INTRODUCTION
Reactive oxygen species are reactive atoms that have unpaired electrons. They can cause oxidative stress in DNA, lipids, proteins, or carbohydrates, eventually leading to neurodegenerative disease and cancer (Halliwell, 2012). The source of free radicals may come from external factors, such as junk food, alcohol, drugs, tobacco, environmental pollution, and heavy metals. However, free radicals can also be produced in the human body from endogenous reactions, such as phagocytosis, mitochondria by producing adenosine triphosphate, and β-oxidation of fatty acids (Jakubczyk et al., 2020; Liguori et al., 2018; Namkoong et al., 2018; Petruk et al., 2018). Therefore, an antioxidant that can slow or inhibit cellular damage in the human body is urgently needed (Lobo et al., 2010). Antioxidants may vary in the mechanism of actions and composition, which can scavenge free radicals, chelate metals, and inhibit enzymes (Gioti et al., 2009; González-Palma et al., 2016).
Plants are potential sources of antioxidants. Phenolic compounds, vitamins, and carotenoids have been reported as the primary contributors to antioxidant activities (Indrianingsih et al., 2015b; Olugbami et al., 2015; Rodríguez-García et al., 2019). The utilization of plants includes different parts of them, such as flowers, leaves, bark, fruits, and roots. Several customers and manufacturers use plant extracts for medicinal use and cosmetic products (Sitarek et al., 2020). Antioxidants from plants are preferred over synthetic antioxidants because of their safety and nutritional benefits (Deng et al., 2011).
Diabetes mellitus (DM) is a disease diagnosed with hyperglycemia. It is caused by either insulin resistance or an insulin production problem, and the symptoms include weight loss, thirst, and frequent urination (Klein et al., 2007). DM complications could damage the kidneys, eyes, nerves, and heart (Sukardiman and Ervina, 2020). According to the WHO, DM patients are estimated to reach approximately 342 million by 2030 and will be a burden for developing countries (Sukardiman and Ervina, 2020). One treatment to overcome DM, primarily type 2, is consuming an α-glucosidase inhibitor. It can postpone glucose absorption and prevent increasing blood glucose levels (Sy et al., 2005). Plants are a potential alternative to treat DM. Besides managing stable blood glucose levels, it can also prevent complications, an advantage over synthetic drugs (Sukardiman and Ervina, 2020). Approximately 1,200 plants were found to have an ability to lower blood glucose levels in ethnopharmacological surveys (Pandey et al., 2011).
Quercus gilva Blume is an evergreen tree grown in Japan and South Korea (Kim and You, 2012; Noshiro and Sasaki, 2011). In Japan, Q. gilva was used as a raw material for agriculture tools, such as spades, axe handles, and hoes. The previous study has reported the bioactivity of phenolic compounds from the bark of Q. gilva, such as anti-inflammatory, antiurolithiasis, and antioxidative agents (Youn et al., 2017). Several studies also identified terpenes from the fruit of Q. gilva and antioxidative agents from the branches of Q. gilva (Itokawa et al., 1978; Moon et al., 2009). The bioactive compounds from Quercus species leaves were phenolic compounds, vitamins, aliphatic alcohols, fatty acids, and sterol (Lämke and Unsicker, 2018; Vinha et al., 2016). Several phenolic constituents in the Quercus species leaves are tannins, flavonoids (epicatechin, quercetin, rutin, kaempferol, and naringin), and phenolic acids (ellagic acid, gallic acid, gentisic acid, p-coumaric acid, vanillic acid, caffeic acid, and ferulic acid) (Brossa et al., 2009; Cantos-Villar et al., 2003; Jong et al., 2012). Our previous study isolated three compounds, namely, catechin, epicatechin, and tiliroside, from Q. gilva leaves (Indrianingsih et al., 2015). However, the isolation of polyphenol and study of its bioactivity on the Q. gilva leaves have not yet been done. Polyphenols such as procyanidins were often obtained as a mixture constituent, such as oligomeric and stereochemical mixtures. It was hard to isolate it as a pure compound (Oizumi et al., 2010). The synthesis of procyanidin itself also had several problems, such as the needed large amount of nucleophile at low temperature and the formation of side products in oligomeric form (Kozikowski et al., 2001).
In the present study, we isolated the polyphenol compound from methanolic extract of Q. silva leaves and studied its bioactivity, such as antioxidant and antidiabetic activity.
MATERIALS AND METHODS
Plant materials, reagents, and general instrumentation
Quercus gilva leaves were harvested from Ehime University Garden, Matsuyama, Japan. Samples had been saved in the Faculty of Agriculture, Ehime University, Japan. The α-glucosidase enzyme, 1,1-diphenyl-2-picrylhydrazyl (DPPH), β-carotene, p-nitrophenyl α-D-glucopyranoside (p-NPG), potassium ferricyanide [K3Fe(CN)6], hydrogen peroxide (H2O2), ferric chloride (FeCl3), trichloroacetic acid, n-hexane, chloroform, toluene, methanol, acetone ethanol, and ethyl acetate were obtained from Wako, Ltd. (Japan). Gallic acid, quercetin, and Tween 40 were obtained from Sigma-Aldrich, Ltd. (Japan). Nuclear magnetic resonance (NMR) spectra were recorded on a JEOL JNM-AL 500 spectrometer (Tokyo, Japan) using tetramethylsilane as the internal standard. Gas chromatography-mass spectrometry and fast atom bombardment-mass spectroscopy (FAB-MS) were performed using equipment from Shimadzu, Japan.
Compound isolation from Q. gilva
Leaves powder of Q. gilva (1.1 kg) was macerated with 8.8 l of methanol at room temperature. After 2 days of immersion, the filtrate was evaporated, separated, and isolated using bioassay-guided isolation. The solvent for silica column chromatography was started from n-hexane (100%), followed by n-hexane:ethyl acetate (50:50) and ethyl acetate:methanol (50:50), and finally methanol (100%). Five fractions F1–F5 were obtained, and further repeated silica column chromatography of F5 resulted in F51 to F53. Compound 1 was isolated as a light brown powder (20 mg) from silica column chromatography of F53.
Compound 1 (light brown powder): Catechin (4α→8)-Catechin (Procyanidin B3). UV, λmax 280.5 nm; Electrospray ionization–mass spectrometry (ESI-MS, positive ion mode) m/z 579 [M + H]+. 13C-NMR (125 MHz, CD3OD) δ 157.9 (C-5u), 157.2 (C-5t), 156.7 (C-7u), 156.4 (C-7t), 146.9 (C-3u?), 146.7 (C-3?t), 146.6 (C-4?u), 146.4 (C-4?t), 133.1 (C-1?u, C-1?t), 120.6 (C-6?u), 120.2 (C-6?t), 116.8 (C-2?u), 116.4 (C-2?t), 116.3 (C-5?u), 116.1 (C-5?t), 108.0 (C-8t), 98.4 (C-10u; C-10t), 96.9 (C-6t), 96.8 (C-6u), 96.7 (C-8u), 83.2 (C-3u), 74.5 (C-2u), 69.7 (C-2t), 68.2 (C-3t), 39.3 (C-4t), 29.6 (C-4u).
HRFAB-MS: [M + H]+: m/z 579 for C30H26O12.
DPPH assay
The antioxidant activity of the isolated compound was performed by the DPPH assay (Indrianingsih et al., 2021). The sample was diluted in methanol in several concentrations, reacted with DPPH (1.01 mM) at room temperature for 30 minutes in dark conditions. The absorbance of the final solution was measured using a UV spectrophotometer at 517 nm. The radical scavenging activity of compound 1 was calculated using
(1)
where A0 is the absorbance of the control and A1 is the absorbance of the sample. The measurement was conducted in triplicate. Quercetin was used as the positive standard in the DPPH assay.
H2O2 assay
The capability of compound 1 in the H2O2 radical scavenging assay was evaluated according to the literature (Indrianingsih et al., 2015) with slight adjustments. H2O2 was diluted in phosphate buffer solution (PBS) (pH 7.4) to obtain the concentration of 40 mmol/l. Compound 1 (4 ml in Aquades) was reacted for 10 minutes with 0.6 ml of H2O2. The absorbance of the final solution was measured at 230 nm using a UV spectrophotometer.
Reducing power assay
According to the literature, the reducing power assay was conducted (Indrianingsih et al., 2015). Compound 1 (20 g/ml) in PBS (2.5 ml) was reacted with K3Fe(CN)6 (2.5 ml), left to stand for 20 minutes at 50°C, and added with trichloroacetic acid (2.5 ml). After centrifugation for approximately 8 minutes, the upper layer (2.5 ml) was reacted with Aquades and FeCl3 (0.5 ml). The final solution was evaluated at 700 nm using a spectrophotometer.
β-Carotene bleaching assay
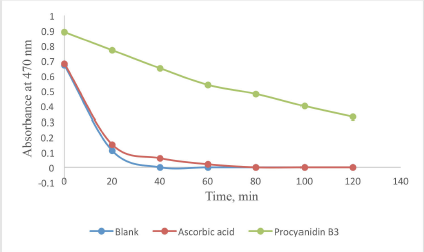
According to a previous study, the capability of compound 1 in preventing β-carotene bleaching was performed (Indrianingsih et al., 2015). β-carotene (0.2 mg/ml) in CHCl3 was reacted with linoleic acid (20 mg) and Tween 40 (200 mg). After evaporation, distilled water (50 ml) was added, and 4.8 ml of the solution was reacted with 0.2 ml of compound 1 solution in methanol. This solution was incubated at 50C. The absorbance of the solution was evaluated at 20 minutes intervals at 470 nm.
α-Glucosidase inhibitory assay
The α-glucosidase assay was conducted according to the literature (Indrianingsih et al., 2015). Several concentrations of compound 1 in dimethyl sulfoxide (10 µl) were mixed with PBS (pH 7, 490 µl) and 250 µl of p-NPG (3 mmol/l). After being reacted for 5 minutes at 37°C, 250 µl of the α-glucosidase enzyme (0.065 IU/ml) was added and left to stand for 15 minutes. One milliliter of Na2CO3 (0.2 mol/l) was added to stop the reaction, and the absorbance of the final solution was evaluated at 400 nm.
REFERENCES
Arya V, Yadav S, Kumar S, Yadav JP. Antioxidant activity of organic and aqueous leaf extracts of Cassia occidentalis L. in relation to their phenolic content. Nat Prod Res, 2011; 25:1473–79. CrossRef
Bendary E, Francis RR, Ali HMG, Sarwat MI, Hady SE. Antioxidant and structure-activity relationships (SARs) of some phenolic and anilines compounds. Ann Agric Sci, 2013; 58(2):173–81. CrossRef
Brossa R, Casals I, Pintó-Marijuan M, Fleck I. Leaf flavonoid content in Quercus ilex L. resprouts and its seasonal variation. Trees, 2009; 23:401–8. CrossRef
Cantos E, Espín JC, López-Bote C, de la Hoz L, Ordóñez JA, Tomás-Barberán FA. Phenolic compounds and fatty acids from acorns (Quercus spp.), the main dietary constituent of free-ranged Iberian pigs. J Agric Food Chem, 2003; 51(21):6248–55. CrossRef
Deng J, Cheng W, Yang G. A novel antioxidant activity index (AAU) for natural products using the DPPH assay. Food Chem, 2011; 125(4):1430–5. CrossRef
Eurich DT, McAlister FA, Blackburn DF, Majumdar SR, Tsuyuki RT, Varney J, Johnson JA. Benefits and harms of antidiabetic agents in patients with diabetes and heart failure: a systematic review. BMJ, 2007; 335(7618):497. CrossRef
Fu CL, Wang HY, Ng WL, Song LX, Huang DJ. Antioxidant activity and proanthocyanidin profile of Selliguea feei rhizomes. Molecules, 2013; 18:4282–92. CrossRef
Gioti EM, Fiamegos YC, Skalkos DC, Stalikas CD. Antioxidant activity and bioactive components of the aerial parts of Hypericum perforatum L. from Epirus, Greece. Food Chem, 2009; 117:398–404. CrossRef
González-Palma I, Escalona-Buendía HB, Ponce-Alquicira E, Téllez-Téllez M, Gupta VK, Díaz-Godínez G, Soriano-Santos J. Evaluation of the antioxidant activity of aqueous and methanol extracts of Pleurotus ostreatus in different growth stages. Front Microbiol, 2016; 7:1099. CrossRef
Halliwell B. Free radicals and antioxidants: updating a personal view. Nutr Rev, 2012; 70(5):257–65. CrossRef
Indrianingsih AW, Tachibana S, Dewi RT, Itoh K. Antioxidant and α-glucosidase inhibitor activities of natural compounds isolated from Quercus gilva Blume leaves. Asian Pac J Trop Biomed, 2015a; 5(9):748–55. CrossRef
Indrianingsih AW, Wulanjati MP, Windarsih A, Bhattacharjya DK, Suzuki T, Katayama T. In vitro studies of antioxidant, antidiabetic, and antibacterial activities of Theobroma cacao, Anonna muricata and Clitoria ternatea. Biocatal Agric Biotechnol, 2021; 33:101995. CrossRef
Indrianingsih, AW, Tachibana S, Itoh K. In vitro evaluation of antioxidant and α-glucosidase inhibitory assay of several tropical and subtropical plants. Proc Environ Sci, 2015b; 28:639–48. CrossRef
Itokawa H, Tachi Y, Kamano Y, Iitaka Y. Structure of gilvanol, a new triterpene isolated from Quercus gilva Blume. Chem Pharm Bull, 1978; 26:331–3. CrossRef
Jakubczyk K, Ka?du?ska J, Dec K, Kawczuga D, Janda K. Antioxidant properties of small-molecule non-enzymatic compounds. Pol Merkur Lekarski, 2020; 48(284):128–32.
Jong J, Bimal K, Hyeun C, Kyung J, Ki S, Young S, Taek S, Ji L, Hye K, Min C. Comparison of phenolic compounds content in indeciduous Quercus species. J Med Plants Res, 2012; 6:5228–39. CrossRef
Katalinic V, Mozina SS, Skroza D, Generalic I, Abramovic H, Milos M, Ljubenkov I, Piskernik S, Pezo I, Terpinc P, Boban M. Polyphenolic profile, antioxidant properties and antimicrobial activity of grape skin extracts of 14 Vitis vinifera varieties grown in Dalmatia (Croatia). Food Chem, 2010; 119(2):715–23. CrossRef
Kedar SB, Singh RP. Genesis and development of DPPH method of antioxidant assay. J Food Sci Technol, 2011; 48(4):412–22. CrossRef
Kim H, You Y. Ecophysiological responses of Quercus gilva, endangered species and Q. glauca to long-term exposure to elevated CO2 concentration and temperature. J Ecol Environ, 2012; 35:203–12. CrossRef
Kiss AK, Derwi?ska M, Dawidowska A, Naruszewicz M. Novel biological properties of Oenothera paradoxa defatted seed extracts: effects on metallopeptidase activity. J Agric Food Chem, 2008; 56(17):7845–52. CrossRef
Klein O, Lynge J, Endahl L, Damholt B, Nosek L, Heise T. Albumin-bound basal insulin analogues (insulin detemir and NN344): comparable time-action profiles but less variability than insulin glargine in type 2 diabetes. Diabetes Obes Metab, 2007; 9(3):290–9. CrossRef
Köhler N, Wray V, Winterhalter P. Preparative isolation of procyanidins from grape seed extracts by high-speed counter-current chromatography. J Chromatogr A, 2008; 1177(1):114–25. CrossRef
Kozikowski AP, Tuckmantel W, Hu Y. Studies in polyphenol chemistry and bioactivity. 3.1,2 Stereocontrolled synthesis of epicatechin-4α,8-epicatechin, an unnatural isomer of the B-type procyanidins. J Org Chem, 2001; 66:1287–96. CrossRef
Kresty LA, Howell AB, Baird M. Cranberry proanthocyanidins mediate the growth arrest of lung cancer cells through modulation of gene expression and rapid induction of apoptosis. Molecules, 2011; 16:2375–90. CrossRef
Kumar RS, Rajkapoor B, Perumal P. Antioxidant activities of Indigofera cassioides Rottl. Ex. DC. using various in vitro assay models. Asian Pac J Trop Biomed, 2012; 2(4):256–61. CrossRef
Lämke JS, Unsicker SB. Phytochemical variation in treetops: causes and consequences for tree-insect herbivore interactions. Oecologia, 2018; 187(2):377–88. CrossRef
Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, Gargiulo G, Testa G, Cacciatore F, Bonaduce D, Abete P. Oxidative stress, aging, and diseases. Clin Interv Aging, 2018; 13:757–72. CrossRef
Liu L, Sun Y, Laura T, Liang X, Ye H, Zeng X. Determination of polyphenolic content and antioxidant activity of kudingcha made from Ilex kudingcha CJ Tseng. Food Chem, 2009; 112:35–41. CrossRef
Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn Rev, 2010; 4(8):118–26. CrossRef
Määttä-Riihinen KR, Kähkönen MP, Törrönen AR, Heinonen IM. Catechins and procyanidins in berries of vaccinium species and their antioxidant activity. J Agric Food Chem, 2005; 53(22):8485–91. CrossRef
Mohammed SA, Yaqub AG, Sanda KA, Nicholas AO, Arastus W, Muhammad M, Abdullahi S. Review on diabetes, synthetic drugs and glycemic effects of medicinal plants. J Med Plants Res, 2013; 7(36):2628–37.
Moon MY, Baik JS, Kim SS, Jang WJ, Kim MS, Lee NH. Identification of antioxidative constituents from the branches of Quercus gilva Blume. J Soc Cosmet Sci Korea, 2009; 35:251–6.
Namkoong J, Kern D, Knaggs HE. Assessment of human skin gene expression by different blends of plant extracts with implications to periorbital skin aging. Int J Mol Sci, 2018; 19(11):3349. CrossRef
Noshiro S, Sasaki Y. Identification of Japanese species of evergreen Quercus and Lithocarpus (Fagaceae). IAWA J, 2011; 32:383–93. CrossRef
Oizumi Y, Mohri Y, Hirota M, Makabe H. Synthesis of procyanidin B3 and its anti-inflammatory activity. the effect of 4-alkoxy group of catechin electrophile in the Yb(OTf)(3)-catalyzed condensation with catechin nucleophile. J Org Chem, 2010; 75(14):4884–6. CrossRef
Olugbami JO, Gbadegesin MA, Odunola OA. In vitro free radical scavenging and antioxidant properties of ethanol extract of Terminalia glaucescens. Pharmacogn Res, 2015; 7(1):49–56. CrossRef
Osman AM. Multiple pathways of the reaction of 2,2-diphenyl-1-picrylhydrazyl radical (DPPH) with (+)-catechin: evidence for the formation of a covalent adduct between DPPH·and the oxidized form of the polyphenol. Biochem Biophys Res Commun, 2011; 412(3):473–8. CrossRef
Pandey A, Tripathi P, Pandey R, Srivatava R, Goswami S. Alternative therapies useful in the management of diabetes: a systematic review. J Pharm Bioallied Sci, 2011; 3(4):504–12. CrossRef
Perron NR, Brumaghim JL. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem Biophys, 2009; 53(2):75–100. CrossRef
Petruk G, Del Giudice R, Rigano MM, Monti DM. Antioxidants from plants protect against skin photoaging. Oxid Med Cell Longev, 2018; 2018:1454936. CrossRef
Rodríguez-García CM, Ruiz-Ruiz JC, Peraza-Echeverría L, Peraza-Sánchez SR, Torres-Tapia LW, Pérez-Brito D, Tapia-Tussell R, Herrera-Chalé FG, Segura-Campos MR, Quijano-Ramayo A. Antioxidant, antihypertensive, anti-hyperglycemic, and antimicrobial activity of aqueous extracts from twelve native plants of the Yucatan coast. PLoS One, 2019; 14(3):e0213493. CrossRef
Singhal M, Paul A, Singh HP. Synthesis and reducing power assay of methyl semicarbazone derivatives. J Saudi Chem Soc, 2014; 18(2):121–7. CrossRef
Sitarek P, Merecz-Sadowska A, Kowalczyk T, Wieczfinska J, Zajdel R, ?liwi?ski T. Potential synergistic action of bioactive compounds from plant extracts against skin infecting microorganisms. Int J Mol Sci, 2020; 21(14):5105. CrossRef
Sukardiman, Ervina M. The recent use of Swietenia mahagoni (L.) Jacq. as antidiabetes type 2 phytomedicine: a systematic review. Heliyon, 2020; 6:e03536. CrossRef
Sy GY, Cissé A, Nongonierma RB, Sarr M, Mbodj NA, Faye B. Hypoglycaemic and antidiabetic activity of acetonic extract of Vernonia colorata leaves in normoglycaemic and alloxan-induced diabetic rats. J Ethnopharmacol, 2005; 98(1–2):171–5. CrossRef
Vinha A, Barreira J, Costa A, Oliveira M. A new age for Quercus spp. fruits: review on nutritional and phytochemical composition and related biological activities of acorns. Compr Rev Food Sci Food Saf, 2016; 15(6):947–81. CrossRef
Wang CM, Hsu YM, Jhan YL, Tsai SJ, Lin SX, Su CH, Chou CH. Structure elucidation of procyanidins isolated from Rhododendron formosanum and their antioxidative and anti-bacterial activities. Molecules, 2015; 20:12787–803. CrossRef
Wang HY, Song LX, Feng SB, Liu YC, Zuo G, Lai FL, He GY, Chen MJ, Huang DJ. Characterization of proanthocyanidins in stems of Polygonum multiflorum Thunb as strong starch hydrolase inhibitors. Molecules, 2013; 18:2255–65. CrossRef
Youn SH, Kwon JH, Yin J, Tam LT, Ahn HS, Myung SC, Lee MW. Anti-inflammatory and anti-urolithiasis effects of polyphenolic compounds from Quercus gilva Blume. Molecules, 2017; 22:1121. CrossRef
Zang X, Shang M, Xu F, Liang J, Wang X, Mikage M, Cai S. A-type proanthocyanidins from the stems of Ephedra sinica (Ephedraceae) and their antimicrobial activities. Molecules, 2013; 18(5):5172–89. CrossRef
Zechel DL, Withers SG. Glycosidase mechanisms: anatomy of a finely tuned catalyst. Acc Chem Res, 2000; 33:11–8. CrossRef