Sa’ariwijaya MSF, Cheah YH. Xanthorrhizol: a review of its pharmacological activities and anticancer properties. Cancer Cell Int, 2015; 15(100):1–15. CrossRef
Park JH, Jung YJ, Shrestha S, Lee SM, Lee TH, Lee CH, Han D, Kim J, Baek NI. Inhibition of NO production in LPS-stimulated RAW264.7 macrophage cells with curcuminoids and xanthorrhizol from the rhizome of Curcuma xanthorrhiza Roxb. and quantitative analysis using HPLC. J Korean Soc Appl Biol Chem, 2014; 57(3):407–12. CrossRef
Peyrat-Maillard MN, Cuvelier ME, Berset C. Antioxidant activity of phenolic compounds in 2,2′-azobis (2-amidinopropane) dihydrochloride (AAPH)-induced oxidation: synergistic and antagonistic effects. J Am Oil Chem Soc, 2003; 80(10):1007. CrossRef
Pulido-Moran M, Moreno-Fernandez J, Ramirez-Tortosa C, Ramirez-Tortosa M. Curcumin and health. Molecules, 2016; 21(3):1–22. CrossRef
Rahmat E, Lee J, Kang Y. Javanese turmeric (Curcuma xanthorrhiza Roxb.): ethnobotany, phytochemistry, biotechnology, and pharmacological activities. Evid Based Complement Alternat Med, 2021; 2021:1–15. CrossRef
Sandeep IS, Sanghamitra N, Sujata M. Differential effect of soil and environment on metabolic expression of turmeric (Curcuma longa cv. Roma). Indian J Exp Biol, 2015; 53(6):406–11
Saldago E, Arana G. Physalis angulata L. (Bolsa Mullaca): a review of its traditional uses, chemistry and pharmacology. Bol Latinoam Caribe Plant Med Aromat, 2012; 12(5):431–45.
Sandur S, Pandey M, Sung B. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis, 2007; 28(8):1765–73. CrossRef
Soares M, Tomassini TCB, Ribeiro dos Santos R, Bellintani M. Inhibition of macrophage activation and lipopolysaccaride-induced death by seco-steroids purified from Physalis angulata L. Eur J Pharmacol, 2003; 459(1):107–12. CrossRef
Sukmawati, Kosman R, Saharudin N. Kombinasi Ekstrak Etanol Daun Kemangi (Ocimum bacilicum L.) Dan Daun Salam (Syzygium polyanthum (Wight) Walp) Sebagai Antiinflamasi Pada Tikus (Rattus Norvegicus) Jantan Yang Diinduksi Karagen. As-Syifaa, 2018; 10(1):1–10. CrossRef
Sun CP, Nie XF, Kang N, Zhao F, Chen LX, Qiu F. A new phenol glycoside from Physalis angulata. Nat Prod Res, 2017a; 31(9):1059–65. CrossRef
Sun CP, Oppong MB, Zhao F, Chen LX, Qiu F. Unprecedented 22,26-seco physalins from Physalis angulata and their anti-inflammatory potential. Org Biomol Chem, 2017b; 15(41):8700–4. CrossRef
Sutrisna E, Indwianiastuti, Haryadi. The Ethanol Extract of Physalis angulata Linn Inhibits COX-2 Activity in MCF-7 Cell In Vitro. International Conference: Research and Application on Traditional Complementary and Alternative Medicine in Health Care (TCAM), Surakarta: 2012, p. 12–7.
Tanaka T, Narazaki M, Masuda K, Kishimoto T. Regulation of IL-6 in immunity and diseases. Adv Exp Med Biol, 2016; 941:79–88. CrossRef
Trujillo J, Granados-Castro LF, Zazueta C, Andérica-Romero AC, Chirino YI, Pedraza-Chaverrí J. Mitochondria as a target in the therapeutic properties of curcumin. Arch Pharm (Weinheim), 2014; 347(12):873–84. CrossRef
Tuan Anh HL, Le Ba V, Do TT, Phan VK, Pham Thi HY, Bach LG, et al. Bioactive compounds from Physalis angulata and their anti-inflammatory and cytotoxic activities. Journal of Asian Natural Products Research, 2021; 23(8):809-17. CrossRef
Wagner H, Bladt S. Plant drug analysis; a thin layer chromatography atlas. 2nd edition, Springer-Verlag Berlin Heidelberg, New York, NY, 2001.
Williamson EM. Synergy and other interactions in phytomedicines. Phytomedicine, 2001; 8(5):401–9. CrossRef
Yang YJ, Yi L, Wang Q, Xie BB, Dong Y, Sha CW. Anti-inflammatory effects of physalin E from Physalis angulata on lipopolysaccharide-stimulated RAW 264.7 cells through inhibition of NF-jB pathway. Immunopharmacol Immunotoxicol, 2017; 39(2):74–9. CrossRef
Yuan H, Ma Q, Cui H, Liu G, Zhao X, Li W, Piao G. How can synergism of traditional medicines benefit from network pharmacology? Molecules, 2017; 22(7):1135. CrossRef
Zhang L, Virgous C, Si H. Synergistic anti-inflammatory effects The data represent the mean ± SD of triplicate experiments.