INTRODUCTION
Burns are one of the most common causes of skin injury [1]. A total of 8,955,228 new burn injury cases were identified globally in 2019 [2]. Burns can be caused by several factors including high temperature, chemicals, electricity, physical friction, and exposure to light irradiation. Invasion of harmful microorganisms will pass through into injured skin due to loss of skin physical barrier function, hence causing infection and even developing into sepsis [3]. Molecular and cellular processes during the inflammatory stage of skin healing are largely initiated by a group of protein mediators known as proinflammatory cytokines [4]. Fatty acids are precursors of these mediators that are able to modulate immune cells by regulating the migration of neutrophils and cytokine production at the wound site [5].
Catfish is one of the most affordable sources of omega-3 fatty acids. Every year, a large amount of the catch is discarded as processing residues including fins, bones, head, skin, and innards [6]. Crude fish oil contains small amounts of nontriglyceride substances. Such impurities reduce the palatability of fish oil by producing an unpleasant taste and odor, and also reducing stability and storage life [7]. The use of catfish oil in the health sector including supplements for various benefits has been reported. Its exploration for topical burn therapy was also described. One big problem with using fish oil for pharmaceutical purposes is the lack of organoleptic properties, in particular the unpleasant smell. Various ways to reduce this unpleasant organoleptic property of fish oil include β-cyclodextrin inclusion complexation, physical adsorption, enzymatic hydrolysis, and high vacuum distillation [6,8–10]. Out of these various techniques, the adsorption using clay seems to be a more attractive way because low cost, green process that avoids chemical contact that has the potential to harm human health and environmental issues as well.
As previously reported, fish oil purification was carried out with activated clay to adsorb some contaminants and remove phospholipids without changing the quality of the oil [11]. Bentonite contains alumina and silicates that effectively attract adsorbates. Due to the presence of an intermediate layer structure that can be modified easily, bentonite exhibits excellent adsorption properties [12]. This property makes bentonite a useful material for purification applications, including the clarification in the oil industry.
One of the challenges in developing oily material for topical preparation is its greasy feeling. The development of oil-in-water (O/W) nanoemulsion systems seems to be a very attractive alternative for transparent water-based semisolid dosage forms. Nanoemulsion is a nano-carrier system with broad flexibility, both as a finished and intermediate formulation. Its incorporation into water-based gel will form a nanoemulgel. Nanoemulgel is a novel approach for topical delivery of hydrophobic drugs. Hydrogel systems provide an important role, especially in skin wounds for topical application. Hydrogel systems have nonadhesive properties and provide moisture retention which makes them ideal as carriers in drug delivery for wound healing applications [13]. In this report, the process of optimizing nanoemulgel formulation from catfish oil and a series of physicochemical evaluations to assess the success of the formulation development are described.
MATERIALS AND METHODS
Materials
Catfish oil (PT. Rumah Inovasi Natura, Tulungagung, Indonesia), bentonite (Sumber Berlian Kimia, Jakarta, Indonesia), standardized docosahexaenoic acid (DHA) (Hubei Fuxing Biotechnology, China), cremophor RH40 (BASF, Ludwigshafen, Germany), Polyethylene glycol (PEG) 400 (Brataco, Jakarta, Indonesia), CMC-Na (Nitra Kimia, Yogyakarta, Indonesia), Carbopol Ultrez 10 (Nusa Kimia, Jakarta, Indonesia), glycerin, methylparaben, propylparaben, Sudan III, 96% ethanol, and distilled water (Brataco, Jakarta, Indonesia).
Male albino rabbits (Oryctolagus cuniculus) with a body weight of 1.8–2 kg were used. The albino rabbits were obtained from an animal farm in Lembang, Indonesia. Animals were housed according to a standard protocol for animal handling. The animal experimentation for dermal acute irritation test has received ethical approval from the Ethics Commission of the Faculty of Dentistry, Universitas Airlangga No. 747/HRECC.FODM/IX/2022.
Extraction of catfish oil
Catfish oil extraction was carried out according to Nazir et al. [14] with slight modification. The wet rendering method was started with a steaming process at 105°C for 30 minutes, followed by a pressing procedure. The pressed liquid in the form of water and oil was separated using a separating funnel, followed by distillation. Figure 1 shows the extraction process and characterization of catfish oil nanoemulgel.
Catfish oil purification
Before being used for purification, the particle size of bentonite was reduced by using a milling machine (Retsch Emax Ball Mill, Haan, Germany) at 1,000 rpm for 1 hour. Then, the bentonite was activated by placing it in an oven set at 100°C for 1 hour. To improve the organoleptic properties of catfish oil, the oil was heated at 80°C for 15 minutes. Then, the activated bentonite was added to the hot oil and stirred for 20 minutes. To precipitate the bentonite, the mixture was centrifuged at 10,000 rpm for 10 minutes, with double repetition.
Bentonite characterization
Particle size and polydispersity index
The particle size and polydispersity index of bentonite were measured using a particle size analyzer (Horiba SZ-100, Kyoto, Japan).
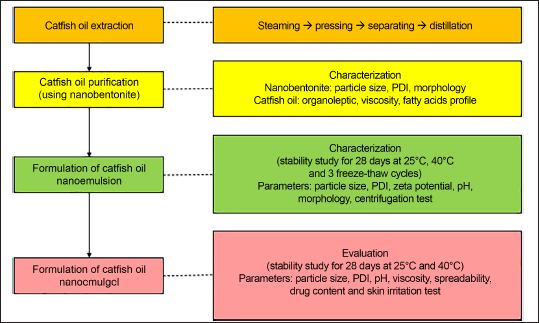 | Figure 1. Flow chart extraction process and characterization of catfish oil nanoemulgel. [Click here to view] |
Morphology
To morphology of bentonite was observed using a scanning electron microscope (SEM). The samples were fixed on a brass stub using double-sided tape and further gold-coated in a vacuum by a sputter coater. The analysis was taken at an excitation voltage of 10 kV and at 10,000× and 40,000× magnifications by using JSM-360LA SEM (Jeol, Tokyo, Japan).
Characterization of catfish oil
Viscosity measurement
The viscosity of purified catfish oil was measured using a Brookfield DV-I+ viscometer (Brookfield Engineering Laboratories, MA, USA) with spindle S21 at room temperature (25°C). The spindle was rotated at 100 rpm and the corresponding viscosity reading was noted.
Fatty acid analysis
Fatty acid was analyzed according to Harahap et al. [15] with slight modification. Catfish oil equivalent to 50 mg of fat is put into a 20 ml vial. Methyl tertiary butyl ether was then added and vortexed for 10 seconds to allow the transesterification reaction. Subsequently, hexane was added, and then a neutralization solution was added and centrifuged. The organic phase was put into a 2 ml vial and then injected into a gas chromatography system (FID Clarus 580 Perkin Elmer, MA, USA).
Formulation of catfish oil nanoemulsion
The development of catfish oil nanoemulsion was done using a spontaneous emulsification method consisting of catfish oil, cremophor RH40, and PEG 400, which serve as oil phase, surfactant, and cosurfactant, respectively. The oil mixture was produced by mixing catfish oil, cremophor RH40, and PEG using a magnetic stirrer at 500 rpm for 2 hours and further, sonication for 1 hour. To produce nanoemulsion, distilled water was added in a ratio of 1:10 under magnetic stirring until a clear system was produced [16]. Characterization of visual appearance and droplet size was carried out to obtain the optimum formula.
Characterization of catfish oil nanoemulsion
Zeta potential
The zeta potential of catfish oil nanoemulsion was analyzed using a zeta potential analyzer (Horiba SZ-100, Kyoto, Japan).
pH measurement
The pH of the catfish oil nanoemulsion was determined using a calibrated pH meter (Mettler Toledo, OH, USA).
Morphology
The morphology of the nanoemulsion was observed using a transmission electron microscope (TEM) (JEOL JEM-1400, Tokyo, Japan). A total sample of 10 μl was dripped into the specimen holder and covered with a 400 mesh grid. After 1 minute, 10 μl of uranyl acetate was dripped on top of the grid and the sample was left to dry for 30 minutes before observation [16].
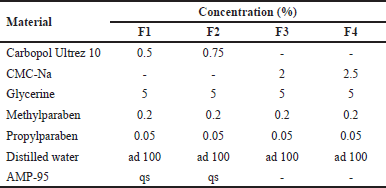 | Table 1. Catfish oil nanoemulgel formula. [Click here to view] |
Centrifugation test
The centrifugation test at 10,000 rpm for 30 minutes was carried out on catfish oil nanoemulsion which was previously added with a few drops of Sudan III. After centrifugation, the visual appearance of the nanoemulsion was observed.
Accelerated stability test
The nanoemulsion stability test was conducted for 28 days at room temperature (25°C) and 40°C with interval time sampling at 0, 7, 14, 21, and 28 days. The freeze-thaw test was conducted for three cycles. For each cycle, the samples were stored for 48 hours at −20°C and 48 hours at room temperature. Observations at each sampling time included organoleptic, particle size, polydispersity index, zeta potential, pH, and centrifugation test.
Preparation of catfish oil nanoemulgel
Based on the formula presented in Table 1, the base gel was prepared by heating distilled water at 70°C in a glass beaker, then methyl paraben and propyl paraben were added, and stirred using a magnetic stirrer until dissolved. Carbopol Ultrez 10 was dispersed into the solution and allowed for 24 hours for a complete hydration process. Furthermore, glycerin was added into the dispersion system and stirred until homogeneous. AMP-95 was added dropwise under stirring to adjust the pH to 5.5 and produce a base gel with a suitable consistency. The CMC-Na base gel was prepared using the same procedure, but no AMP-95. The formulation was continued by combining the oil phase and base gel in a ratio of 1:10. It was then homogenized using a homogenizer DLAB OS20-Pro (DLAB Scientific, Beijing, China) at 300 rpm until the oil phase was completely dispersed in the base gel to form an emulgel.
Physicochemical evaluation of nanoemulgel
Rheological behavior
Rheological characterization of catfish oil nanoemulgel was carried out at room temperature (25°C) using a Brookfield DV-I+ (Brookfield Engineering Laboratories, MA, USA) viscometer with spindle S28. Spindle speed was gradually increased from 10 to 100 rpm and gradually decreased from 100 to 10 rpm. The viscosity of the sample at each speed was recorded.
Spreadability study
A total of 1 g of catfish oil nanoemulgel was placed between two Petri dishes with a diameter of 20 cm, then pressed with a 125 g weight for 1 minute. The spread diameter of the nanoemulgel was measured with a caliper [17].
Determination of DHA content
A total of 0.5 g of sample was added with 96% ethanol up to 10 ml, then stirred at 500 rpm for 30 minutes. Centrifugation was then carried out at a speed of 15,000 rpm for 15 minutes. The DHA content in the catfish oil nanoemulgel preparation was determined by dilution. A volume of 50 μl of filtered supernatant was added with 96% ethanol to 5 ml and then analyzed using a UV-Vis spectrophotometer Thermo Scientific Evolution 220 (Thermo Fisher Scientific, MA, USA) at a wavelength of 221 nm [18].
Accelerated stability test
The stability test of catfish oil nanoemulgel was conducted for 28 days at room temperature (25°C) and 40°C with interval sampling time at 0, 7, 14, 21, and 28 days. Observations at each sampling time included organoleptic, particle size, polydispersity index, pH, viscosity, spreadability, and determination of DHA content.
In vivo acute skin irritation test
Acute skin irritation tests of the catfish oil nanoemulgel were done using the Organization for Economic Cooperation and Development guidelines. Three rabbits were acclimatized for 7 days and placed in individual cages. A day before testing, the rabbit’s fur was shaved on the back area of approximately 6 cm2. Half a gram of catfish oil nanoemulgel was gently applied at the test site while untreated skin areas served as a control. After 4 hours, the residues of the test preparation were removed immediately using distilled water. The test sites were assessed at a time interval of 1, 24, 48, and 72 hours for dermal reaction using Draize scoring criteria [19].
Statistical analysis
The results were measured in triplicate and expressed as mean value ± standard deviation. The statistical analyses were made by t-test using Minitab version 21.3.1.0. The statistical significance level was set at p-value < 0.05.
RESULT AND DISCUSSION
Purification of catfish oil
Bentonite was heat-activated before being used in the catfish oil purification process. The surface area of bentonite increased as the temperature rose up to 100°C. The increase in heat-activated surface area occurs due to the removal of water molecules and other contaminants attached to the nonactivated bentonite surface. Therefore, it can produce new pores and enhance the availability of adsorption sites [20].
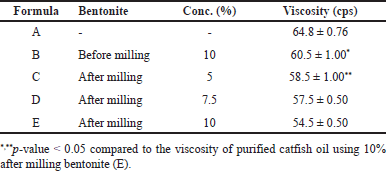
The viscosity of purified catfish oil was influenced by the level and particle size of bentonite. As seen in Table 2, there was a significant difference (p-value < 0.05) between the viscosity of bentonite-purified catfish oil with particle sizes of bentonite at the same level and the same particle size at different levels. The decrease in viscosity of catfish oil reflects the adsorbing power of the adsorbent. The viscosity of catfish oil decreased, in line with the addition of nanobentonite which has a larger total surface area. Likewise, the viscosity of catfish oil also decreased with increasing the bentonite concentration. This is logical because the addition of bentonite amount will provide a higher number of adsorbent active sites [21,22]. In the process of purifying catfish oil, the optimum result is shown by formula E (10% of nanobentonite), resulting in the lowest viscosity with the clearest appearance as depicted in Figure 2.
Bentonite characterization
Particle size and polydispersity index
As presented in Table 3, it is found that the particle size of bentonite before and after milling showed a significant difference (p-value < 0.05). The polydispersity index showed the uniformity of the particle size at a value of <0.5, indicating that the bentonite particle size has good homogeneity.
 | Table 2. Characteristics of catfish oil with various levels and particle size of bentonite in the purification process (mean ± SD, n = 3). [Click here to view] |
 | Figure 2. Visual appearance of catfish oil after adsorption process using nanobentonite. (A) Catfish oil without bentonite. (B) Catfish oil added by 10% bentonite before milling. (C) Catfish oil added by 5% bentonite after milling. (D) Catfish oil added by 7.5% bentonite after milling. (E) Catfish oil added by 10% bentonite after milling. [Click here to view] |
 | Table 3. Particle size and polydispersity index of bentonite (mean ± SD, n = 3). [Click here to view] |
 | Figure 3. SEM image of before milling bentonite. Samples magnifications were 10,000× (left) and 40,000× (right). [Click here to view] |
 | Figure 4. SEM image of after milling bentonite. Samples magnifications were 10,000× (left) and 40,000× (right). [Click here to view] |
Morphology
Figures 3 and 4 show the difference in bentonite morphology before and after milling. Unmilled bentonite had a regular surface and compact structure with narrow inter-particle spaces. Meanwhile, bentonite after milling had a surface that looked irregular, with many flakes with wider inter-particle spaces. Compared to unmilled bentonite, the surface area of milled bentonite was significantly increased as seen in the SEM image.
Characterization of catfish oil
Organoleptic and viscosity
Crude catfish oil is brownish in color, has a strong fishy smell, and is slightly clear. After the purification process, the oil shows yellowish with a weaker fishy smell and clearer. Purification also decreased the oil viscosity significantly, i.e., from 64.8 ± 0.76 to 54.5 ± 0.50 cps.
Fatty acid analysis
Table 4 shows that catfish oil contains polyunsaturated fatty acids including omega-3, 6, and 9 substances. The main content of omega-3 fatty acids are DHA and eicosapentaenoic acid (EPA). Insignificant changes in the bioactive substances after purification suggests that bentonite is a proper mineral for physical adsorption for purification purpose.
Nanoemulsion of catfish oil preparation
Catfish oil nanoemulsion formulation was performed using cremophor RH40 as a surfactant and PEG 400 as a cosurfactant. The surfactant and cosurfactant applied in the formula are well-established combinations as reported in our previous work [23]. Cremophor RH40 is a nonionic surfactant with an Hydrophilic–lipophilic balance (HLB) value of 14–16. Cremophor RH40 is nontoxic, stable over a wide temperature range, and shows little tendency to foam [24]. In addition, PEG 400 is a nonionic surfactant with an HLB value of 11.3 [25]. Formulations using surfactants with high HLB can form O/W nanoemulsions. In addition to the HLB value, the molecular structure of the surfactant is also an important factor in forming nanoemulsions. Cremophor RH40 has an alkyl branch structure, where the structure affects the penetration of the interface layer resulting in the formation of its own nanoemulsion [26]. PEG 400 has an affinity to the oil phase and water phase, so it can help surfactants strengthen the nanoemulsion structure [27].
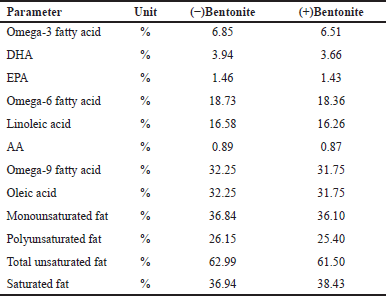 | Table 4. Comparison of the fatty acid profile of catfish oil before and after adding bentonite. [Click here to view] |
The ternary phase diagram was determined by mixing catfish oil with a mixture of cremophor RH40 and PEG 400 surfactants. One part of catfish oil was mixed with a mixture of surfactants with a ratio variation of 8:1; 7:2: 6:3; and 5:4. Meanwhile, two parts of catfish oil were mixed with surfactant mixtures with variations in the ratio of 7:1; 6:2, and 5:3. To produce nanoemulsion, the oil phase was diluted with distilled water using a fixed ratio of 1:10. The surfactant blend ratio was chosen with decreasing surfactant concentration with respect to increasing cosurfactant concentration. One axis of the ternary phase diagram represents the oil phase, another represents the surfactant and the third represents the cosurfactant as shown in Figure 5. Nanoemulsion was identified as the point in the phase diagram, where a clear and transparent formulation was obtained based on visual observation [28].
In the optimization of the catfish oil nanoemulsion formula, the clarity, particle size, and polydispersity index were important parameters to observe. The nanoemulsion characterization is presented in Table 5. As seen in Figure 6, formula F1 produced good nanoemulsion characteristics. This is due to the relatively appropriate proportion of surfactant to cosurfactant [29]. This formula had the smallest droplet size of 21.03 ± 0.23 nm and a narrow size distribution. Increasing the oil phase resulted in a proportional increase in particle size due to a simultaneous decrease in surfactant/cosurfactant proportion, giving a cloudy visual appearance, as observed in formulas F5–F7. The increase in surfactant to cosurfactant led to a decrease in droplet size.
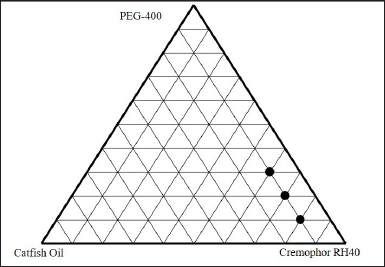 | Figure 5. Ternary phase diagram. [Click here to view] |
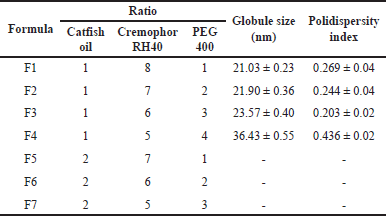 | Table 5. Characteristics of catfish oil nanoemulsions with various oil-surfactant-cosurfactant ratios (mean ± SD, n = 3). [Click here to view] |
 | Figure 6. Visual appearance of nanoemulsion formula F1–F7. [Click here to view] |
Characterization of catfish oil nanoemulsion
Droplet size, polydispersity index, zeta potential, and pH
The physical characteristic of catfish oil nanoemulsion showed an average droplet size of 21.03 ± 0.23 nm, with a polydispersity index was 0.269 ± 0.04 indicating a homogeneous dispersion. The nanoemulsion exhibited a negative surface charge by a zeta potential value of −15 ± 2.57 mV due to the presence of anionic fatty acid groups from the catfish oil [18]. The use of cremophor RH40 as a nonionic surfactant was able to stabilize the emulsion system through a strong steric repulsion mechanism. The presence of ethylene oxide groups and long hydrocarbon chains also contributed to the stabilization via sterical effect [30]. Since the target application of this preparation was for topical use, the formulation was designed to have a suitable pH for the skin, i.e., between 4.5 and 6.5 [31]. In this case, the pH of the nanoemulsion was 6.83 ± 0.02.
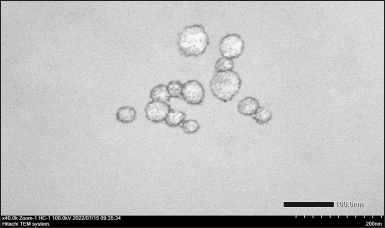 | Figure 7. TEM image of catfish oil nanoemulsion (magnification: 40,000×). [Click here to view] |
 | Figure 8. Centrifugation test result of catfish oil nanoemulsion. [Click here to view] |
Morphology
As depicted in Figure 7, TEM analysis shows spherical nanoemulsion droplets with a size of <50 nm. This result is in line with the observation by using a particle size analyzer.
Centrifugation test
The centrifugation challenge is a common procedure to test the physical stability of a liquid–liquid dispersion system. The stability test of catfish oil nanoemulsion is presented in Figure 8. The nanoemulsion containing Sudan III was pinkies and monodispersed. This indicates that the oil droplets are properly dispersed in the nanoemulsion [32]. According to Stokes’ law, centrifugation in nanoemulsions can increase the rate of globule migration toward the superficial layer of the nanoemulsion system. As a result, there is a possibility of increased aggregation of globules that can break the surfactant monolayer and form coagulation. The presence of cosurfactants in nanoemulsion formulations can optimize the function of surfactants to protect the globules from aggregation [33].
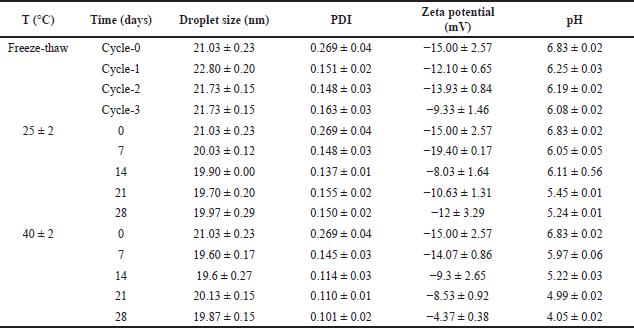 | Table 6. Nanoemulsion stability test over time at various temperatures (mean ± SD, n = 3). [Click here to view] |
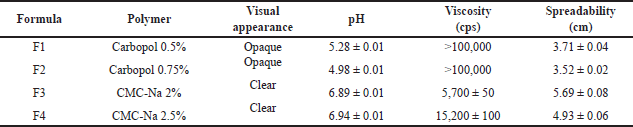 | Table 7. Characteristics of catfish oil nanoemulgel with various polymers (mean ± SD, n = 3). [Click here to view] |
Accelerated stability test
The study was to observe the changing of the droplet size after 28 days of storage at room temperature as well as at 40°C. As presented in Table 6, the nanoemulsion droplet size after 28 days remained constant at various storage conditions, with a PDI value smaller than 0.3, which was an indicator of good stability. Based on this observation, from these data, the zeta potential and pH tend to decrease significantly during the storage at various conditions, mainly at 40°C. The decrease in pH is suggested by the production of acidic compounds due to the decomposition of hydroperoxides in the oxidation reaction. High temperatures accelerated the deterioration of the nanoemulsion. However, the decrease in pH did not significantly affect the overall quality of the nanoemulsion and its use for topical purposes [34,35].
Preparation of catfish oil nanoemulgel
Based on the characterization data presented in Table 7, formula F3 produced the most suitable characteristics as a nanoemulgel for topical use. The formula produced transparent and homogeneous consistency as depicted in Figure 9.
 | Figure 9. Visual appearance of nanoemulgel formula F1–F4. [Click here to view] |
Physicochemical evaluation of nanoemulgel
Droplet size, polydispersity index, pH, viscosity, spreadability, and rheological behavior
The physicochemical properties of the catfish oil nanoemulgel are presented in Table 8 (day 0). Nanoemulgel has nanoemulsion droplet size on the nanoscale, namely in the range of 100–200 nm. Nanoemulgel has PDI < 0.5 showing a narrow and uniform size distribution of the nanoemulsion globules contained. Nanoemulgel pH is greater than the normal skin pH range; however, nanoemulgel preparation approaching neutral pH does not cause irritation. Irritation may occur on topical preparation with a pH of 9 or greater [36]. Nanoemulgel has a spreadability of 5.69 ± 0.08, which was in the specification range of less than 7 cm. The suitable range of spreadability for semifluid preparation should be 5–7 cm [17].
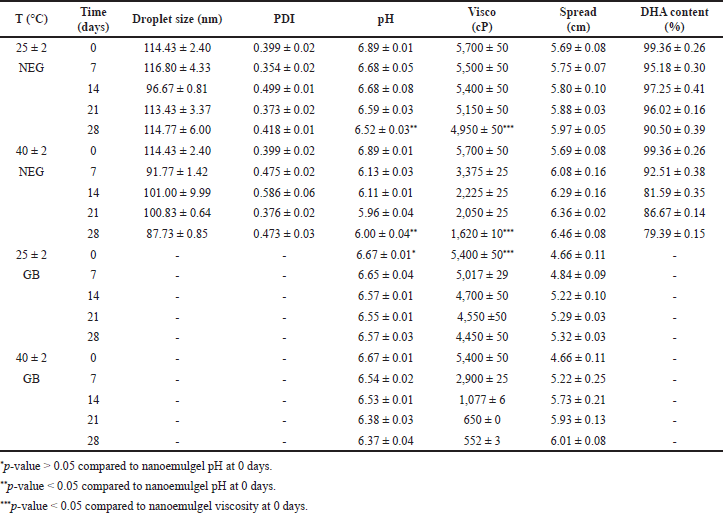 | Table 8. Nanoemulgel stability test over time at various temperatures (mean ± SD, n = 3). [Click here to view] |
There were no significant differences in characteristics between base gel and after the addition of catfish oil nanoemulsion. In the case of the rheological profile, the nanoemulgel underwent a transition from gel to sol and showed shear thinning after the application of shear stress. However, a slow reversion to gel started after the removal of the stress. This indicates pseudoplastic behavior with thixotropy properties [37]. Topical preparations with pseudoplastic properties have a thick viscosity in the container but are easy to pour and provide good spreadability so that they can increase permeation after topical administration [38].
Accelerated stability and acute dermal irritation tests
As presented in Table 8, nanoemulsion droplet size in nanoemulgel was relatively stable at both temperatures during the storage period, i.e., in the range of 80–120 nm. Nanoemulgel has a polydispersity index <0.5, this shows the homogeneity of the nanoemulsion droplet size in nanoemulgel at both temperatures during the storage.
The nanoemulgel pH was not significantly different compared to the base gel with a p-value > 0.05; however, pH decreased significantly with a p-value < 0.05 at both temperatures. The pH of the catfish oil nanoemulgel tends to be a more stable nanoemulsion that has a liquid consistency. Based on Stokes’ law, preparations with higher viscosity will increase system stability [39]. Nanoemulgel viscosity and spreadability have differed significantly compared to base gel with a p-value < 0.05. The viscosity of the nanoemulgel decreased significantly with a p-value < 0.05 at both temperatures but can provide good spreadability for topical application.
The content of the main active component, DHA during storage at room temperature was relatively stable. Higher viscosity tends to protect DHA in the nanoemulgel system from degradation via inhibition of the migration and transfer of reactive species during oxidation [40]. When the nanoemulgel was stored at a higher temperature of 40°C for 28 days, DHA levels were degraded by 19.97%. This phenomenon occurred due to the presence of ethylene double bonds in the DHA molecule that are broken by methylene, making it sensitive to oxygen and temperature [41].
Rabbits showed no skin irritation signs after applying catfish oil nanoemulgel. The treated skin had no erythema and edema compared to control. Erythema and edema score was 0 in each rabbit at any interval time of the observation. Catfish oil nanoemulgel does not cause irritation characterized by a Primary Irritation Index score was 0.
CONCLUSION
Catfish oil purification has been successfully carried out with the aim of improving the organoleptic properties of catfish oil without significantly reducing DHA levels. The catfish oil purifying process was conducted using nanobentonite adsorbent with an average particle size of 508.47 ± 10.47 nm at 10%. The optimum nanoemulsion was produced from catfish oil, cremophor RH40, and PEG 400 in the ratio of 1:8:1. The catfish oil nanoemulsion showed an average droplet size of 21.03 ± 0.23 nm, PDI of 0.269 ± 0.04, pH of 6.83 ± 0.02, and zeta potential of −15 ± 2.57 mV. The nanoemulsion was successfully incorporated in the 2% Sodium Carboxymethylcellulose (CMC-Na) gel with a particle size of 114.4 ± 2.40 nm, PDI 0.399 ± 0.02, pH 6.89 ± 0.01, viscosity 5,700 ± 50 cps, spreadability 5.69 ± 0.08 cm, physicochemically stable at a storage temperature of 25°C and no indication of irritation. Overall, nanoemulgel catfish oil is promising to be an alternative for burn wound healing.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
FUNDING
There is no funding to report..
ETHICAL APPROVALS
The study protocols were approved by the ethics committee of the Faculty of Dental Medicine, Health Research Ethical Clearance Comission, Airlangga University (Approval No. 747/HRECC.FODM/IX/2022).
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Heidari M, Bahramsoltani R, Abdolghaffari AH, Rahimi R, Esfandyari M, Baeeri M, et al. Efficacy of topical application of standardized extract of Tragopogon graminifolius in the healing process of experimental burn wounds. J Tradit Complement Med. 2019;9(1):54–9. CrossRef
2. Yakupu A, Zhang J, Dong W, Song F, Dong J, Lu S. The epidemiological characteristic and trends of burns globally. BMC Public Health. 2022 Aug 22;22(1):1596. CrossRef
3. Zwierello W, Piorun K, Skorka-Majewicz M, Maruszewska A, Antoniewski J, Gutowska I. Burns: classification, pathophysiology, and treatment: a review. Int J Mol Sci. 2023 Feb 13;24(4):3749. CrossRef
4. McDaniel JC, Belury M, Ahijevych K, Blakely W. Omega-3 fatty acids effect on wound healing. Wound Repair Regen. 2008;16(3):337–45. CrossRef
5. Ishak WM, Katas H, Yuen NP, Abdullah MA, Zulfakar MH. Topical application of omega-3-, omega-6-, and omega-9-rich oil emulsions for cutaneous wound healing in rats. Drug Deliv Transl Res. 2019;9(2):418–33. CrossRef
6. Haiyee ZA, Yahya NI, Rashid NA, Hashim DM. Characterisation of catfish (Clarias batrachus) oil: β-cyclodextrin inclusion complex. Malays J Anal Sci. 2016;20(4):838–43. CrossRef
7. Rizliya V, Mendis E. Biological, physical, and chemical properties of fish oil and industrial applications. In: Kim SK, editor. Seafood processing by-products: trends and applications. New York, NY: Springer New York; 2013. Vol. 11, pp 285–313. CrossRef
8. Sathivel S, Prinyawiwatkul W. Adsorption of FFA in crude catfish oil onto chitosan, activated carbon, and activated earth: a kinetics study. J Am Oil Chem Soc. 2004;81(5):493–6. CrossRef
9. De Oliveira DA, Minozzo MG, Licodiedoff S, Waszczynskyj N. Physicochemical and sensory characterization of refined and deodorized tuna (Thunnus albacares) by-product oil obtained by enzymatic hydrolysis. Food Chem. 2016 Sep 15;207:187–94. CrossRef
10. Dinamarca E, Garrido F, Valenzuela A. Simple high vacuum distillation equipment for deodorizing fish oil for human consumption. Lipids. 1990 Mar;25(3):170–1. CrossRef
11. Alfio VG, Manzo C, Micillo R. From fish waste to value: an overview of the sustainable recovery of omega-3 for food supplements. Molecules. 2021;26(4):1002. CrossRef
12. Nadhiro U, Subekti S, Tjahjaningsih W. Quality characteristics of Bali sardinella (Sardinella lemuru) oil purified with bentonite as an adsorbent. IOP Conf Ser Earth Environ Sci. 2018;137(1):012012. CrossRef
13. Algahtani MS, Ahmad MZ, Nourein IH, Albarqi HA, Alyami HS, Alyami MH, et al. Preparation and characterization of curcumin nanoemulgel utilizing ultrasonication technique for wound healing: in vitro, ex vivo, and in vivo evaluation. Gels. 2021 Nov 14;7(4):213. CrossRef
14. Nazir N, Diana A, Sayuti K. Physicochemical and fatty acid profile of fish oil from head of tuna (Thunnus albacares) extracted from various extraction method. Int J Adv Sci Eng Inf Technol. 2017;7(2):709–15. CrossRef
15. Harahap IA, Sobral MM, Casal S, Pinho S, Faria MA, Suliburska J, et al. Fat oxidation of fatty fish vs. meat meal diets under in vitro standardized semi-dynamic gastric digestion. Front Nutr. 2022 Jun 30;9:901006. CrossRef
16. Rachmawati H, Budiputra DK, Mauludin R. Curcumin nanoemulsion for transdermal application: formulation and evaluation. Drug Dev Ind Pharm. 2015;41(4):560–6. CrossRef
17. Garg A, Aggarwal D, Garg S, Singla AK. Spreading of semisolid formulations: an update. Pharm Technol North America. 2002;26(9):84.
18. Ahmad J, Gautam A, Komath S, Bano M, Garg A, Jain K. Topical nano-emulgel for skin disorders: formulation approach and characterization. Recent Pat Antiinfect Drug Discov. 2019;14(1):36–48. CrossRef
19. Organization for Economic Co-Operation and Development (OECD) [Internet]. Testing guideline 404: OECD guidelines for testing of chemicals, adopted guideline 404: acute dermal irritation/corrosion. J. Chem. OECD; 2002 [cited 2023 Oct 17]. CrossRef
20. Toor M, Jin B, Dai S, Vimonses V. Activating natural bentonite as a cost-effective adsorbent for removal of Congo-red in wastewater. J Ind Eng Chem. 2015;21:653–61. CrossRef
21. Nasuha N, Hameed BH, Din AT. Rejected tea as a potential low-cost adsorbent for the removal of methylene blue. J Hazard Mater. 2010;175(1–3):126–32. CrossRef
22. Tsai WT, Lai CW, Hsien KJ. Effect of particle size of activated clay on the adsorption of paraquat from aqueous solution. J Colloid Interface Sci. 2003;263(1):29–34. CrossRef
23. Rachmawati H, Novel MA, Ayu S, Berlian G, Tandrasasmita OM, Tjandrawinata RR, et al. The in vitro–in vivo safety confirmation of PEG-40 hydrogenated castor oil as a surfactant for oral nanoemulsion formulation. Sci Pharm. 2017;85(2):18. CrossRef
24. Soliman SM, Abdelmalak NS, El-Gazayerly ON, Abdelaziz N. Novel non-ionic surfactant proniosomes for transdermal delivery of lacidipine: optimization using 23 factorial design and in vivo evaluation in rabbits. Drug Deliv. 2016;23(5):1608–22. CrossRef
25. Bali V, Ali M, Ali J. Study of surfactant combinations and development of a novel nanoemulsion for minimising variations in bioavailability of ezetimibe. Colloids Surf B Biointerfaces. 2010;76(2):410–20. CrossRef
26. Weerapol Y, Limmatvapirat S, Nunthanid J, Sriamornsak P. Self-nanoemulsifying drug delivery system of nifedipine: impact of hydrophilic–lipophilic balance and molecular structure of mixed surfactants. AAPS PharmSciTech. 2014;15(2):456–64. CrossRef
27. Chen YS, Chiu YH, Li YS, Lin EY, Hsieh DK, Lee CH, et al. Integration of PEG 400 into a self-nanoemulsifying drug delivery system improves drug loading capacity and nasal mucosa permeability and prolongs the survival of rats with malignant brain tumors. Int J Nanomed. 2019;14:3601–13. CrossRef
28. Azeem A, Rizwan M, Ahmad FJ, Iqbal Z, Khar RK, Aqil M, et al. Nanoemulsion components screening and selection: a technical note. AAPS PharmSciTech. 2009;10(1):69–76. CrossRef
29. Jyothi BJ, Sreelakshmi K. Design and evaluation of self-nanoemulsifying drug delivery system of flutamide. J Young Pharm. 2011;3(1):4–8. CrossRef
30. Hoeller S, Sperger A, Valenta C. Lecithin based nanoemulsions: a comparative study of the influence of non-ionic surfactants and the cationic phytosphingosine on physicochemical behaviour and skin permeation. Int J Pharm. 2009;370(1–2):181–6. CrossRef
31. Ali SM, Yosipovitch G. Skin pH: from basic science to basic skin care. Acta Derm Venereol. 2013;93(3):261–7. CrossRef
32. Liang J, Li H, Yan J, Hou W. Demulsification of oleic-acid-coated magnetite nanoparticles for cyclohexane-in-water nanoemulsions. Energy Fuels. 2014;28(9):6172–8. CrossRef
33. Adi AC, Christanto C, Rachmawati H, Adlia A. Vitamin E-based folic acid nanoemulsion: formulation and physical evaluation for oral administration. Pharma Nanotechnol. 2019;7(4):304-13. CrossRef
34. Bernardi DS, Pereira TA, Maciel NR, Bortoloto J, Viera GS, Oliveira GC, et al. Formation and stability of oil-in-water nanoemulsions containing rice bran oil: in vitro and in vivo assessments. J Nanobiotechnol. 2011;9:44. CrossRef
35. Cheong AM, Tan CP, Nyam KL. Physicochemical, oxidative and anti-oxidant stabilities of kenaf seed oil-in-water nanoemulsions under different storage temperatures. Ind Crops Prod. 2017;95:374–82. CrossRef
36. Paudel KS, Milewski M, Swadley CL, Brogden NK, Ghosh P, Stinchcomb AL. Challenges and opportunities in dermal/transdermal delivery. Ther Deliv. 2010;1(1):109–31. CrossRef
37. Algahtani MS, Ahmad MZ, Ahmad J. Nanoemulgel for improved topical delivery of retinyl palmitate: formulation design and stability evaluation. Nanomaterials. 2020;10(5):848. CrossRef
38. Md S, Alhakamy NA, Aldawsari HM, Kotta S, Ahmad J, Akhter S, et al. Improved analgesic and anti-inflammatory effect of diclofenac sodium by topical nanoemulgel: Formulation development—in vitro and in vivo studies. J Chem. 2020;2020:4071818. CrossRef
39. Chang WC, Hu YT, Huang Q, Hsieh SC, Ting Y. Development of a topical applied functional food formulation: Adlay bran oil nanoemulgel. LWT. 2020;117:108619. CrossRef
40. Wang C, Sun C, Lu W, Gul K, Mata A, Fang Y. Emulsion structure design for improving the oxidative stability of polyunsaturated fatty acids. Compr Rev Food Sci Food Saf. 2020;19(6):2955–71. CrossRef
41. Fournier V, Destaillats F, Juanéda P, Dionisi F, Lambelet P, Sébédio JL, et al. Thermal degradation of long-chain polyunsaturated fatty acids during deodorization of fish oil. Eur J Lipid Sci Technol. 2006;108(1):33–42. CrossRef