INTRODUCTION
With the increasing COVID-19 vaccination rate, many individuals around the globe were prepared to restore their normal lifestyle, particularly those who were fully vaccinated or received the third booster shot (Gowrisankar et al., 2022). However, the end of 2021 reported a new highly contagious variant, later named omicron by the World Health Organization (WHO) (Kannan et al., 2021). International reports warned that this variant is a new severe form of the SARS-CoV-2 virus, and it has a higher risk of transmissibility, posing serious repercussions on the global population’s health, and the economy’s recovery from the third year of the pandemic (Singhal, 2022). The omicron variant was categorized as a variant of concern due to its high number of mutations on its spike protein (Ghosh et al., 2022; Miller et al., 2022). The latter raised global concerns about viral transmissibility, pathogenicity, and immune evasion (Meo et al., 2021). On the other hand, the presumed has brought up some speculations, whether the current COVID-19 vaccines could effectively resist this new strain, and if more robust protection may be achieved by a third or even a fourth dose of vaccine (Chen et al., 2022; Garcia-Beltran et al., 2022). This constant SARS-CoV-2 virus change through mutations has created doubts and uncertainty on the public’s willingness to receive the vaccine for the unvaccinated, or take the booster shot for vaccinated individuals (Kannan et al., 2021; Lu et al., 2021). A recent Chinese study explored the public acceptance of the third dose of the COVID-19 vaccine. Although the majority of the study participants (93.7%) showed a high willingness to receive the third dose of COVID-19 vaccines, older participants and those who had a low educational level, low monthly income, low perception of the benefit of COVID-19 vaccine, high level of barriers, and less knowledge regarding COVID-19 were less likely to accept a third dose of COVID-19 vaccine (Wang et al., 2021). On the other hand, an American study reported that around 38% of the participants enrolled in the study were hesitant toward receiving a booster dose of the COVID-19 vaccine. Being unvaccinated and lack of trust in COVID-19 vaccines were among the factors that were associated with hesitancy to the COVID-19 vaccine booster dose (Yadete et al., 2021).
The omicron variant has fueled the exponential rise in COVID-19 cases in the Middle Eastern region (Reuters, 2022). Despite a 13% decrease in COVID-19-related deaths, cases in the region surged by 89% in the first week of January (Middle East Monitor, 2022). In Jordan, omicron accounts for 90% of the daily COVID-19 cases (Jordan Times, 2022). While in Lebanon, the Ministry of Public Health registered the highest number of COVID-19 cases since the beginning of the pandemic; however, still COVID-19 vaccination remains a personal choice (Al Jazeera, 2022). Nonetheless, in Kuwait, given the rising number of cases, the COVID-19 vaccine booster was made compulsory for all citizens and travelers to the country (Times of India Travel, 2021).
To date, limited studies are available in the literature to address the public’s perceptions and willingness to take or continue COVID-19 vaccination after the surge of this new variant. Given the scant data of COVID-19 vaccine effectiveness on the omicron variant, the main aim of the study is to identify the Middle Eastern public’s willingness to take or continue COVID-19 vaccination with the emerging omicron variant. Highlighting this important aspect is essential in directing international health agencies and the local Ministry of Health in identifying what types of endorsements and awareness messages are necessary to convince unvaccinated individuals to receive the COVID-19 vaccine, and vaccinated ones to take the booster shot.
MATERIALS AND METHODS
A web-based survey, using a cross-sectional design, was conducted during the omicron wave of the COVID-19 pandemic for 3 weeks, during December 2021. A bilingual structured questionnaire was set up using Google Forms and the generated link was shared through various social networking platforms (i.e., Facebook, WhatsApp, Twitter, and Instagram), targeting the general population (age >18 years) residing in the Middle Eastern Arabic-speaking countries, including Lebanon, Jordan, and Kuwait.
The sample size was calculated using the following formula for cross-sectional studies developed by Daniel and Cross (2013). Since the prevalence rate of the COVID-19 vaccine acceptance in some Arab countries varied between 20% and 62.4% (Abu-Farha et al., 2021; Kaadan et al., 2021). We assumed that the expected acceptance rate in the recruited countries would be around 50%, where n is the sample size, Z is the Z-value for 95% confidence limits (which is 1.96 when α = 0.05), P is the estimated prevalence of COVID-19 vaccine acceptance (which is 50%), and d is the desired precision (which is 4% in this study). The required sample size was 600 and increased by 20% for possible dropout and incomplete responses. Thus, the estimated sample size was 720.
A questionnaire was designed by the study’s principal investigator after reviewing relevant studies (Abu-Farha et al., 2021; Zhou et al., 2021). Two experts in public health and pharmacy practice, other than the research team, revised the questionnaire for its comprehensiveness, relevance, ease of completion, and its face and content validity. Afterward, the questionnaire was translated to colloquial Arabic (the participants’ native language) by one author, and back-translated by another author, for validation.
The questionnaire consisted of 15 close-ended questions with predefined options, divided into four sections preceded by the study introduction. The introduction outlined the study’s nature, purpose, anonymity, and estimated time for completion, followed by an informed consent statement. The first section was dedicated to retrieve participants’ sociodemographic data, including age, gender, country of residence, as well as educational and employment status. The second section recorded the participants’ general health status, previous infection with COVID-19, intake of the COVID-19 vaccine, and the number of vaccine doses being received. While the third section was concerned with the participants’ perception of the new variant of the COVID-19 (omicron) that would influence the population’s readiness to receive the COVID-19 vaccine. The fourth section investigated the participants’ willingness to receive the next dose of the COVID-19 vaccine (for those who did not receive any dose yet or took less than three doses). Moreover, participants were inquired about their acceptance to take any newly developed vaccine that tackles the new COVID-19 variant.
A pilot test was carried out among 20 volunteers from the general population in Jordan and Lebanon, using a convenience sampling approach. Ten participants completed the English version and the other 10 completed the Arabic version. The participants were requested to evaluate the questionnaire structure, clarity, cultural acceptability, and to give heir overall impression. Then, some questions were adjusted based on their feedback. Two days later, the questionnaire was retested on the same participants to ensure its reliability and reproducibility. The data obtained from the pilot test were discarded and not included in the final data analysis.
The study design and conduction followed the World Medical Association’s Declaration of Helsinki guidance. In addition, the study’s protocol was approved by the Institutional Review Board of King Abdullah University Hospital, University of Science & Technology, Jordan (Ref: 20210608). Participation in the study was voluntary, and the purpose of the study was explained before accessing the questionnaire. Electronic informed consent of the participants was obtained, and participants had the right to defer from submitting their responses at any time.
Study data were entered into IBM statistical package for social sciences (IBM SPSS Statistics, version 22.0, Chicago, Illinois) for data analysis. Descriptive analyses were presented as mean ± standard deviation (SD) and frequency for continuous variables and categorical variables, respectively.
RESULTS
In this cross-sectional study, 812 individuals were recruited. The mean age of the study sample was 28.52 ± 12.31, and females represent three-quarters of them (n = 599). Around two-thirds of the sample have a diploma or bachelor degree (n = 574, 70.7%), and they were recruited from three different countries: Lebanon (n = 427, 52.6%), Jordan (n = 279, 34.4%), and Kuwait (n = 106, 13.1%). Almost 28% of the sample have a medical-related degree (n = 231, 28.4%), and only12.9% of them have chronic medical conditions. Regarding COVID-19 infection, 32.1% of the participants (n = 261) reported that they have been infected with COVID-19 before taking the vaccine, while 3.8% of them (n = 31) have been infected after taking the vaccine. For more details about the sociodemographic and medical characteristics, refer to Table 1.
Participants were asked about the number of doses they received from COVID-19 vaccines, and 88.7% of the participants reported that they have received at least one dose of the vaccine as follow: 30 participants received only one dose (3.7%), 628 received two doses (77.3%), and 64 participants received three doses (7.9%). On the other hand, 90 participants did not receive any COVID-19 vaccine. All participants were asked about their willingness to get the vaccine or take an additional dose after the new omicron variant. Results showed that 22.2% (20/90) of those who did not receive the vaccine at all were willing to take the vaccine, while a higher percentage of 66.7% of those who have received one dose were willing to take the second dose. Regarding participants who took two doses, 49.2% of them (n = 309) were willing to take the third dose. Finally, 596 participants (73.4%) reported willingness to take any newly developed vaccine that will target the new COVID-19 variant (omicron). Further details are present in Table 2.
DISCUSSION
In spite of the fact that enormous efforts are rendered in the development of effective vaccines against the COVID-19 virus, the vaccination coverage rate among the population is an important factor that decides the success of the immunization process (Feleszko et al., 2021). Vaccinations that were developed before the COVID-19 pandemic took years of thoughtful clinical trials that ensured the safety and efficacy of these vaccines to be ready to be distributed globally. However, the COVID-19 vaccine was developed in such a short period, which may have raised doubts among the public about the safety of the vaccine, leading to hesitancy toward receiving COVID-19 vaccines (Feleszko et al., 2021). Furthermore, after the emergence of the omicron variant, the effectiveness of COVID-19 vaccines against this variant is reduced (Accorsi et al., 2022), which could have increased public suspicion regarding the effectiveness of COVID-19 vaccines. Therefore, this study aimed to evaluate the willingness of the Middle East population to take or continue with COVID-19 vaccination after the emergence of the omicron variant.
At the time of this survey, the number of COVID-19 cases and deaths in Lebanon, Jordan, and Kuwait were over 20,029 cases (population was ~6.8 million) with 109 deaths (World Health Organization, 2022a), 12,856 cases (population was ~10.3 million) with 240 deaths (World Health Organization, 2022b), and 2,812 cases (population was ~4.2 million) and 2 deaths (World Health Organization, 2022c), respectively. The current study revealed that 77% of the Middle Eastern participants received two doses of the COVID-19 vaccine. When comparing with other countries similar vaccination rates of two doses were reported in Canada (77.1%) (Government of Canada, 2022). While in Emirates, Portugal, and United Kingdom, estimates were much higher accounting for 93.7%, 92%, and 84%, respectively. However, relatively lower estimates of two COVID-19 vaccine doses were reported in some other countries such as Russia (52%), India (47%), and Pakistan (34.6%) (Our World in Data, 2022). What is catastrophic, there are some countries where less than 10% of the population is vaccinated such as Syria (4.5%), Yemen (1.2%), Nigeria (2.2%), and Afghanistan (9.6%) (Our World in Data, 2022). This gap could be explained due to inequity in vaccine supply distribution, which remains a main global concern, but also there are other reasons in nations with conflict zones where the COVID-19 pandemic is not a priority (Singhal, 2022). Many of these countries have had a deteriorated healthcare system even before the pandemic, and some of them have electricity shortages to be able to store certain mRNA vaccines at extremely low temperatures in the refrigerators (Aziz et al., 2020).
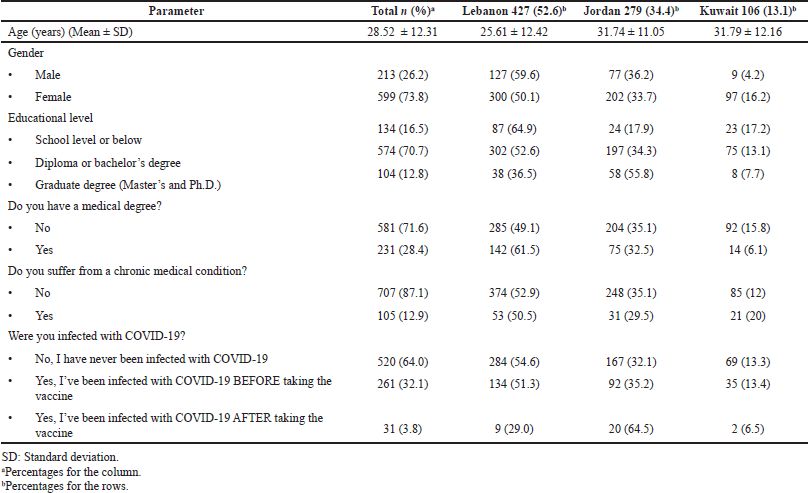 | Table 1. Sociodemographic and medical characteristics of the study sample (N = 812). [Click here to view] |
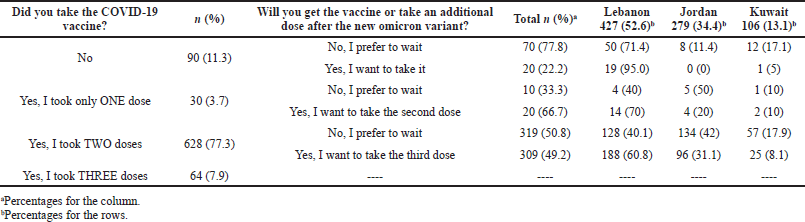 | Table 2. Public willingness to take the COVID-19 vaccine after the emergence of the new omicron variant (N = 812). [Click here to view] |
As for the proportion of individuals who received the booster shot (third dose), it is represented by only 7.9%. This finding reflects a low rate of COVID-19 vaccine boosters received by the participants. A significantly higher proportion of boosted doses received were reported in the United Kingdom (63.4%), Bahrain (53.9%), Emirates (42%), Portugal (39.7%), France (39.2%), USA (38.7%), and Canada (34.5%) (Government of Canada, 2022; Our World in Data, 2022; Wang et al., 2021). A recent laboratory study indicated that three doses of the mRNA COVID-19 vaccine increased neutralizing antibodies titers by 25 times more than compared to two doses against the omicron variant. As such, it indicated that two doses may not be sufficient to prevent the omicron variant infection (Garcia-Beltran et al., 2022). Furthermore, a recent study conducted in Brazil and Scotland demonstrated that the ChAdOx1 nCoV-19 vaccine declines in protecting severe COVID-19, and improving survival were only observed a few months following two vaccine doses (Our World in Data, 2022).
The mentioned countries having a higher vaccination rate are imposing government banning policies and movement restrictions from traveling, shopping, and restaurant visits on the unvaccinated (Wu et al., 2022). Recently in Canada, in Quebec province, they were planning to sweep a health contribution tax to all unvaccinated adults, since 10% of the remaining unvaccinated account for about 50% of those in intensive care units (Government of Canada, 2022), in addition to the financial burden implied on the government and on vaccinated individuals (Government of Canada, 2022). It is also worth mentioning that in this study the surge of the omicron variant slightly increased the willingness of unvaccinated individuals to take the first dose of the COVID-19 vaccine by 22.4%. Among the vaccinated, they reported that the omicron variant makes them more likely to continue taking any developed COVID-19 vaccine targeting this new variant. Our findings have important implications on the COVID-19 vaccination decision. As the pandemic changes, the public’s opinions with the surge of different variants are fluctuating. Therefore, there is an urgent need to raise awareness about the omicron severity, and that the rapidly spreading strain puts the unvaccinated particularly at risk. Vaccine campaigns should elevate the voices of influencing messengers such as healthcare providers caring for severe COVID-19 cases.
Finally, although this study was proactive in investigating the Middle Eastern’s acceptance to receive the COVID-19 vaccine during the omicron pandemic, some limitations must be pointed out. First, this study was limited by its relatively small scale, since it was conducted only in three Middle Eastern Arab countries, where the findings may not be generalizable to the Arab world due to diversity in their economic status and political directions, although they share a prevailing culture. Second, we recruited a convenience sample of participants via social media, which may have introduced selection bias, limiting the generalizability of results to the general population. Third, we also did not retrieve the predictors that might influence participants’ acceptance to receive the COVID-19 vaccine. Nonetheless, this study was only interested in investigating the acceptance rate to receive the COVID-19 vaccine during the omicron outbreak and did not intend to distinguish the causality of the participants’ responses.
CONCLUSION
This study has generally reflected a low rate in unvaccinated individuals’ willingness in favor of taking the COVID-19 vaccine in response to the new omicron variant, but higher acceptability of vaccinated individuals to continue COVID-19 vaccination. Therefore, our findings highlight the need to consider expanding this type of research to other countries. This would provide a clearer picture of the perception of COVID-19 vaccination decisions in the era of new variants. Moreover, conducting qualitative research may help understand the predictors that hinder their acceptance in taking the COVID-19 vaccine and whether the emergence of new variants would change their minds.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The study design and conduction followed the World Medical Association’s Declaration of Helsinki guidance. the study’s protocol was approved by the Institutional Review Board of King Abdullah University Hospital, University of Science & Technology, Jordan (Ref: 20210608). Participation in the study was voluntary, and the purpose of the study was explained before accessing the questionnaire. Electronic informed consent of the participants was obtained, and participants had the right to defer from submitting their responses at any time.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abu-Farha R, Mukattash T, Itani R, Karout S, Khojah H, Al-Mahmood A, Alzoubi KH. Willingness of Middle Eastern public to receive COVID-19 Vaccines. Saudi Pharm J, 2021; 29:734–9. CrossRef
Accorsi EK, Britton A, Fleming-Dutra KE, Smith ZR, Shang N, Derado G, Miller J, Schrag SJ, Verani JR. Association between 3 doses of mRNA COVID-19 vaccine and symptomatic infection caused by the SARS-CoV-2 omicron and delta variants. JAMA, 2022; 327:639–51. CrossRef
Aziz AB, Raqib R, Khan WA, Rahman M, Haque R, Alam M, Zaman K, Ross AG. Integrated control of COVID-19 in resource-poor countries. Int J Infect Dis, 2020; 101:98–101. CrossRef
Chen J, Wang R, Gilby NB, Wei G-W. Omicron variant (B.1.1.529): infectivity, vaccine breakthrough, and antibody resistance. J Chem Inf Model, 2022; In Press:1–11. CrossRef
Daniel, Wayne W., and Chad L. Cross. Biostatistics: a foundation for analysis in the health sciences. John Wiley & Sons, Hoboken, New Jersey, U.S. 2018.
Feleszko W, Lewulis P, Czarnecki A, Waszkiewicz P. Flattening the curve of COVID-19 vaccine rejection—an international overview. Vaccines, 2021; 9:44. CrossRef
Garcia-Beltran WF, St Denis KJ, Hoelzemer A, Lam EC, Nitido AD, Sheehan ML, Berrios C, Ofoman O, Chang CC, Hauser BM, Feldman J, Roederer AL, Gregory DJ, Poznansky MC, Schmidt AG, Iafrate AJ, Naranbhai V, Balazs AB. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell, 2022; 185:457–66. CrossRef
Ghosh A, Kar PK, Gautam A, Gupta R, Singh R, Chakravarti R, Ravichandiran V, Dastidar SG, Ghosh D, Roy S. An insight into SARS-CoV-2 structure, pathogenesis, target hunting for drug development and vaccine initiatives. RSC Med Chem, 2022; 1–36. CrossRef
Government of Canada. Vaccines for COVID-19. CanadaCa, 2022. Available vaihttps://www.canada.ca/en/public-health/services/diseases/coronavirus-disease-covid-19/vaccines.html (accessed 1 March 2022).
Gowrisankar A, Priyanka TMC, Banerjee S. Omicron: a mysterious variant of concern. Eur Phys J Plus, 2022; 137:100. CrossRef
Al Jazeera. ‘Vaccine dictatorship’: Many Lebanese refuse the COVID jab 2022. https://www.aljazeera.com/news/2022/1/14/lebanon-vaccine-hesitancy (accessed 2 March 2022).
Jordan Times. Omicron accounts for 90% of daily COVID cases in Kingdom 2022. Available via https://www.jordantimes.com/news/local/omicron-accounts-90-daily-covid-cases-kingdom (accessed 3 March 2022).
Kaadan MI, Abdulkarim J, Chaar M, Zayegh O, Keblawi MA. Determinants of COVID-19 vaccine acceptance in the Arab world: a cross-sectional study. Glob Heal Res Policy, 2021; 6:23. CrossRef
Kannan S, Shaik Syed Ali P, Sheeza A. Omicron (B.1.1.529)—variant of concern—molecular profile and epidemiology: a mini review. Eur Rev Med Pharmacol Sci, 2021; 25:8019–22.
Lu L, Mok BW-Y, Chen L-L, Chan JM-C, Tsang OT-Y, Lam BH-S, et al. Neutralization of SARS-CoV-2 Omicron variant by sera from BNT162b2 or Coronavac vaccine recipients. Clin Infect Dis, 2021; ciab1041. CrossRef
Meo SA, Meo AS, Al-Jassir FF, Klonoff DC. Omicron SARS-CoV-2 new variant: global prevalence and biological and clinical characteristics. Eur Rev Med Pharmacol Sci, 2021; 25:8012–8.
Middle East Monitor. WHO warns Omicron behind COVID surge across Eastern Mediterranean, 2022. Availble via https://www.middleeastmonitor.com/20220114-who-warns-omicron-behind-covid-surge-across-eastern-mediterranean/ (accessed 3 March 2022).
Miller NL, Clark T, Raman R, Sasisekharan R. Insights on the mutational landscape of the SARS-CoV-2 Omicron variant receptor-binding domain. Cell Reports Med, 2022; 3:100527. CrossRef
Our World in Data. Coronavirus (COVID-19) vaccinations, 2022. Available via https://ourworldindata.org/covid-vaccinations (accessed 2 March 2022).
Reuters. Omicron probably caused COVID-19 surge in Mideast in early Jan -WHO official, 2022. Available via https://www.reuters.com/world/middle-east/covid-19-cases-surged-mideast-early-jan-deaths-fell-who-official-2022-01-13/ (accessed 3 March 2022).
Singhal T. The emergence of omicron: challenging times are here again! Indian J Pediatr, 2022; 1–7. CrossRef
Times of India Travel. Kuwait makes COVID-19 booster dose mandatory for travellers, Kuwait. Timestravel, 2021. Available via https://timesofindia.indiatimes.com/travel/travel-news/kuwait-makes-covid-19-booster-dose-mandatory-for-travellers/as88478268.cms (accessed 2 March 2022).
Wang R, Tao L, Han N, Liu J, Yuan C, Deng L, Han C, Sun F, Chi L, Liu M, Liu J. Acceptance of seasonal influenza vaccination and associated factors among pregnant women in the context of COVID-19 pandemic in China: a multi-center cross-sectional study based on health belief model. BMC Pregnancy Childbirth, 2021; 21:745. CrossRef
World Health Organization. Lebanon: WHO coronavirus disease (COVID-19) dashboard with vaccination data | WHO coronavirus (COVID-19) dashboard with vaccination data. WHO 2022a. Available via https://covid19.who.int/region/emro/country/lb (accessed 3 March 2022).
World Health Organization. Jordan: WHO coronavirus disease (COVID-19) dashboard with vaccination data | WHO coronavirus (COVID-19) dashboard with vaccination data. WHO 2022b. Available via https://covid19.who.int/region/emro/country/jo (accessed 3 March 2022).
World Health Organization. Kuwait: WHO coronavirus disease (COVID-19) dashboard with vaccination data | WHO coronavirus (COVID-19) dashboard with vaccination data. WHO 2022c. Available via https://covid19.who.int/region/emro/country/kw (accessed 3 March 2022).
Wu M, Wall EC, Carr EJ. Three-dose vaccination elicits neutralising antibodies against omicron. Lancet Glob Heal, 2022; 399:715–7. CrossRef
Yadete T, Batra K, Netski DM, Antonio S, Patros MJ, Bester JC. Assessing acceptability of COVID-19 vaccine booster dose among adult americans: a cross-sectional study. Vaccines, 2021; 9:1424. CrossRef
Zhou Y, Zhang J, Wu W, Liang M, Wu Q-S. Willingness to receive future COVID-19 vaccines following the COVID-19 epidemic in Shanghai, China. BMC Public Health, 2021; 21:1103. CrossRef