INTRODUCTION
Mollusks represent the second-largest invertebrate animal phylum after Arthropoda (Baldwin, 2003; Rusmore-Villaume, 2008). They are also considered one of the most important elements in food chains as a balanced source of protein (Haszprunar and Wanninger, 2012) and represent the most prominent members of marine faunal ecosystems. Some species have direct and indirect commercial importance and even medical benefits to humans (Rusmore-Villaume, 2008) and are utilized as prospective resources of bioactive products in numerous cultures worldwide (Khan et al., 2009).
Recently, the molluscan natural products (high content of proteins, vitamins, and trace elements) have become very valuable foodstuffs, and their synthetically formulated structural analogs have been recognized in clinical trials as promising therapeutic agents for many diseases (Alves and Diederich, 2021; Tabakaeva et al., 2018). Marine bivalves are considered a rich source of antimicrobial peptides that possess various biological activities such as antibacterial, antioxidant, anticoagulant, anti-inflammatory, and anticarcinogenic (Galdiero et al., 2015). The main cause of their bioactivities is the presence of biologically active peptides (Alves and Diederich, 2021). Bivalves occurring in Egypt represent a neglected animal group, and little is known about them or their diversity and importance (Temraz, 2016). Therefore, more light should be shed on their chemical and biological aspects.
In particular, Paratapes undulatus, common name undulate Venus, is a species of saltwater clams. These clams are a popular food in Egypt, inhabiting the inshore shallow sandy seabed in Timsah Lake, located near Ismailia City at the midpoint of the Suez Canal and about 80?km south of Port Said, Egypt (Loftus et al., 2015). Lately, a broad spectrum of naturally occurring bioactive molecules separated from the mollusks was utilized in the manufacture of several medications (Alves and Diederich, 2021). The strong impact of antimicrobial resistance on healthcare and the economy has led to a crucial demand to secure unique molecules with novel modes of action to reduce the harmful effects of infectious diseases (Ghareeb et al., 2015). Several antimicrobial compounds have been isolated from peptides subunits of marine bivalves due to their thorough capability to resist infection with different types of viruses (Khan et al., 2009). They also exhibit a wide range of antibacterial activities against Gram-positive and Gram-negative bacteria, as well as yeast (Burge et al., 2014; Schmitt et al., 2012). The rapid and increasing development of antimicrobial resistance is one of the current global health challenges. Pathogenic microorganisms have the capability to hinder the effect of antimicrobial agents, which leads to raising the accompanying hazards of infections via pathogenic microorganisms like bacteria, fungi, viruses, and parasites. Furthermore, the appearance of novel pathogens like SARS, H1N1, and various types of influenza has become a vital communal health risk. To overcome this phenomenon, scientists have worked to discover novel antimicrobial and antiviral pharmaceuticals from natural resources like plants, marine organisms, mollusks, and fungal extracts (Abdel-Aziz et al., 2018, 2021; Elkhouly et al., 2021a; Ghareeb et al., 2014a, 2019; Hamed et al., 2020).
On the other hand, overproduction of reactive species led to the initiation of oxidative stress that in turn initiates several dangerous disorders such as “cancer, cardiovascular, and inflammation.” Moreover, the accompanying destructive side effects can be reduced using naturally occurring antiradical ingredients that act as strong free radical scavengers (Ghareeb et al., 2018; Sobeh et al., 2018).
In the current study, we aimed to investigate for the first time the chemical and biological profiles of the soft parts of the marine clams P. undulatus and the scientific evidence regarding their chemical composition and associated antibacterial, antibiofilm, and antioxidant activities.
MATERIALS AND METHODS
Chemicals, reagents, and instruments
All solvents, standards, and reagents are of high analytical grade. Methanol and sulfuric acid were obtained from El-Nasr Pharmaceutical Chemicals Company (Cairo, Egypt). Nutrient agar and Nutrient Broth media were purchased from HiMedia Laboratories Pvt. Ltd. (Mumbai, India). 2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical, ascorbic acid, gallic acid, Folin–Ciocalteu’s reagent, sodium carbonate, sodium phosphate, and ammonium molybdate were purchased from Sigma-Aldrich (Steinheim, Germany). For antioxidant assays, the absorbance was measured using a spectrophotometer (UV-VS spectrophotometer, Milton Roy 601, CO). The obtained extract was concentrated under vacuum using a rotary evaporator, Buchi R-300 (Flawil, Switzerland).
Collection of P. undulatus
Venus clams (P. undulatus) were collected from Timsah Lake which is located close to Ismailia City at the midpoint of the Suez Canal and about 80?km south of Port Said, Egypt (Fig. 1) (El-Serehy et al., 2018). The collected samples were cleaned with distilled water to eliminate any sands or contaminations and then kept in a refrigerator at −18°C till being used.
Preparation of the methanolic extract of P. undulatus clams (Born, 1778)
Paratapes undulatus clams are a popular food in Egypt, so after being washed with distilled water, their shells were opened with stainless steel knives and their soft bodies were separated from these shells by forceps. The collected soft bodies (250 g) were homogenized and then were extracted four times with methanol (2 l) at room temperature (25°C ± 2°C). The combined extracts were concentrated to afford 19.0 g methanol extract.
Antibacterial activity assay
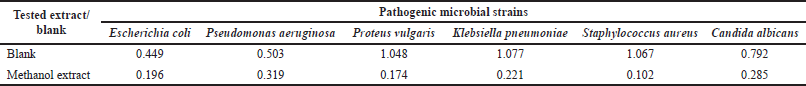
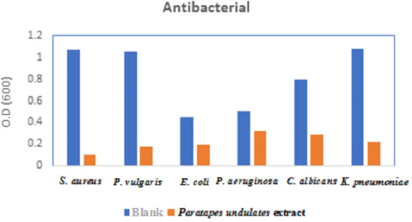
The antibacterial activity of the tested extract was evaluated against some pathogenic microbial strains including four Gram-negative bacteria (Escherichia coli ATCC 25955, Pseudomonas aeruginosa ATCC 10145, Proteus vulgaris, and Klebsiella pneumoniae), one Gram-positive bacterium (Staphylococcus aureus NRRL B-767), and one yeast (Candida albicans ATCC 10231) according to the reported procedures (Elkhouly et al., 2021b). Ciprofloxacin and nystatin were utilized as a control.
Antibiofilm activity assay
The biofilm inhibitory potential was determined against four pathogenic microbial strains, including S. aureus and Bacillus subtilis as Gram-positive bacteria as well as P. aeruginosa and E. coli as Gram-negative bacteria, according to the reported procedures (El-Shazly et al., 2021; Hamed et al., 2020).
Total phenolic content (TPC) using Folin–Ciocalteu’s assay
The TPC was evaluated using Folin–Ciocalteu’s reagent according to the reported procedures (El-Neekety et al., 2016; Hathout et al., 2016). Briefly, 100 μl of the tested sample (500 μg/ml) was mixed with 500 μl of Folin–Ciocalteu’s reagent and 1.5 ml of sodium carbonate (20%). The reaction mixture was shaken and completed to 10 ml using distilled water. The mixture was allowed to stand for 120 minutes. Afterward, the absorbance was recorded at 765 nm. All measurements were performed in triplicate. The TPC was presented as mg gallic acid equivalent (GAE) per g extract.
Antioxidant activity evaluation
Free radical scavenging activity using DPPH
Free radical scavenging activity was evaluated using the DPPH assay according to the reported procedures (Ghareeb et al., 2016; Shirwaikar et al., 2006). Briefly, various serial concentrations from the tested sample (1.5 ml) were added to a (1.5 ml) solution of 0.1 mmol/l DPPH. Equivalent volumes of methanol and DPPH acted as the control. After incubation for 20 minutes at 37°C in the absence of light, the absorbance was recorded at 517 nm. The test was carried out in triplicate. The free radical scavenging activity was expressed in IC50 value (concentration from tested sample required to scavenge 50% of the radical).
 | Figure 1. A map of Timsah Lake with the inset showing the position of the lake on the Suez Canal.
[Click here to view] |
Total antioxidant capacity (TAC) using phosphomolybdenum assay
The TAC was evaluated using the phosphomolybdenum assay based on the reported procedures (Prieto et al., 1999). Basically, this assay is based on the reduction of molybdenum (VI) to molybdenum (V) by the tested sample and consequent development of a green color [phosphate = molybdenum (V)] complex at low pH with maximum absorption at 695 nm. Briefly, 0.5 ml of the tested sample (500 µg /ml) in methanol was mixed with 5 ml from the test reagent [0.6 M H2SO4, 28 mM NaH2PO4, and 4 mM (NH4)6Mo7O24]. The tubes containing the tested samples and reagents were capped and incubated in a water bath at 95°C for 1.5 hours. After cooling, the absorbance was recorded at 695 nm against a blank. The blank comprised all reagents and solvents without the tested sample. All experiments were carried out in triplicate. The TAC was presented as the number of ascorbic acid equivalent (AAE) (Elkhouly et al., 2021; Prieto et al., 1999).
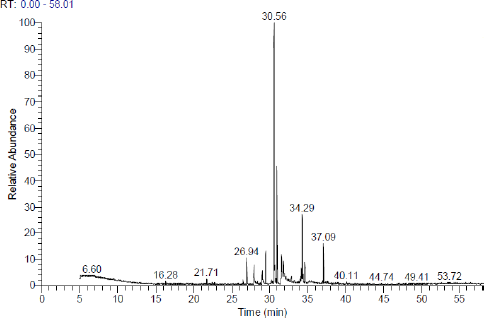
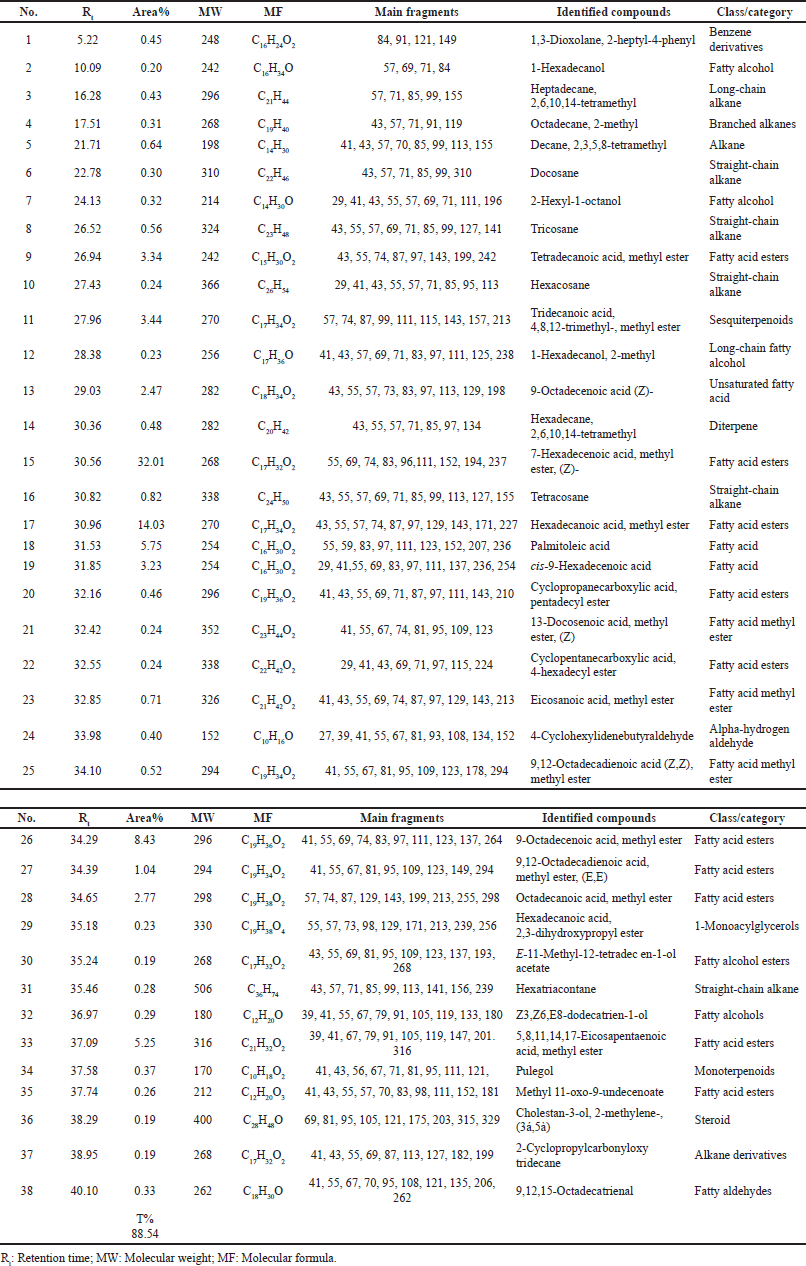
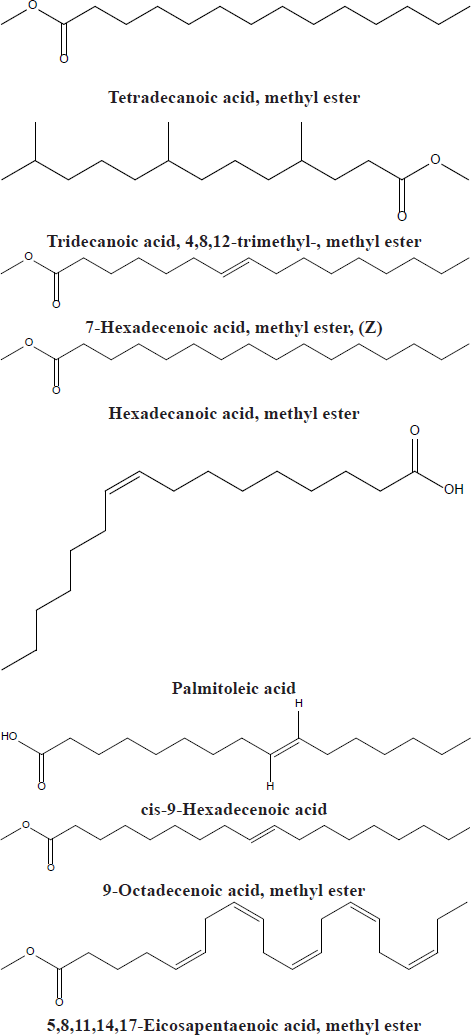
Chemical characterization using GC-MS analysis
GC-MS examination was conducted according to the reported procedures (Khalaf et al., 2020), using a Thermo Scientific, Trace GC Ultra/ISQ Single Quadrupole MS, TG-5MS fused silica capillary column (30 m, 0.251 mm, and 0.1 mm film thickness). An electron ionization system with ionization energy of 70 eV was utilized for GC/MS recognition. Helium gas was utilized as the carrier gas at a regular flow rate of 1 ml/minute. The injector and MS transfer line temperature was set at 280°C. The oven temperature was instructed to an initial temperature of 50°C (hold 2 minutes) to 150°C at an increasing rate of 7°C/minute, then to 270°C at an increasing rate of 5°C/minute (hold 2 minutes), and subsequently to 310°C as the definitive temperature at a growing level of 3.5°C/minute (hold 10 minutes). The quantitative determination of all the identified compounds was examined using a percent relative peak area. A tentative recognition of the components was accomplished based on the comparison of the irrelative retention time and mass spectra with those of the National Institute of Standards and Technology (NIST), Wiley library data of the GC-MS technique.
Statistical analysis
All investigations were carried out thrice, and the obtained results are recorded as the average and standard deviation (SD). Significant variations were explored using the one-way analysis of variance. Variances at p < 0.05 were considered significant.
REFERENCES
Abdel-Aziz MS, Ghareeb MA, Saad AM, Refahy LA, Hamed AA. Chromatographic isolation and structural elucidation of secondary metabolites from the soil-inhabiting fungus Aspergillus fumigatus 3T-EGY. Acta Chromatogr, 2018; 30(4):243–9. CrossRef
Abdel-Aziz MS, Ghareeb MA, Hamed AA, Rashad EM, El-Sawy ER, Saad IM, Ghoneem KM. Ethyl acetate extract of Streptomyces spp. isolated from Egyptian soil for management of Fusarium oxysporum: the causing agent of wilt disease of tomato. Biocatal Agric Biotechnol, 2021; 37:102185. CrossRef
Abdel-Wareth MTA, El-Hagrassi AM, Abdel-Aziz MS, Nasr SM, Ghareeb MA. Biological activities of endozoic fungi isolated from Biomphalaria alexandrina snails maintained in different environmental conditions. Int J Environ Sci, 2019; 76(5):780–99. CrossRef
Abubakar L, Mwangi C, Uku J, Ndirangu S. Antimicrobial activity of various extracts of the sea urchin Tripneustes gratilla (Echinoidea). African J Pharmacol Ther, 2012; 1:19–23.
Alves C, Diederich M. Marine natural products as anticancer agents. Mar Drugs, 2021; 19:447.
Baldwin CC. FAO species identification guide for fishery purposes: the living marine resources of the Western central pacific. Copeia, 2003: (1):212–4. CrossRef
Burge C, Eakin CM, Friedman CS, Froelich B, Hershberger PK, Hofmann EE, Petes LE, Prager KC, Weil E, Willis BL, Ford SE, Harvell CD. Climate change influences on marine infectious diseases: implications for management and society. Ann Rev Mar Sci, 2014; 6:249–77. CrossRef
Donia M, Hamann MT. Marine natural products and their potential applications as anti-infective agents. Lancet Infect Dis, 2003; 3:338–48. CrossRef
Eghianruwa QA, Osoniyi OR, Maina N, Wachira S. Bioactive peptides from marine molluscs-a review. Int J Biochem Res Rev, 2019; 27(4):1–12. CrossRef
El-Neekety AA, Abdel-Aziz MS, Hathout AS, Hamed AA, Sabry BA, Ghareeb MA, Aly SE, Abdel-Wahhab MA. Molecular identification of newly isolated non-toxigenic fungal strains having antiaflatoxigenic, antimicrobial and antioxidant activities. Der Pharm Chem, 2016; 8:121–34.
El-Serehy HA, Abdallah HS, Al-Misned FA, Al-Farraj SA, Al-Rasheid KA. Assessing water quality and classifying trophic status for scientifically based managing the water resources of the Lake Timsah, the lake with salinity stratification along the Suez Canal. Saudi J Biol Sci, 2018; 25:1247–56. CrossRef
El-Shazly MAM, Hamed AA, Kabary HA, Ghareeb MA. LC-MS/MS profiling, antibiofilm, antimicrobial and bacterial growth kinetic studies of Pluchea dioscoridis extracts. Acta Chromatogr, 2021; 1–13; doi:10.1556/1326.2021.00956 CrossRef
Elkhouly HI, Hamed AA, El Hosainy AM, Ghareeb MA, Sidkey NM. Bioactive secondary metabolite from endophytic aspergillus tubenginses ash4 isolated from hyoscyamus muticus: antimicrobial, antibiofilm, antioxidant and anticancer activity. Pharmacogn J, 2021a; 13:434–42. CrossRef
Elkhouly HI, Sidkey NM, Ghareeb MA, El Hosainy AM, Hamed AA. Bioactive secondary metabolites from endophytic Aspergillus terreus AH1 isolated from Ipomoea carnea growing in Egypt. Egypt J Chem, 2021b; 64(12): 7511–20.
Galdiero S, Falanga A, Berisio R, Grieco P, Morelli G, Galdiero M. Antimicrobial peptides as an opportunity against bacterial diseases. Curr Med Chem, 2015; 22:1665–77. CrossRef
Ghareeb MA, Shoeb HA, Madkour HFM, Refaey LA, Mohamed MA, Saad AM. Antioxidant and cytotoxic activities of Tectona grandis linn. leaves. Int J Phytopharm, 2014a; 5:143–57.
Ghareeb MA, Shoeb HA, Madkour HMF, Refahy LA, Mohamed MA, Saad AM. Antioxidant and cytotoxic activities of flavonoidal compounds from Gmelina arborea (Roxb.). Glob J Pharmacol, 2014b; 8:87–97.
Ghareeb MA, Refahy LA, Saad AM, Osman NS, Abdel-Aziz MS, El-Shazly MA, Mohamed AS. In vitro antimicrobial activity of five Egyptian plant species. J Appl Pharm Sci, 2015; 5:045–9.
Ghareeb M, Saad A, Ahmed W, Refahy L, Nasr S. HPLC-DAD-ESI-MS/MS characterization of bioactive secondary metabolites from Strelitzia nicolai leaf extracts and their antioxidant and anticancer activities in vitro. Pharmacogn Res, 2019; 10:368. CrossRef
Ghareeb MA, Mohamed T, Saad AM, Refahy LA, Sobeh M, Wink M. HPLC-DAD-ESI-MS/MS analysis of fruits from Firmiana simplex (L.) and evaluation of their antioxidant and antigenotoxic properties. J Pharm Pharmacol, 2018; 70:133–42. CrossRef
Ghareeb MA, Saad AM, Abdou AM, Refahy LA, Ahmed WS. A new kaempferol glycoside with antioxidant activity from Chenopodium ambrosioides growing in Egypt. Orient J Chem, 2016; 32:3053–61. CrossRef
Giftson H, Patterson J. Evaluation of antibacterial activity of crude extracts of gastropod, Harpa davidis, roding 1798, from Kanyakumari coast against isolated human and fish pathogens. Asian J Pharm Clin Res, 2016; 9(3):159–62.
González VL, Andrade SCS, Bieler R, Collins TM, Dunn CW, Mikkelsen PM, Taylor JD, Giribet G. A phylogenetic backbone for Bivalvia: an RNA-seq approach. Proc R Soc B Biol Sci, 2015; 282:20142332. CrossRef
Hamed AA, Soldatou S, Mallique QM, Arjunan S, Miranda KJ, Casolari F, Pavesi C, Diyaolu OA, Thissera B, Eshelli M, Belbahri L, Luptakova L, Ibrahim NA, Abdel-Aziz MS, Eid BM, Ghareeb MA, Rateb ME, Ebel R. Screening fungal endophytes derived from under-explored egyptian marine habitats for antimicrobial and antioxidant properties in factionalised textiles. Microorganisms, 2020; 8:1–19. CrossRef
Hathout AS, EL-Neekety AA, Abdel Aziz MS, Sabry BA, Hamed AA, Ghareeb MA, Aly SE. Novel Egyptian bacterial exhibiting antimicrobial and antiaflatoxigenic activity. J appl Pharm Sci, 2016; 6:001–10. CrossRef
Haszprunar G, Wanninger A. Molluscs. Curr Biol, 2012; 22(13):R510–4. CrossRef
Hussein HM, Hameed IH, Ibraheem OA. Antimicrobial activity and spectral chemical analysis of methanolic leaves extract of Adiantum capillus-veneris using GC-MS and FT-IR spectroscopy. Int J Pharmacogn Phytochem Res, 2016; 8:369–85.
Ibrahim H, Ahmed HO, Abd El Razek FA, Elmasry E. Proteolysis and heat-sensitive antibacterial agents from several levantine sponge species. Int J Adv Res, 2018; 6:14–27. CrossRef
Ibrahim HAH. Antibacterial carotenoids of three Holothuria species in Hurghada, Egypt. Egypt. J Aquat Res, 2012; 38:185–94. CrossRef
Ibrahim HAH, Abd-Elnaby F. Antimicrobial characteristics of marine polychaetes collected from Alexandria beaches. Sect Title Nonmamm Biochem, 2010; 36:557–67.
Ibrahim HAH, Elatriby DE, Hamed MM. Antimicrobial activity of some Egyptian marine invertebrates, red sea. Egypt. J Aquat Biol Fish, 2020; 24:321–40. CrossRef
Idsoe O, Guthe T, Willcox RR, de Weck AL. Nature and extent of penicillin side-reactions, with particular reference to fatalities from anaphylactic shock. Bull World Health Organ, 1968; 38:159–88.
Joy M, Chakraborty K, Pananghat V. Comparative bioactive properties of bivalve clams against different disease molecular targets. J Food Biochem, 2016; 40:593–602. CrossRef
Kandhasamy M, Arunachalam KD. Evaluation of in vitro antibacterial property of seaweeds of southeast coast of India. African J Biotechnol, 2008; 7:1958–61. CrossRef
Karawita R, Siriwardhana N, Lee KW, Heo MS, Yeo IK, Lee YD, Jeon YJ. Reactive oxygen species scavenging, metal chelation, reducing power and lipid peroxidation inhibition properties of different solvent fractions from Hizikia fusiformis. Eur Food Res Technol, 2005; 220:363–71. CrossRef
Khalaf OM, Abdel-Aziz MS, El-Hagrassi AM, Osman AF, Ghareeb MA. Biochemical aspect, antimicrobial and antioxidant activities of Melaleuca and Syzygium species (Myrtaceae) grown in Egypt. J Phys Conf Ser, 2021; 1879:022062. CrossRef
Khan MK, Sajid MS, Khan MN, Iqbal Z, Iqbal MU. Bovine fasciolosis: prevalence, effects of treatment on productivity and cost benefit analysis in five districts of Punjab, Pakistan. Res Vet Sci, 2009; 87:70–5. CrossRef
Lavanya R, Veerappan N. Antibacterial potential of six seaweeds collected from Gulf of Mannar of southeast coast of India. Adv Biol Res, 2011; 5:38–44.
Loftus E, Rogers K, Lee-Thorp J. A simple method to establish calcite: aragonite ratios in archaeological mollusc shells. J Quat Sci, 2015; 30:731–5. CrossRef
Madkour HMF, Ghareeb MA, Abdel-Aziz MS, Khalaf OM, Saad AM, El-Ziaty AK, and Abdel-Mogib M. Gas chromatography-mass spectrometry analysis, antimicrobial, anticancer and antioxidant activities of n-hexane and methylene chloride extracts from Senna italica. J Appl Pharm Sci, 2017; 7:023–32.
Nair DG, Weiskirchen R, Al-Musharafi SK. The use of marine-derived bioactive compounds as potential hepatoprotective agents. Acta Pharmacol Sin, 2015; 36:158–70. CrossRef
Odeleye T, White WL, Lu J. Extraction techniques and potential health benefits of bioactive compounds from marine molluscs: a review. Food Funct, 2019; 10:2278–89. CrossRef
Omar HH. Antibacterial activity of extracts of marine algae from the Red Sea of Jeddah, Saudi Arabia. African J Biotechnol, 2012; 11:13576–85. CrossRef
Panayotova V, Merdzhanova A, Dobreva DA, Bratoeva K, Makedonski L. Nutritional composition, bioactive compounds and health-beneficial properties of black sea Shellfish. J IMAB-Annu Proc (Sci Pap), 2020; 26(3):3293–7. CrossRef
Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem, 1999; 269(2):337–41. CrossRef
Rockenbach II, Rodrigues E, Gonzaga LV, Caliari V, Genovese MI, Gonalves AEDSS, Fett R. Phenolic compounds content and antioxidant activity in pomace from selected red grapes (Vitis vinifera L. and Vitis labrusca L.) widely produced in Brazil. Food Chem, 2011; 127:174–9. CrossRef
Rusmore-Villaume ML. Seashells of the Egyptian Red Sea - The Illustrated Handbook, 2008. The American University in Cairo Press, Cairo, Egypt.
Schmitt P, Rosa RD, Duperthuy M, de Lorgeril J, Bachère E, Destoumieux-Garzón D. The antimicrobial defense of the Pacific oyster, Crassostrea gigas. How diversity may compensate for scarcity in the regulation of resident/pathogenic microflora. Front Microbiol, 2012; 3:160. CrossRef
Shawky BT, Nagah M, Ghareeb MA, El-Sherbiny GM, Moghannem SAM, and Abdel-Aziz MS. Evaluation of antioxidants, total phenolics and antimicrobial activities of ethyl acetate extracts from fungi grown on rice straw. J Renew Mater, 2019; 7(7):667–82. CrossRef
Shirwaikar A, Shirwaikar A, Rajendran K, Punitha ISR. In vitro antioxidant studies on the benzyl tetra isoquinoline alkaloid berberine. Biol Pharm Bull, 2006; 29:1906–10. CrossRef
Smith P, Hiney MP, Samuelsen OB. Bacterial resistance to antimicrobial agents used in fish farming: a critical evaluation of method and meaning. Annu Rev Fish Dis, 1994; 4:273–313. CrossRef
Sobeh M, Mahmoud MF, Hasan RA, Abdelfattah MAO, Sabry OM, Ghareeb MA, El-Shazly AM, Wink M. Tannin-rich extracts from Lannea stuhlmannii and Lannea humilis (Anacardiaceae) exhibit hepatoprotective activities in vivo via enhancement of the anti-apoptotic protein Bcl-2. Sci Rep, 2018; 8:9343. CrossRef
Sreejamole KL, Radhakrishnan CK. Antioxidant and cytotoxic activities of ethyl acetate extract of the Indian green mussel Perna viridis. Asian J Pharm Clin Res, 2013; 6:197–201.
Tabakaeva OV, Tabakaev AV, Piekoszewski W. Nutritional composition and total collagen content of two commercially important edible bivalve molluscs from the Sea of Japan coast. J Food Sci Technol, 2018; 55:4877–86. CrossRef
Temraz TA. Egyptian biodiversity strategy and action plan (2015-2030). FAO, 2016. Available via https://www.fao.org/faolex/results/details/en/c/LEX-FAOC156958/
Wright AD, McCluskey A, Robertson MJ, MacGregor KA, Gordon CP, Guenther J. Anti-malarial, anti-algal, anti-tubercular, anti-bacterial, anti-photosynthetic, and anti-fouling activity of diterpene and diterpene isonitriles from the tropical marine sponge Cymbastela hooperi. Org Biomol Chem, 2011; 9:400–7. CrossRef
Youssef DTA, Shaala LA, Asfour HZ. Bioactive compounds from the Red Sea marine sponge Hyrtios species. Mar Drugs, 2013; 11:1061–70. CrossRef