INTRODUCTION
Tretinoin (TRT) is linked to various biological functions and has an essential function in treating pathological conditions and a significant impact on inflammatory mechanisms regulation and cancer prevention. The interaction of peroxisomes proliferator-activated receptors and retinoic acid receptor (RAR) enhances the cellular activities of TRT. Targeted genes’ postactivation receptor results in a homeostatic regulatory system which binds to RAR and normalizes keratinization and follicular epithelial differentiation. It also promotes follicular epithelial mitotic activity and loosely adherent corneocytes. Corneocyte shedding is the primary mechanism of comedolytic action.
Furthermore, polyphenolic curcumin (CUR), derived naturally, has anti-acne, psoriasis, dermatitis, wound healing, facial photoageing, anti-inflammatory, and anti-cancer properties (Desam and Rajab, 2021; Gillis and Goa, 1995; Kim and Weinkle, 2021; Kohli et al., 2005). When employed in a co-delivery or combinational strategy, this substance enhances cancer signaling pathways in diverse malignancies in melanoma tumors, reduces anti-cancer drug adverse effects, and alters efficacy (Batra et al., 2019). Controlled drug release has long been a problem, and nanotechnology-mediated drug delivery is now being employed to overcome it. Furthermore, it results in the co-administration of primary and adjuvant therapies, reducing or eliminating medication-related side effects, and improving drug performance. Nanocarrier-mediated delivery can help reduce skin discomfort associated with the immediate release of certain active pharmaceutical ingredients by regulating the release rate and improving the drug’s skin permeation, resulting in a more successful treatment outcome and fewer side effects. The nanoemulsion (NE) drug delivery technology has been intensively researched to improve the biopharmaceutical efficiency of poorly soluble topical medicines (Hussain et al., 2017; Thakur et al., 2013; Waghule et al., 2020). The ease with which it may be processed and manufactured and its long shelf-life have piqued interest in the development of NE-based topical treatments. NE is formed by spreading colloidal oil droplets ranging in size from 20 to 200 nm in an immiscible aqueous solution (Chen et al., 2011). Because the low viscosity of NE makes it difficult for the patients to apply it directly to their skin, integrating it into a hydrogel system is functional. TRT is encapsulated in an NE system, allowing deeper skin penetration while also shielding the skin from direct TRT contact (Lai et al., 2013). Incorporating TRT–CUR-loaded NE into the gelling system also enables for exact monitoring of TRT–CUR release from the NE gel system and a reduction in TRT-related skin reactions (Chen et al., 2020). The goal of this research is to improve topical TRT administration while minimizing unwanted side effects. TRT-NE was modified to improve TRT distribution to the skin. To help with skin responses, CUR was included in the formulation. This hydrogel formulation hydrates the skin while also increasing the efficacy of NE-mediated encapsulated medicinal delivery.
MATERIALS AND METHODS
Materials
TRT and CUR were procured from Cure Tech Skin Care, Himachal Pradesh, India. Oleic acid, Tween 80, propylene glycol, castor oil, span 60, ethanol, methanol, triethanolamine, carbopol 934, disodium hydrogen phosphate, and potassium di-hydrogen orthophosphate were purchased from Loba chemicals, Mumbai. All other chemicals were of analytical grade reagents.
Methods
TRT–CUR NE formulation
Design-Expert 12, the design of experts allowed the evaluation of important impacts without aliasing to other effects. It was used to figure out what the most important aspects were that influenced the responses (Beg et al., 2019; Singh et al., 2017). The amounts of oil X1 (10%–15%), surfactant X2 (10%–15%), and cosurfactant X3 (25%–30%) were used to fabricate NE as independent variable in the design. Based on the statistical data analysis, the Box–Behnken design (BBD) was employed; all three elements (X1, amount of oil, X2, quantity of surfactant, and X3, quantity of cosurfactant) were found to be significant (Bhoop et al., 2013). BBD was able to improve these analytical criteria even more (Table 1). BBD is a form of response surface design that is beneficial for controlling the experimental periphery and avoiding over-grouping. Three important factors and three levels were used to evaluate key effects, interaction effects, and quadratic effects in a BBD with 17 runs alongside 5 center point optimization designs.
The reproducibility of the following TRT–CUR–nanoemulgel (NEG) formulation process was assessed using the five center point run. Design-Expert version 12 was used to conduct the research (Stat-Ease Inc., Minneapolis, Minnesota):
Y = b0 + b1X1 + b2X2 + b3X3 + b12X1X2 + b13X1X3 + b23X2X3 + b11>X12 + b22X22 + b33X32
Preparation of TRT–CUR-loaded NEG
TRT–CUR–NEG was formulated in the following two phases:
Phase 1: Formation of NE. Based on solubility studies, oil, surfactant, and co-surfactant were utilized to make NE. Oleic acid, Tween 80, and propylene glycol were used as an oil, surfactant, and co-surfactant, respectively, and deionized water was used as an aqueous phase. TRT (0.025%) and CUR (0.025%) were disseminated in a specific amount of oleic acid using an ultrasonicator to obtain a homogenous solution (Azami et al., 2018; Chandrashekhar et al., 2015; Heng et al., 2017; Rahman et al., 2015). In deionized water that had been stored, a specific amount of surfactant was disseminated. The oily phase containing drugs TRT and CUR was dispersed in an aqueous phase for 30 minutes using a probe ultrasonicator. An exterior cool jacketed bath with ice was used to regulate the lower temperature of the formulation, and a homogenous NE was created (Saani et al., 2019).
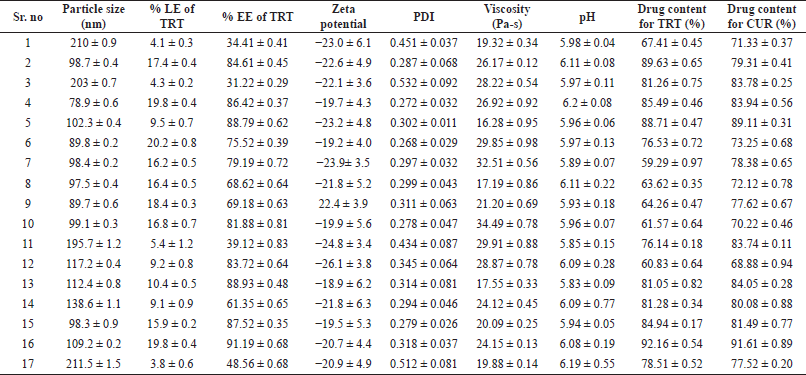 | Table 1. NE’s % L.E, % E.E, mean particle size, PDI, and zeta potential, viscosity, pH, and drug content. [Click here to view] |
Phase 2: NEG formation. The hydrogel was made with carbopol-934 and deionized water, and triethanolamine was employed to maintain the pH range of 5–6. The amount of carbopol 934 required for a 1% w/v solution was diffused in water and kept for 24 hours. The NE was then mixed with the hydrogel to formulate an NEG (Elmataeeshy et al., 2018; Md et al., 2020).
TRT–CUR–NE Characterization
Particle size analysis
At a temperature of 25°C and angle of 90°C, the particle size, zeta potential, and PDI of TRT–CUR–NE were measured in triplicate using dynamic light scattering by zeta sizer (Malvern Instruments Ltd. UK). Before analysis, all of the formulations were diluted with deionized water. Droplet size, PDI, and zeta potential were provided by the equipment’s software (Gurpreet, 2018; Hamid et al., 2021).
Entrapment efficiency (% EE) and loading efficiency (% LE)
TRT–CUR–NEs were assessed for % EE and % LE by calculating the amount of un-entrapped drug in an aqueous phase, removing it by centrifugation at 5,000 rpm for 25 minutes, and filtering the supernatant. After that, the filtered supernatant is diluted and evaluated using a UV spectrophotometer with maximum wavelengths of 421 and 359 nm, respectively, for CUR and TRT (Artiga et al., 2018; Hamid et al., 2021; Jaiswal et al., 2015). Then, % EE and % LE for entire experimental runs were determined via the following equations:
Characterization of TRT–CUR–NEG
pH and spreadability studies
For topical formulations, pH measurement was required to ensure that the formulation does not irritate the skin. At room temperature, pH was determined with a digital pH meter (Campani et al., 2016). The spreadability of the NEG is determined by placing 1.0 g of TRT–CUR–NEG in the center of a glass slide with 1 cm2 marks. After that, another slide was placed on top of it, followed by a nearly 100 g weight. The spreading coefficient was then determined as a function of the spreading area covered by each sample (Karri et al., 2015; Sahu et al., 2018).
Rheological studies
For topical formulations, pH measurement is required to ensure that the formulation does not irritate the skin. The pH was determined with a digital pH meter (Campani et al., 2016). The NEG spreadability is determined by placing 1.0 g of TRT–CUR–NEG in the center of a glass slide with 1 cm2 marks. After that, another slide was placed on it, followed by a nearly 100 g weight. The spreading coefficient was then determined as a function of the spreading area covered by each sample (Karri et al., 2015; Sahu et al., 2018).
Drug content uniformity
Samples weighing 500 mg were obtained from various regions of the gel, such as the upper layer, middle layer, and bottom layer. Each sample was extracted using a 2:8 mixture of methanol and water, then centrifuged for 15 minutes at 3,000 rpm. The supernatant was filtered with Whatmann filter paper of 0.45 μm and the filtrate was measured with a UV spectrophotometer at max 421 and 359 nm or CUR and TRT, correspondingly. The process was carried out thrice and the uniformity of the material is recorded as the average content (Ahmad et al., 2019).
In-vitro release study
The drug release from TRT–CUR–NEG was calculated using a Franz diffusion cell with a glass cylinder open at both ends. NEG equivalent to 2 mg of TRT and CUR was placed equally over the dialysis membrane surface (saturated in PBS 7.4 pH for 24 hours). The donor compartment was filled with PBS (7.4 pH), which mimics the blood or plasma. The drug molecules were believed to be directly taken up by the systemic circulatory system through NEG. As a result, testing drug release in a 7.4 pH phosphate buffer is necessary. For 24 hours, the entire assembly was placed on a magnetic stirrer and kept at 37°C ± 2°C. At defined time intervals, for instance, 0, 0.25, 0.5, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 12, 18, and 24, aliquots of 1.5 ml sample were obtained, and fresh PBS was added to maintain the sink conditions. The samples were then tested using a UV spectrophotometer at 421 and 359 nm for CUR and TRT, respectively (Dhawan et al., 2014). The in vitro release study was repeated thrice to obtain accurate data.
Determination of TRT–CUR release kinetics
The data from the drug release study was fitted into various mathematical models to determine NEG’s release kinetics and mechanism. Three kinetics models can be used to examine the release kinetics, i.e., zero, first, and Higuchi model. A cumulative % drug release and time are drawn in a zero-order graph, the log cumulative % drug release and time is plotted in a first-order graph, and the cumulative % drug release and the square root of time are plotted in the Higuchi model graph. The fourth model, Korsmeyer–Peppas, was used to determine the drug release mechanism, graph among log cumulative % drug release, and log time was created (Gadkari et al., 2019; Jain et al., 2015).
Studies on physical stability
For determining the physical stability of NE, thermodynamic stability studies are essential. The following thermodynamic stability tests were carried out to assess the stability of NE formulations.
Freeze thaw cycle
For 24 hours, the NE formulations were held at 25°C. After that, the formulations were withdrawn and stored at room temperature. The thermodynamically stable NEs were recovered to their original temperature within 2–3 minutes. Three times, this cycle was repeated to attain precision.
Heating and cooling cycle
Six cycles were carried out in the refrigerator between 4°C and 45°C, with at least 48 hours of storage at each temperature, and the improved formulations were evaluated for stability at these temperatures.
Centrifugation
In this phase, the NEs were centrifuged at 5,000 rpm for 30 minutes to check for creaming, phase separation, and cracking (Ma et al., 2021; Ojha et al., 2021).
RESULTS AND DISCUSSION
Screening of factors by design of expert
The crucial elements for the formulation chosen were based on the trials, and the literature review has a significant impact on the particle size and % LE of TRT. The studied factors influenced both responses, with values ranging from 78.9 ± 0.6 to 210 ± 0.9 nm and 3.8% ± 0.6% to 20.2% ± 0.8% for particle size and % LE, respectively. It explains that the screening parameters, such as the amount of oil, surfactant, and co-surfactant, substantially impact the particle size of the TRT–CUR–NE and % TRT.
Optimization of formulation using Design-Expert
BBD focused on the essential components’ significant effect, interaction, and quadratic effect on particle size and % LE.
Effect of critical factors on particle size (Y1)
The F-value obtained from the optimized model after ignoring the insignificant expressions of the model for particle size was observed to be significant, i.e., F-value = 8,564.51 and pcal value = 0.0001, whereas lack of fit was insignificant, i.e., F-value = 2.18, based on the results of analysis of variance (ANOVA) for BBD. The adjusted R2 and predicted R2 regression coefficients were determined to be 0.9998 and 0.9990, respectively, implying that the predicted value is 95% similar to the experimental value. 78.9 ± 0.6 nm was the smallest particle size measured in the fourth run, and 210 ± 0.9 nm was the biggest particle size observed in the first run, according to the design.
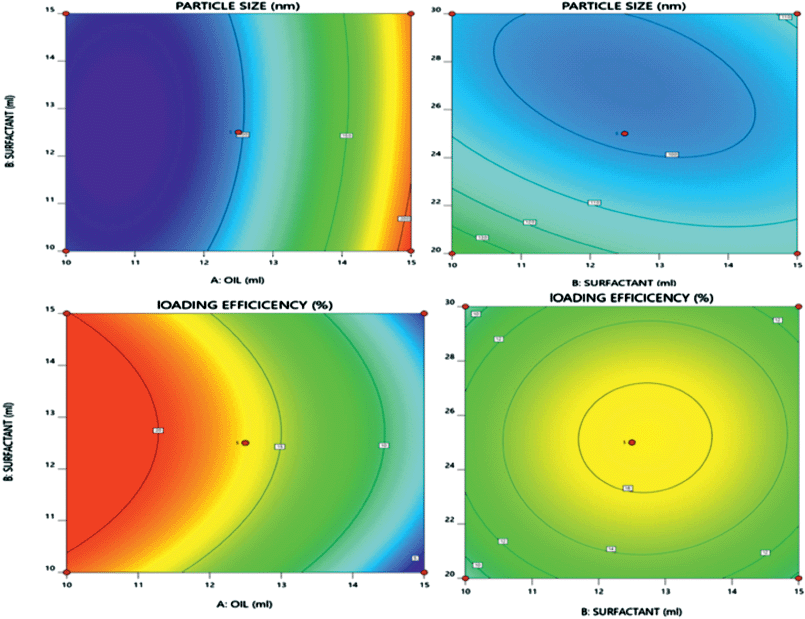 | Figure 1. 3D response surface plots presenting effect of critical factors on particle size and % LE TRT in TRT–CUR–NE. [Click here to view] |
Particle size (Y1) = 98.80 + 56.55 × X1 − 3.65 × X2 − 9.55 × X3 – 3.98 × X1 X2 + 5.88 × X1 X3 + 7.32 × X2 X3 + 40.37 × X12 + 8.22 × X22 + 11.87 × X32>
Effect of critical factors on % LE (Y2)
The F-value obtained from the optimized model after ignoring the insignificant expressions of the model for particle size was observed to be significant, i.e., F-value = 132.41 and pcal value < 0.0001, whereas lack of fit was insignificant, i.e., F-value = 1.88 and pcal value = 0.2734, based on the results of ANOVA for BBD. The adjusted R2 and predicted R2 regression coefficients were determined to be 0.9867 and 0.9415, respectively, implying that the predicted value is 95% similar to the experimental value. The minimum % LE seen in the first run was 4.1% ± 0.3%, while the highest % LE observed in the sixth run was 20.2% ± 0.8% (Table 1).
LE = 16.54 – 7.35 × X1 + 0.3250 × X2 + 0.2250 × X3 + 0.4 × X1 X2 + 0.05 × X1 X3 + 0.2 × X2 X3 – 1.30 × X12 – 3.75 × X22 – 3.24 × X32>
Figure 1 and Equation (3) reveal that increasing the amount of oil had a favorable influence on the particle size of TRT–CUR–NE because increasing the amount of oil caused the internal phase viscosity to rise, preventing the particles from fracturing (Schreiner et al., 2020). The amount of surfactant and co-surfactant had a negative impact on particle size, as the particle size decreased upon increasing the amount of surfactant and co-surfactant (Yeo et al., 2021).
On the other hand, an increased amount of oil exhibited a substantial negative influence on the % LE of TRT in TRT–CUR–NE during emulsification, as shown in Figure 1 and Equation (4). The % LE dropped as the % of oil increased, possibly due to supersaturation of the internal phase, causing TRT release from the internal phase to the external phase during emulsification (Diwan et al., 2020).
Optimization of TRT–CUR–NE
The desirability function was examined using Design-Expert software ver.12 to identify the best facts for TRT–CUR–NE formulation. For the optimized TRT–CUR–NE, the desirability function value was found to be 1.0. The best conditions for the preparation of TRT–CUR–NE were determined to have the minimum particle size and the maximum % of LE. The software predicted the trial circumstances, such as the amount of oil (10.0%), surfactant (12.158%), and co-surfactant (26.906%). New TRT–CUR–NEs (n = 3) were produced using the conditions projected by the software to check the reasonableness of the aforementioned controlled circumstances, and the responses had been determined. After that, a Wilcoxon’s signed-rank test value 0.05 was used to see a substantial difference between anticipated and experimental particle size and % LE of the NEs. The hypothesized model was viable because there was no significant difference between the projected and measured particle size and % LE values.
Analysis of particle size
The optimized TRT-CUR-NE’s mean particle size, PDI, and zeta potential, viscosity, and pH were 77.6 ± 2.1 nm, −20.7 ± 4.4 mV, and 0.268 ± 0.029, respectively (n = 3). The viscosity and pH were found to be in the range of 58.96 ± 0.64 Pas to 37.85 ± 0.33 Pas and 5.83 ± 0.09 to 6.2 ± 0.08, respectively. A low PDI score indicates a tapering particle size distribution and a consistent NE formulation. The zeta potential, which indicates charge borne on the surface of particles, was negative for TRT–CUR–NE, which might be assigned to the drug substance or oleic acid because of the presence of Tween 80 as a non-ionic surfactant (Table 1 and Figs. 2 and 3).
Drug EE (%) and LE (%)
The % EE and % LE of the NE were computed using Equations (3 and 4). The surfactant and co-surfactant enabled the increase in % EE and % LE. However, the increase in oil content has a detrimental impact on both entrapment and LE. For the optimized TRT–CUR–NE of TRT, EE and LE were 85.92% ± 2.6% and 19.6% ± 1.2%, respectively. For the optimized TRT–CUR–NE, the % EE and % LE of CUR were 88.31% ± 3.2% and 18.7% ± 2.5%, respectively.
Drug content of TRT and CUR
The maximum amount of drug content for TRT and CUR was 92.16 ± 0.54 and 91.61 ± 0.89 respectively.
Physical stability of NE
After physical stability testing, no significant changes were observed in the optimized formulation, as shown in Table 2 (Zhang et al., 2018).
Characterization of TRT–CUR–NEG
pH, spreadability, and drug content of TRT–NEG
The pH of optimized TRT–CUR–NEG was 6.1 ± 0.4, signifying the similarity with the skin pH. Spreadability was 6.2 ± 0.6 g cm/second, indicating that the ease in spreading the NEG devoid of high friction. Drug content of top, middle, and bottom layers was found to be 86.73 ± 0.62, 89.78 ± 0.39, 92.13 ± 0.46, respectively, and demonstrated that the loss of medication due to the inclusion of NEG was negligible.
 | Table 2. Results of physical stability. [Click here to view] |
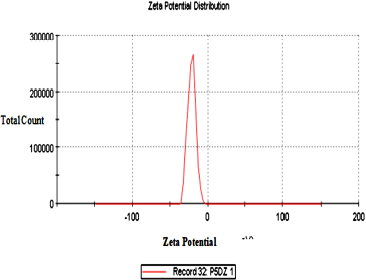 | Figure 2. Zeta potential of TRT–CUR–NE. [Click here to view] |
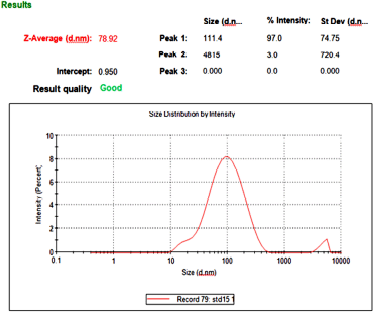 | Figure 3. Particle size of TRT–CUR–NE. [Click here to view] |
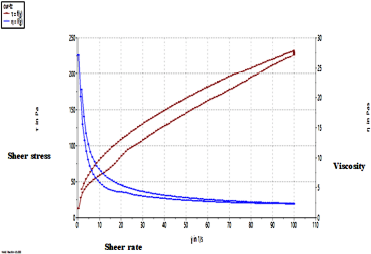 | Figure 4. Rheogram depicting the pseudoplastic nature of TRT–CUR–NE. [Click here to view] |
pH and viscosity
The pH of optimized TRT–CUR–NEG was 5.9 ± 0.4, signifying the similarity with the skin pH. The average viscosity was found to be 48.29 ± 2.7 Pas.
Rheological measurements
The rheogram in Figure 4 depicts the pseudoplastic nature of TRT–CUR–NEG, demonstrating the shear-thinning makeup of NEG. the mean viscosity was measured to be 48.29 ± 2.7 Pas. The TRT–CUR–NEG exhibited pseudoplastic characteristics, unique to hydrophilic polymeric formulations, with both non-Newtonian and non-linear interaction of shear stress and shear rate. Upon application to the biological surface, such performance was thought critical because it would aid in dissemination. In hydrophilic polymeric systems, pseudoplastic quality is frequent, which increases spreadability during application. Greater pseudo-plasticity correlates to easy spreadability (Ali et al., 2016; Ghica et al., 2016).
In vitro drug release
Figure 5 shows the in vitro release profiles of plain TRT–CUR gel and TRT–CUR–NEG in 7.4 pH phosphate buffer. At 24 hours, the release from the plain gel was very modest and showed that TRT and CUR diffusions from a simple gel base were slowed because the gelling ingredient kept the drug in its polymeric structure, preventing it from diffusing. The surfactant and co-surfactant increased TRT and CUR diffusion across the skin by inducing transdermal partitioning into the skin layers in TRT–CUR–NEG (Algahtani et al., 2020). Following 24 hours, the TRT–CUR–NEG had shown 91.2% ± 0.86% cumulative release for CUR with 92.46% ± 0.69% cumulative release for TRT, as shown in Figure 5. The plain gel and TRT–CUR–NE showed 24.48% ± 0.29% and 74.36% ± 0.56% cumulative release for TRT, respectively. The flux finds out to be 0.78 μg/sq.cm/hour and 0.55 μg/sq.cm/hour for TRT and CUR, respectively.
Determination of CUR and TRT release kinetics
Mathematical modeling is an essential aspect of the investigation for characterizing the kinetics of drug release from the formulation. The optimized CUR–TRT–NEG’s release data were fixed to the zero, first-order, Higuchi, and Korsmeyer–Peppas model. For the CUR release, the R2 values obtained from the graphs are 0.958, 0.9597, and 0.9635, respectively, for the zero, first, and Higuchi model of CUR, while TRT values are 0.9585, 0.9555, and 0.9629, respectively. The drug molecules have been released according to the Higuchi model. The mode of release was corroborated using Korsmeyer–Peppas model, which yielded n values of 0.5955 and 0.5757 respectively for CUR and TRT, which fall between 0.45 and 0.89, indicating that the formulation followed a non-Fickian release pattern in Table 3 (Costa and Lobo, 2001; Lokhandwala et al., 2013; Samaha et al., 2009).
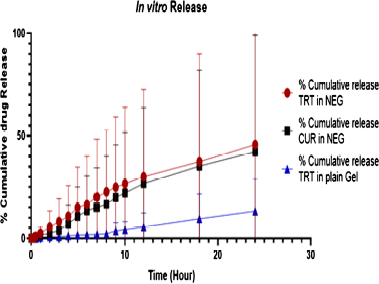 | Figure 5. % cumulative release of TRT and CUR from plain gel, TRT-CUR NEG versus time. [Click here to view] |
 | Table 3. Results of mathematical modeling. [Click here to view] |
Anti-bacterial activity
The in vitro anti-acne activity of optimized TRT–CUR–NEG (diameter of zone of inhibition 32.21 ± 21 mm) against Propionibacterium acne was found to be comparable to that of marketed revize gel (diameter of zone of inhibition 26.77 ± 48 mm).
Physical stability testing
After physical stability testing, no significant changes were observed in the optimized formulation in Figure 6, illustrating that the parameters of TRT–CUR–NEG like pH and drug content had shown little changes, which confirmed the physical stability of the formulation (Aithal et al., 2020).
DISCUSSION
The TRT–CUR–NE was formulated according to BBD and 17 formulations were made and characterized to select the optimized formulation with the lowest droplet size (78.9 ± 0.6) and highest % LE (19.8 ± 0.4). The particle size is determined by the amount of surfactant and cosurfactant used, and the drug is mostly present at the interfacial surface, where surfactant molecules are arranged between the oily and aqueous interfaces, but there is insufficient LE in both the oily and aqueous phases at the same time. The quadratic equation for both droplet size and % LE exhibited positive control of oil content on droplet size and negative control over % LE while in contrast to this increase in the extent of surfactant and cosurfactant paraded negative impact on droplet size and positive impact on % LE. The % LE dropped as the % of oil increased, possibly due to supersaturation of the internal phase, causing TRT release from the internal phase to the external phase during emulsification (Diwan et al., 2020). A low PDI score indicates a tapering particle size distribution and a consistent NE formulation. The zeta potential, which indicates charge borne on the surface of particles, was negative for TRT–CUR–NE, which might be assigned to the drug substance or oleic acid because of the presence of tween 80 as a non-ionic surfactant. It explains that the screening parameters, such as the amount of oil, surfactant, and co-surfactant, substantially impact the particle size of the TRT-CUR-NE and % TRT. Further, the R2 value obtained for droplet size, i.e., 0.99876 and % LE, i.e., 0.9587; the p-value for both was < 0.05, illustrating the significance of the design. The optimized TRT-CUR-NE was then mixed with the hydrogel composed of carbopol 934 and distilled water, and the NEG was then characterized for pH, viscosity, spreadability, and drug content. The pH of the NEG was observed to be within the range of skin pH, i.e., 5.9 ± 0.4. The in vitro release studies of Plain gel, TRT-CUR-NE, and TRT-CUR-NEG showed a % cumulative drug release of 28.64% ± 0.31%, 80.32% ± 0.42%, and 89.64% ± 0.97% after 24 hours, respectively. Upon fitting the mathematical models, the Higuchi model described the release of TRT and CUR from TRT–CUR–NEG based on R2 value, i.e., 0.9629 and 0.9935, respectively, for TRT and CUR. Hence, the TRT–CUR–NEG was observed to possess better topical formulation’s requisite characteristics and significant release kinetics and indicating that the formulation followed a non-Fickian release pattern
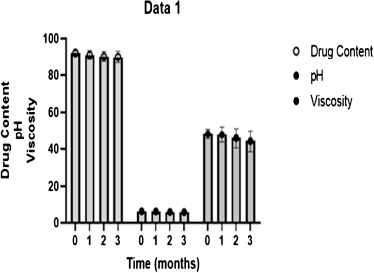 | Figure 6. Stability analysis of TRT–CUR–NEG. [Click here to view] |
CONCLUSION
Thus, the TRT–CUR–NEG could be an effective approach to be delivered topically for improved performance and fewer side effects. Also, a hydrogel system that can hydrate the skin and improve the effectiveness of NEG mediated encapsulated therapeutic delivery and anti-acne activity. Furthermore, the co-additive formulation of TRT–CUR possesses all requisite characteristics; however, there is some lack of studies which may ascertain the validity of this co additive addition for various skin ailments.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Ahmad J, Gautam A, Komath S, Bano M, Garg A, Jain K. Topical nano-emulgel for skin disorders: formulation approach and characterization. Recent Pat Antiinfect Drug Discov, 2019; 14(1):36–48. CrossRef
Algahtani MS, Ahmad MZ, Ahmad J. Nanoemulgel for improved topical delivery of retinyl palmitate: formulation design and stability evaluation. Nanomaterials, 2020; 10(5):848. CrossRef
Ali I, Shah LA. Rheological investigation of the viscoelastic thixotropic behavior of synthesized polyethylene glycol-modified polyacrylamide hydrogels using different accelerators. Polym Bull, 2021; 78(3):1275–91. CrossRef
Aithal GC, Narayan R, Nayak UY. Nanoemulgel: a promising phase in drug delivery. Curr Pharm Des, 2020; 26(2):279–91. CrossRef
Artiga-Artigas M, Lanjari-Pérez Y, Martín-Belloso O. Curcumin-loaded nanoemulsions stability as affected by the nature and concentration of surfactant. Food Chem, 2018; 266:466–74. CrossRef
Azami SJ, Teimouri A, Keshavarz H, Amani A, Esmaeili F, Hasanpour H, Elikaee S, Salehiniya H, Shojaee S. Curcumin nanoemulsion as a novel chemical for the treatment of acute and chronic toxoplasmosis in mice. Int J Nanomedicine, 2018; 13:7363. CrossRef
Batra H, Pawar S, Bahl D. Curcumin in combination with anti-cancer drugs: a nanomedicine review. Pharmacol Res, 2019; 139:91–105. CrossRef
Beg S, Swain S, Rahman M, Hasnain MS, Imam SS. Application of design of experiments (DoE) in pharmaceutical product and process optimization. In: Beg S, Hasnain MS (eds.). Pharmaceutical quality by design. Academic Press, Cambridge, MA, pp 43–64, 2019. CrossRef
Bhoop BS, Beg S, Raza K. Developing “optimized” drug products employing “designed” experiments. Chem Ind Digest, 2013; 23:70–6.
Campani V, Biondi M, Mayol L, Cilurzo F, Pitaro M, De Rosa G. Development of nanoemulsions for topical delivery of vitamin K1. Int J Pharm, 2016; 511(1):170–7. CrossRef
Chandrashekhar B, Anitha M, Ruparelia M, Vaidya P, Aamir R, Shah S, Thilak S, Aurangabadkar S, Pal S, Saraswat A, Sanmukhani JJ. Tretinoin nanogel 0.025% versus conventional gel 0.025% in patients with acne vulgaris: a randomized, active controlled, multicentre, parallel group, phase IV clinical trial. J Clin Diagn Res, 2015; 9(1):WC04. CrossRef
Chen H, Khemtong C, Yang X, Chang X, Gao J. Nanonization strategies for poorly water-soluble drugs. Drug Discov Today, 2011; 16(7-8):354–60. CrossRef
Chen J, Ma Y, Tao Y, Zhao X, Xiong Y, Chen Z, Tian Y. Formulation and evaluation of a topical liposomal gel containing a combination of zedoary turmeric oil and tretinoin for psoriasis activity. J Liposome Res, 2020; 31(2):1–15. CrossRef
Costa P, Lobo JMS. Modeling and comparison of dissolution profiles. Eur J Pharm Sci, 2001; 13(2):123–33. CrossRef
Dhawan B, Aggarwal G, Harikumar S. Enhanced transdermal permeability of piroxicam through novel nanoemulgel formulation. Int J Pharm Investig, 2014; 4(2):65. CrossRef
Diwan R, Khan S, Ravi PR. Comparative study of cilnidipine loaded PLGA nanoparticles: process optimization by DoE, physico-chemical characterization and in vivo evaluation. Drug Deliv Transl Res, 2020; 10(5):1442–58. CrossRef
Desam NR, Al-Rajab AJ. The importance of natural products in cosmetics. In: Pal D, Nayak AK (eds.). Bioactive natural products for pharmaceutical applications, Springer, Australia, pp 643–85, 2021. CrossRef
Elmataeeshy ME, Sokar MS, Bahey-El-Din M, Shaker DS. Enhanced transdermal permeability of terbinafine through novel nanoemulgel formulation; development, in vitro and in vivo characterization. Future J Pharm Sci, 2018; 4(1):18–28. CrossRef
Gadkari P, Patil P, Saudagar R. Formulation, development and evaluation of topical nanoemulgel of tolnaftate. J drug deliv ther, 2019; 9(2-s):208–13.
Ghica MV, Hîrj?u M, Lupuleasa D, Dinu-Pîrvu CE. Flow and thixotropic parameters for rheological characterization of hydrogels. Molecules, 2016; 21(6):786. CrossRef
Gillis JC, Goa KL. Tretinoin. Drugs, 1995; 50(5):897–923. CrossRef
Heng M. Topical curcumin: a review of mechanisms and uses in dermatology. Int J Dermatol Clin Res, 2017; 3(1):010–7. CrossRef
Hussain Z, Thu HE, Ng SF, Khan S, Katas H. Nanoencapsulation, an efficient and promising approach to maximize wound healing efficacy of curcumin: a review of new trends and state-of-the-art. Colloids Surf B Biointerfaces, 2017; 150:223–41. CrossRef
Jain K, Sood S, Gowthamarajan K. Optimization of artemether-loaded NLC for intranasal delivery using central composite design. Drug deliv, 2015; 22(7):940–54. CrossRef
Jaiswal M, Dudhe R, Sharma P. Nanoemulsion: an advanced mode of drug delivery system. Biotech, 2015; 5(2):123–7. CrossRef
Karri VNR, Raman SK, Kuppusamy G, Mulukutla S, Ramaswamy S, Malayandi R. Terbinafine hydrochloride loaded nanoemulsion based gel for topical application. J Pharm Investig, 2015; 45(1):79–89. CrossRef
Kim A, Weinkle SH. Cosmeceuticals using vitamin A and its derivatives plus new delivery methods for them. Aesthet Dermatol, 2021; 5:26–37 CrossRef
Kohli K, Ali J, Ansari M, Raheman Z. Curcumin: a natural antiinflammatory agent. Indian J Pharmacol, 2005; 37(3):141. CrossRef
Lai F, Pireddu R, Corrias F, Fadda AM, Valenti D, Pini E, Sinico C. Nanosuspension improves tretinoin photostability and delivery to the skin. Int J Pharm, 2013; 458(1):104–9. CrossRef
Lokhandwala H, Deshpande A, Deshpande S. Kinetic modeling and dissolution profiles comparison: an overview. Int J Pharm Bio Sci, 2013; 4(1):728–73.
Ma Q, Zhang J, Lu B, Lin H, Sarkar R, Wu T, Li X. Nanoemulgel for improved topical delivery of desonide: formulation design and characterization. AAPS Pharm Sci Tech, 2021; 22(5):1–14. CrossRef
Md S, Alhakamy NA, Aldawsari HM, Kotta S, Ahmad J, Akhter S, Shoaib Alam M, Khan MA, Awan Z, Sivakumar PM. Improved analgesic and anti-inflammatory effect of diclofenac sodium by topical nanoemulgel: formulation development—in vitro and in vivo studies. J Chem, 2020; 2020: 1–10. CrossRef
Ojha B, Jain VK, Gupta S, Talegaonkar S, Jain K. Nanoemulgel: a promising novel formulation for treatment of skin ailments. Polym Bull, 2021; 79:1–25. CrossRef
Rahman SA, Abdelmalak NS, Badawi A, Elbayoumy T, Sabry N, Ramly AE. Formulation of tretinoin-loaded topical proniosomes for treatment of acne: in-vitro characterization, skin irritation test and comparative clinical study. Drug Deliv, 2015; 22(6):731–9. CrossRef
Sahu S, Katiyar SS, Kushwah V, Jain S. Active natural oil-based nanoemulsion containing tacrolimus for synergistic antipsoriatic efficacy. Nanomedicine, 2018; 13(16):1985–98. CrossRef
Samaha D, Shehayeb R, Kyriacos S. Modeling and comparison of dissolution profiles of diltiazem modified-release formulations. Dissolution Technol, 2009; 16(2):41–6. CrossRef
Saani SM, Abdolalizadeh J, Heris SZ. Ultrasonic/sonochemical synthesis and evaluation of nanostructured oil in water emulsions for topical delivery of protein drugs. Ultrason Sonochem, 2019; 55:86–95. CrossRef
Schreiner TB, Santamaria-Echart A, Ribeiro A, Peres AM, Dias MM, Pinho SP, Barreiro MF. Formulation and optimization of nanoemulsions using the natural surfactant saponin from quillaja bark. Molecules, 2020; 25(7):1538. CrossRef
Singh B, Saini S, Lohan S, Beg S. Systematic development of nanocarriers employing quality by design paradigms. In: MishraV, Kesharwani P, Amin MCM, Iyer A (eds.). Nanotechnology-based approaches for targeting and delivery of drugs and genes, Academic Press, Cambridge, MA, , pp 110–48, 2017. CrossRef
Thakur A, Walia MK, Kumar S. Nanoemulsion in enhancement of bioavailability of poorly soluble drugs: a review. Pharmacophore, 2013; 4(1):15–25.
Voorhees J. Clinical effects of long-term therapy with topical tretinoin and cellular mode of action. J Int Med Res, 1990; 18:26C–8C.
Waghule T, Gorantla S, Rapalli VK, Shah P, Dubey SK, Saha RN, Singhvi G. Emerging trends in topical delivery of Curcumin through lipid nanocarriers: effectiveness in skin disorders. AAPS Pharm Sci Tech, 2020; 21(7):1–12. CrossRef
Yeo E, Chieng CJY, Choudhury H, Pandey M, Gorain B. Tocotrienols-rich naringenin nanoemulgel for the management of diabetic wound: fabrication, characterization and comparative in vitro evaluations. Curr Res Pharmacolo Drug Dis, 2021; 2:100019. CrossRef
Zhang Z, McClements DJ. Overview of nanoemulsion properties: stability, rheology, and appearance. In: Jafari SM, McClements DJ, (eds.). Nanoemulsions, Academic Press, Cambridge, MA, pp 21–49, 2018. CrossRef