INTRODUCTION
On account of its biodiversity, the Nakhon Si Thammarat area in Thailand is an important reservoir of natural resources. Nakhon Si Thammarat comprises several environmental habitats, ranging from the Khao Luang National Park to the Gulf of Thailand, which provide a correspondingly wide variety of natural resources (Sutthipibul, 2013). In traditional Thai medicine, some Annonaceous plants have been used to cure pathological conditions, including snakebites, diarrhea, dysentery, arthritic pain, rheumatism, and neuralgia, as well as for analgesic, astringent, and weight-loss purposes (Choudhary et al., 2015). Recently, some Annonaceous plants have even been used to treat cancer and provide other health benefits. However, safety profiles and scientific data for these plants are needed to consolidate their promising potential use. For example, multidrug-resistant bacteria represent a severe problem in the global healthcare system. Opportunistic infections by such bacteria cause severe human health issues and worldwide economic problems as affected patients require special treatment and long hospital stays for the administration of drugs with higher antibacterial activity and intensive health interventions (Aslam et al., 2018). Many other kinds of microorganisms also affect human health and create public health problems, such as Staphylococcus aureus, Escherichia coli, Enterococcus faecalis, and Acinetobacter baumannii, which can be found in contaminated food and cause health conditions including food poisoning, gastritis, colitis, and severe sepsis (Castellanos et al., 2019). Klebsiella pneumoniae and Pseudomonas aeruginosa are pathogens that also cause opportunistic infections, whereby the latter is often drug-resistant (Azimi et al., 2019). Candida albicans is a yeast that causes infections, especially in patients with weakened immune systems (Mayer et al., 2013). Degenerative diseases associated with free radicals, including atherosclerosis, cancer, inflammatory joint disease, asthma, diabetes, senile dementia, and degenerative eye disease, also represent serious problems for the general population (Furman et al., 2019). Therefore, it is important and urgent for phytochemists to investigate bioactive compounds from, for example, Annonaceous plants with respect to their potential to be used against pathogens and to protect human health from degenerative diseases. In this study, we have collected several samples of Annonaceous plants during excursions to various areas of Nakhon Si Thammarat. The antimicrobial activities of the leaves of the samples were determined using a broth microdilution assay, and their antioxidant properties were screened using a 2,2-diphenyl-1-picrylhydrazyl- (DPPH-)-based free radical scavenging assay.
MATERIALS AND METHODS
The leaves of 20 species of Annonaceous plants were collected in the Sichon, Thasala, and Muang districts of Nakhon Si Thammarat (Thailand) between June and August 2021. Voucher specimens were identified and deposited at Walailak Botanical Park, Walailak University. All samples were dried using a hot-air oven at 50°C and then ground into a powder using a mill. The samples were extracted in refluxing methanol (samples 1–10: 3 × 4 l; samples 11–20: 3 × 0.5 l; 3 hours). The combined methanolic extracts were filtered before all volatiles were removed from the filtrate under reduced pressure to yield the crude methanolic extract.
Phytochemical screening
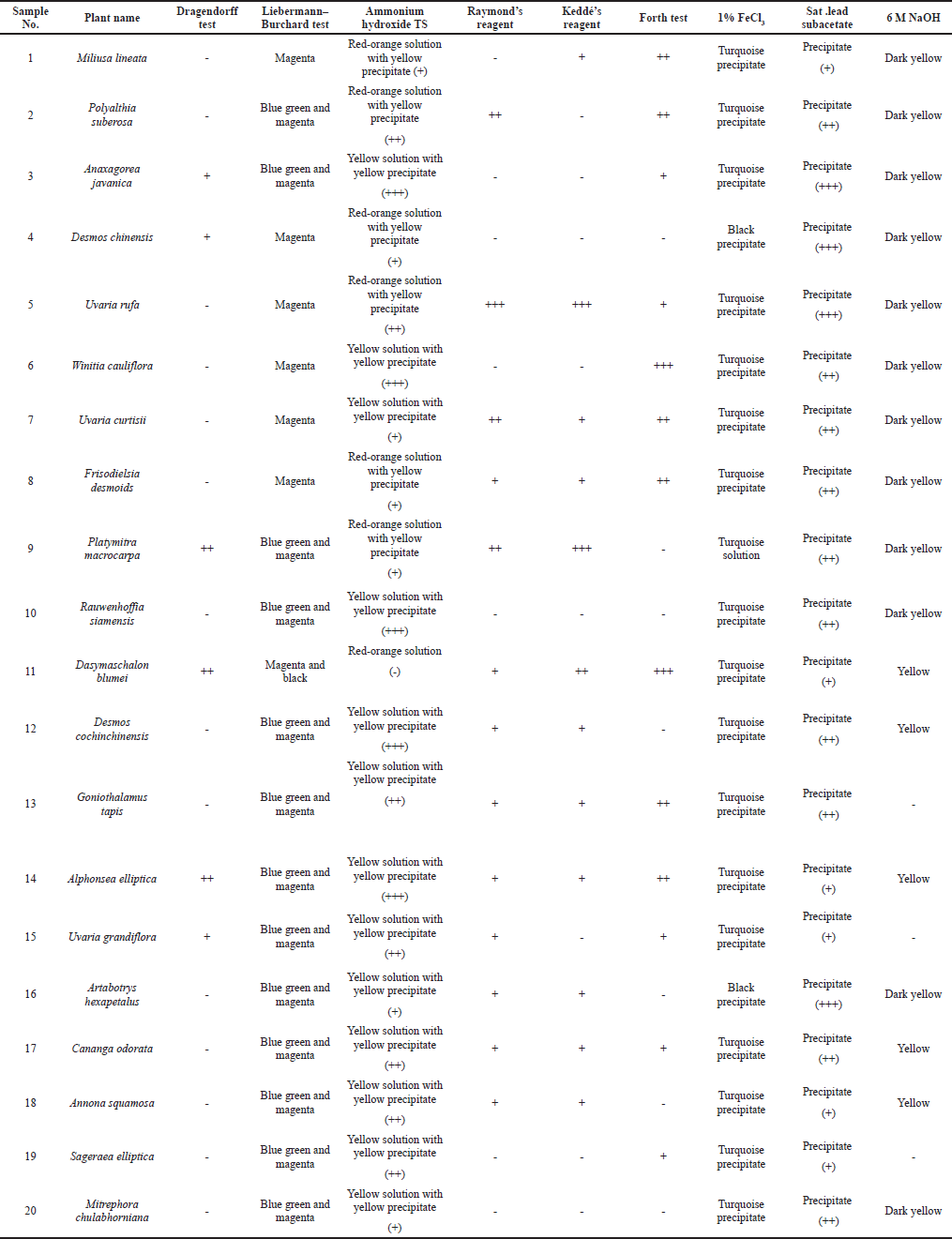
Test for alkaloids
A small amount of each crude methanolic extract was individually boiled with a 10% sulfuric acid solution (5 ml) for 10 minutes. The filtrates were treated with Dragendorff’s reagent. If a sample contains alkaloids, an orange-red amorphous precipitate is formed during the reaction (Raal et al., 2020).
Test for flavonoids
A small amount of each methanolic leaf extract was individually dissolved in methanol (3 ml). Each methanol extract was treated with a few drops of ammonium hydroxide test solution (TS). If the sample contains flavones or flavonols, a yellow precipitate is formed and the solution will turn its color. In the case of flavanones, an orange-red solution is observed, while in the case of chalcones or aurones, the TS changes color to magenta (Hossain et al., 2013).
Test for tannins
A small amount of each crude methanol extract was individually boiled with distilled water (5 ml) before a saturated lead subacetate solution (1 ml) was added to 2 ml of the filtrate of each sample. The formation of a precipitate indicates the presence of tannins. Additionally, a small sample (2 ml) of the filtrate of each extract was treated with a few drops of a 1% ferric chloride solution. The formation of a greenish-black (turquoise) precipitate indicates the presence of condensed tannins, while the formation of a deep blue precipitate indicates the presence of hydrolyzable tannins (Heera et al., 2012).
Test for anthraquinones
Modified Borntrager’s test – To a small sample (1 ml) of each methanol extract, 1 ml of a 5% ferric chloride solution and 1 ml of 10% sulfuric acid were added before the resulting solution was heated to ~90°C for 10 minutes in a water bath. After cooling, 1 ml of chloroform was added. The sample was shaken well, and the organic layer was separated. An equal volume of 10% sodium hydroxide solution was then added. The formation of a rose-pink/red precipitate at the ammonia layer indicates the presence of anthraquinones (Auwal et al., 2014).
Test for steroids and terpenoids
Liebermann -Burchard test – A small amount (1 ml) of the methanolic extract of each sample was dried in a porcelain dish. Then, chlorophylls were washed out using a small amount (1–2 ml) of chloroform before a few drops of acetic anhydride were added to the sample and mixed well. One drop of concentrated sulfuric acid was added next to the sample, and both fractions were allowed to mix. If a sample contains steroids, a green to blue-green color will emerge during the reaction. If the sample contains terpenoids, a purple-red or magenta color will emerge during the reaction (Mujeeb et al., 2014).
Test for coumarins
A small amount of each crude methanolic extract (1–2 g) was individually boiled with distilled water (5 ml). A small amount of the filtrate of each sample (2 ml) was treated with 1 ml of a 6 M sodium hydroxide solution. If a sample contains coumarins, the solution will turn yellow to dark yellow during the reaction (Stefanachi et al., 2018).
Test for saponin glycosides
A small amount of each crude methanolic extract was individually boiled with distilled water (5 ml). A small sample of the filtrate (2 ml) of each sample was shaken for 30 seconds. If a sample contains saponin glycosides, it exhibits stable honeycomb-shaped bubbles (El Hazzam et al., 2020).
Biological evaluation
Antioxidant assay
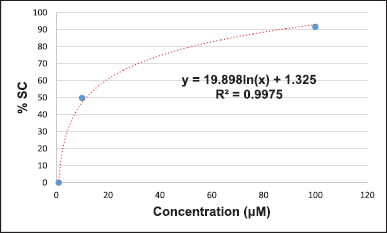
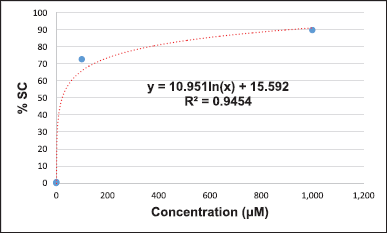
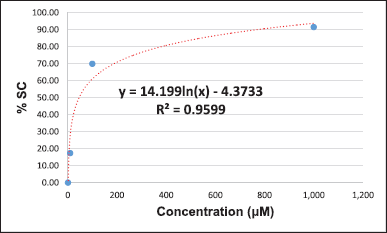
The DPPH-based free radical scavenging assay was chosen to evaluate the potential of the crude methanolic extracts to serve as antioxidants. We slightly modified the process for the preparation of the TS, positive control, and samples from the method described by Braca et al. (2001). l-ascorbic acid was used as a positive control. l-ascorbic acid (10 mg) was dissolved in methanol (10 ml) to prepare a 1,000 μg/ml solution, which was then diluted using the serial dilution method with the same solvent to give solutions of concentrations of 100, 10, 1, and 0.1 μg/ml. All samples were dissolved and diluted with methanol to give concentrations of 1,000, 100, 10, 1, and 0.1 μg/ml. About 100 μl of the positive control and the various concentrations of each sample were mixed with 100 μl of DPPH. About 100 μl of DPPH mixed with 100 μl of methanol was used as a control. About 200 μl methanol was used as the blank control. The blank sample was prepared by mixing 100 μl of the sample with 100 μl of methanol. All reactions were carried out within 30 minutes in the dark before the absorbance at 517 nm was measured using a microplate reader. All data were used for the calculation of %SC50 (Braca et al., 2001).
% SC = [Abcontrol – Abcontrol blank] – [Absample – Absample blank] × 100
[Abcontrol – Abcontrol blank].
Antimicrobial assay
The potentials of all the samples to serve as antimicrobial agents were measured using a broth microdilution assay to determine the minimum inhibition concentration (MIC) and minimum microbicidal concentration (MMC). Resazurin was used as the indicator. The test microbes were E. coli ATCC 25922 (Croxen et al., 2013), S. aureus ATCC 25923 (Tong et al., 2015), P. aeruginosa (Duraisingham et al., 2014), E. faecalis (Anderson et al., 2016), A. baumannii (Howard et al., 2012), K. pneumoniae (Paczosa et al., 2016), and C. albicans (Martins et al., 2014). Pseudomonas aeruginosa, E. faecalis, A. baumannii, K. pneumoniae, and C. albicans were collected and isolated from patients at Maharaj Nakhon Si Thammarat Hospital. The identified microbes were cultured on trypticase soy agar at 37°C, except for C. albicans, which was cultured on Sabouraud dextrose agar (SDA) at 25°C. A single colony of each of the bacteria was inoculated into 2X Mueller–Hinton broth (MHB), and C. albicans was inoculated into 2X Sabouraud dextrose broth. Before the assay, all microbes were incubated for 3 hours using the same conditions as previously described. All microbes were then diluted with a 0.85% sodium chloride solution using aseptic techniques to give concentrations equal to 0.5 on the McFarland scale. The bacteria were diluted 100 times with the same solution before the assay. All samples were prepared as solutions with dimethyl sulfoxide and 2X MHB to give a concentration of 1,000 μg/ml and diluted using the serial dilution method. About 10 μl of microbes was added to each concentration of each sample. All test solutions were mixed well and incubated at the same temperature for 24 hours. A resazurin solution was used as an indicator; 10 μl of the solution was added to each well and then incubated for 3 hours at the same temperature. In the presence of living microbes, resazurin irreversibly changes color from deep blue to pink, which is detectable via visual observation. The lowest concentration of each sample that inhibited the microbe was the MIC. The solutions in the wells for which the color of resazurin did not change were dropped on MHA (for bacteria) or SDA (for yeast and fungi) and then incubated for 3 hours at the same temperature. The lowest concentration where microbe growth did not occur is the MMC. Erythromycin and ketoconazole were used as positive controls for the bacteria and fungi, respectively (Balouiri et al., 2016).
RESULTS AND DISCUSSION
Twenty species of Annonaceous plants were collected from the Sichon (1–4, 6–9, and 19–20), Thasala (5, 10–13, and 16–17), and Muang (14–15 and 18) districts of Nakhon Si Thammarat (Thailand). Some Annonaceous plant pictures in this research were shown in Figure 1. Voucher specimens were identified and deposited at Walailak University. The leaves of the samples were cleaned and dried at 50°C. Each sample was milled into a powder, which was subsequently extracted with refluxing methanol (samples 1–10: 3 × 4 l; samples 11–20: 3 × 0.5 l). The combined methanol fractions were filtered before all volatiles were removed from the filtrate under reduced pressure to yield the crude methanolic extract. The percent yield of all samples is shown in Table 1.
The phytochemical screening of the methanolic leaf extracts was carried out using a variety of tests. Summary of the phytochemical test results of all samples is shown in Table 2. The detail of test results was presented in Supplementary Materials. Dragendorff’s reagent was used for alkaloid testing. A small amount of each methanolic leaf extract was individually boiled with 10% H2SO4 (5 ml) in a water bath for 10 minutes. After filtration, the filtrates (1 ml) were treated with five drops of Dragendorff’s reagent. An orange-red amorphous precipitate was observed for samples 3, 4, 9, 11, 14, and 15, which indicates the presence of alkaloids. We used Borntrager’s test and a modified Borntrager’s test to confirm the absence of anthraquinone in all samples. The Liebermann–Burchard test was modified for steroid and terpenoid testing. All samples were treated with chloroform to remove some chlorophylls. The obtained results indicated that samples 1–20 contained terpenoids and that samples 2, 3, 9, 10, and 12–20 also contained steroids. Raymond’s reagent and Keddé’s reagent were applied to test for the C-methylene of the lactone ring of acetogenins. These reagents have been used to detect the C-methylene of the unsaturated lactone ring of cardiac glycosides. In our investigation, a precipitate occurred in the reactions of samples 1–2, 5, 7–9, and 11–18, which suggests that these contain acetogenins (Akaike, 2001). The froth test (El Hazzam et al., 2020) was used to test for saponin glycosides. A small amount of each methanolic leaf extract was individually boiled with distilled water (5 ml). After filtration, the filtrates (2 ml) were shaken for 30 seconds and examined. Honeycomb-shaped bubbles that remained stable for more than 30 minutes after shaking indicate the presence of saponin glycosides. Saponin glycosides were observed for samples 1–3, 5–8, 11, 13–15, 17, and 19. The other filtrates were tested for tannins using 1% ferric chloride and saturated lead subacetate solutions. Methanolic leaf extracts 1–20 were found to contain condensed tannins based on the test using ammonium hydroxide TS, in which all samples changed color to red-orange and formed a yellow precipitate. These results confirm the presence of flavonoids in all the samples. To determine the presence of coumarins, 1 ml of each of the filtrates was treated with 1 ml of 6 M sodium hydroxide solution. Samples 1–12, 14, 16–18, and 20 turned yellow during this reaction, which confirms the presence of coumarins.
Antioxidant activity
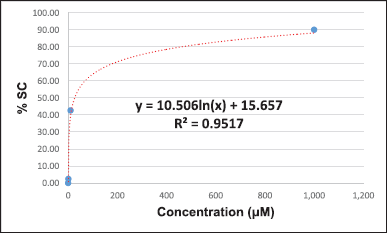
The DPPH-based free radical scavenging assay was used to determine the antioxidant activity of the methanolic leaf extracts relative to that of l-ascorbic acid. The results suggest that sample 1 (%SC50 = 5.39 + 0.12 μg/ml), sample 18 (%SC50 = 7.38 + 0.83 μg/ml), and sample 2 (%SC50 = 11.55 + 0.82 μg/ml) show the highest activity among the tested samples. The DPPH-based free-radical-scavenging assay result of all samples is shown in Table 3. Some calculations of the result were presented in Supplementary Materials.
Antimicrobial activity
The broth microdilution assay was applied to evaluate the antimicrobial activity of the methanolic leaf extracts. Resazurin was used as an indicator. Ampicillin and ketoconazole were used as positive controls for antibacterial and antifungal activity, respectively. The minimum concentration that inhibited the growth of the microbes is referred to as the MIC. The minimum concentration that killed the microbes is referred to as the MMC. A summary of the results is shown in Table 4.
The methanolic leaf extracts showed microbiostatic activity against the tested microbials. Samples 1–10 and 20 inhibited the growth of S. aureus at 250–500 μg/ml (sample 2 was the most active; MIC = 250 μg/ml). Samples 1–10, 12, 13, and 16–20 showed inhibitory activity against E. coli (MIC = 250–500 μg/ml). Samples 1–10 showed inhibitory activity against A. baumannii (MIC = 125 μg/ml). Samples 1–10, 13, and 16–20 showed inhibitory activity against P. aeruginosa (MIC = 125–250 μg/ml), while samples 1–10, 13, 17, 19, and 20 showed inhibitory activity against K. pneumoniae (MIC = 250–500 μg/ml). Samples 1–10 showed inhibitory activity against E. faecalis (MIC = 250–500 μg/ml), whereas sample 20 showed strong activity against E. faecalis (MIC = 50 μg/ml and MMC = 1,000 μg/ml). Only samples 1 and 4 exhibited fungistatic activity against C. albicans (MIC = 125 μg/ml).
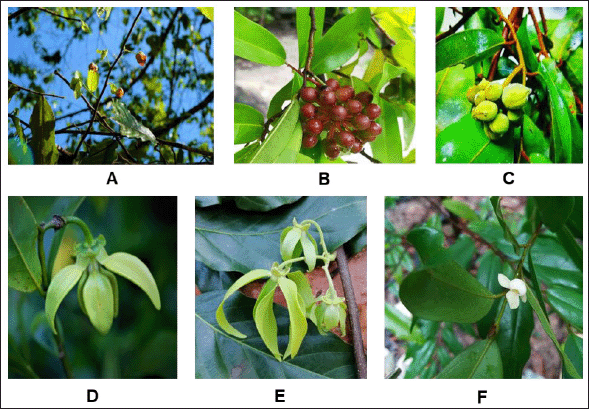 | Figure 1. Some Annonaceous plants in this research; A (Sample 1)-Miliusa lineata (Flowers, leafs, and stem), B (Sample 2)-Polyalthia suberosa (Leafs and fruits), C (Sample 10)-Rauwenhoffia siamensis (fruits, leafs, and stem), D (Sample 16)-Artabotrys hexapetalus (Flower), E (Sample 17)-Cananga odorata (Flower, leafs, and stem), and F (Sample 20)- Mitrephora chulabhorniana (Flower, leafs, and stem). (Damthongdee et al., 2019; Pooma and Suddee, 2014) [Click here to view] |
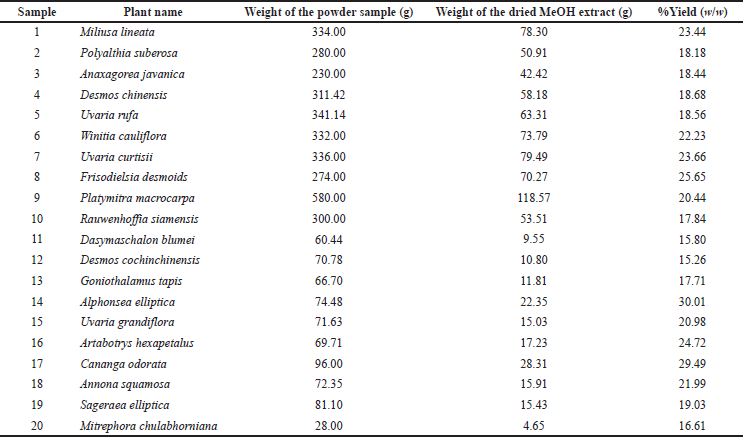 | Table 1. Methanolic leaf extracts of some Annonaceous plants in Nakhon Si Thammarat, Thailand (Sample 1–20).(Pooma and Suddee, 2014) [Click here to view] |
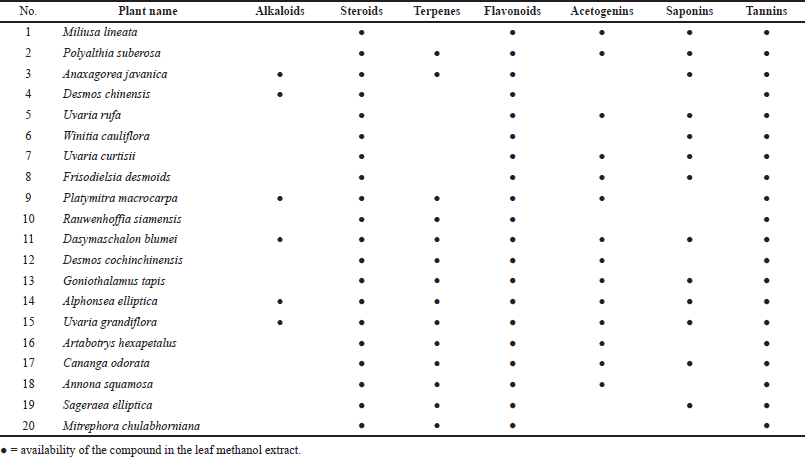 | Table 2. Phytochemical screening of methanolic leaf extracts of some Annonaceous plants in Nakhon Si Thammarat, Thailand (Sample1–20). (Auwal et al., 2014; El Hazzam et al., 2020; Heera et al., 2012; Hossain et al., 2013; Raal et al., 2020; Stefanachi et al., 2018) [Click here to view] |
20 species of Annonaceous plants were collected during an excursion in Nakhon Si Thammarat (Thailand). Voucher specimens were prepared and deposited at Walailak University. Ten samples (1–4, 6–9, and 19–20) were collected from a limestone mountain in the Sichon district. Seven samples (5, 10–13, and 16–17) were collected from the Thasala district. Samples 5 and 10 are ubiquitous native plants all across Nakhon Si Thammarat. Three samples (14, 15, and 18) were collected from the Muang District of Nakhon Si Thammarat. The leaves of all samples were used to prepare methanolic leaf extracts. The average percentage yield (w/w) of the extracts was 20. Phytochemical screening of the methanolic leaf extracts was carried out using a variety of standard tests. The test for alkaloids was conducted using Dragendorff’s reagent. The formation of an orange-red precipitate in the reaction indicates the presence of alkaloids, which occurred for samples 3, 4, 9, 11, 14, and 15. The amount of the orange-red precipitate that formed in samples 9, 11, and 14 was particularly high, which is probably related to the amount of alkaloids present in the sample. The Liebermann -Burchard test was used to gauge the presence of steroids and terpenoids. The blue-green color observed for samples 2, 3, and 9–20 indicated that these samples contained steroids. The purple-red or magenta color observed in all samples indicated that all the samples contained terpenoids. To screen for acetogenins, we applied reagents used to test for the C-methylene of the unsaturated lactone ring in cardiac glycosides, that is, Raymond’s reagent and Keddé’s reagent. The color of the methanolic leaf extracts did not change, albeit a precipitate was formed in the reactions of samples 1, 2, 5, 7–9, and 11–18, indicating that these contain acetogenins. The froth test was used to screen for saponin glycosides, which were found in samples 1–3, 5–8, 11, 13–15, 17, and 19; samples 6 and 11 contained especially high amounts of saponin glycoside, as evident from the high amount of stable honeycomb-shaped bubbles. All samples were treated with a saturated lead subacetate solution to form a precipitate, indicating that all samples contained tannins. To identify the kind of tannins, a 1% ferric chloride solution was used. All samples formed a turquoise/dark-green precipitate, confirming the presence of condensed tannins. These results are all related to the presence of flavonoids. All samples (1–20) reacted with ammonium hydroxide TS to give a red-orange solution and a yellow precipitate. The condensed tannins have a core flavonoid structure. The water-soluble part of the methanolic leaf extracts was also tested for coumarins. The occurrence of yellow/dark-yellow color in the reaction of almost all samples (1–12, 14, 16–18, and 20) indicates the presence of coumarins, which is not surprising, considering that the leaves of almost all Annonaceous plants have a unique smell that is related to the presence of coumarins.
 | Table 3. Antioxidant activity of the methanolic leaf extracts of some Annonaceous plants in Nakhon Si Thammarat, Thailand (Extract 1–20) and l-ascorbic acid. (Akaike, 2001; Braca et al., 2001) [Click here to view] |
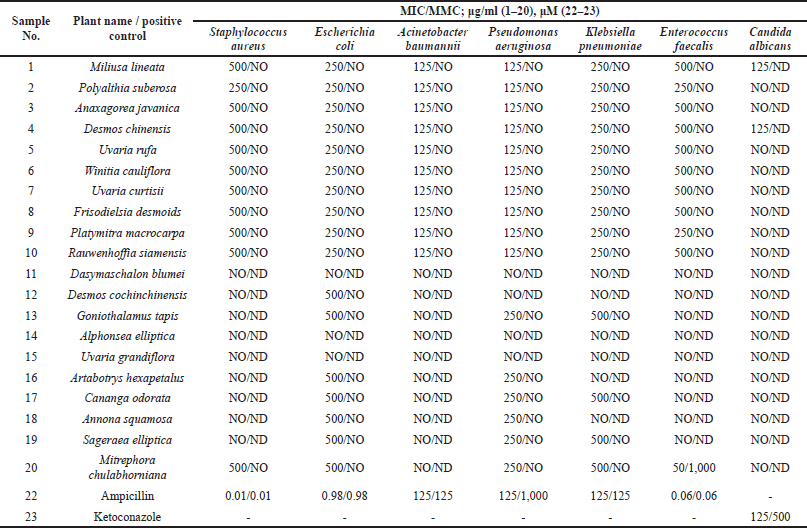 | Table 4. Results of a broth microdilution assay to examine the antimicrobial activity of the methanolic leaf extracts of some Annonaceous plants in Nakhon Si Thammarat, Thailand (Sample 1–20) and the positive controls ampicillin (22) and ketoconazole (23). [Click here to view] |
The presence of phytochemicals in the methanolic leaf extracts (1–20) is related to their antioxidant activity. The DPPH-based free radical scavenging assay was used to determine their antioxidant activities compared to that of l-ascorbic acid as a positive control. The %SC50 values of the samples were 5.39–74.42 μg/ml, whereby sample 1 was the most active (%SC50 = 5.39 μg/ml) and sample 3 was the least active (%SC50 = 74.42 μg/ml) among all the tested samples. The level of antioxidant activity is related to the number of hydroxyl groups, which contain lone pairs of electrons, in their structures. These lone-pair electrons of the hydroxyl groups can react with free radicals to protect against the negative health consequences of oxidative stress.
The methanolic leaf extracts showed microbiostatic activity against the test microbials. Samples 1–10 and 20 inhibited the growth of S. aureus at 250–500 μg/ml (sample 2 was the most active; MIC = 250 μg/ml). Samples 1–10, 12, 13, and 16–20 showed inhibitory activity against E. coli (MIC = 250–500 μg/ml). Samples 1–10 showed inhibitory activity against A. baumannii (MIC = 125 μg/ml). Samples 1–10, 13, and 16–20 showed inhibitory activity against P. aeruginosa (MIC = 125–250 μg/ml). Samples 1–10, 13, 17, 19, and 20 showed inhibitory activity against K. pneumoniae (MIC = 250–500 μg/ml). Samples 1–10 showed inhibitory activity against E. faecalis (MIC = 250–500 μg/ml), while sample 20 showed strong activity against E. faecalis (MIC = 50 μg/ml and MMC = 1,000 μg/ml). Only samples 1 and 4 showed fungistatic activity against C. albicans (MIC = 125 μg/ml). Based on these results, it seems feasible to conclude that the antimicrobial activity is related to the presence of flavonoids in the methanolic leaf extracts. The specificity toward the microbes depends on the structures of the compounds contained in the methanolic leaf extracts. Among these samples, sample 20 was particularly interesting as it showed bactericidal activity against E. faecalis (MIC = 50 μg/ml and MMC = 1,000 μg/ml), as were samples 1 and 4, which showed fungistatic activity against C. albicans (MIC = 125 μg/ml). This is the first time to report on the biological activities of all samples, especially sample 20.
CONCLUSION
Flavonoids were found in the methanolic leaf extracts of all Annonaceous plants in this study. Alkaloids, acetogenins, coumarins, and saponins are distributed in some kinds of Annonaceous plants. The specific activities of the methanolic leaf extracts are related to the types and amounts of their components. In this study, the antioxidant and antimicrobial activities of the methanolic leaf extracts of 20 Annonaceous plants were determined. All samples contained flavonoids, and all showed antioxidant activity in the DPPH assay. The potency of each sample depends on the substituent groups in the structure of the flavonoids, such as hydroxyl groups, which contain lone pairs of electrons and are able to react with free radicals. Based on the antimicrobial activity results of the test samples, the antimicrobial activity can be expected to be related to the presence of flavonoids in the methanolic leaf extracts. The specificity of the antimicrobial activity of the methanolic leaf extracts toward the microbes depends on the structures of the compounds they contain. Among these samples, extract 20 was particularly interesting as it showed bactericidal activity against E. faecalis (MIC = 50 μg/ml and MMC = 1,000 μg/ml), as were extracts 1 and 4, which showed fungistatic activity against C. albicans (MIC = 125 μg/ml). These three samples can thus be considered promising potential candidates for further investigations in order to obtain more scientific data and harvest the health benefits of the active compounds. This research was run on the theme of the discovery of the polypharmacophore from natural products. The main activity was antimicrobial activity. The microbial infection related to NO and oxygen free radicals leads to degenerative symptoms. The sample which showed antimicrobial activities with antioxidant activity will be a good candidate for medicine with synergistic activity. Among 20 species of Annonaceous plants from Nakhon Si Thammarat, samples 1 and 2 were those candidates. On the other hand, this is the first report on the biological activities of sample 20. However, all results in this research will be the guide for drug discovery of Annonaceous plants.