INTRODUCTION
The N-heterocycle is well known for its biological activity that increasingly attracts the development of novel biological entities, such as oxazole, oxazolone, benzoxazole, and oxadiazole (Vo and Bode, 2014). This class of compounds exhibits a wide variety of biological activities, including antidepressant, anticancer, antimicrobial, anti-inflammatory, anti-HIV, and analgesic effects (World Cancer Research Fund International, 2018). Currently, several available drugs contain N-heterocycle, including reclazepam, which is a benzodiazepine that has been used as an anxiolytic, thozalinone, an antidepressant, and cyclosporine, which is an immunosuppressant drug that is commonly utilized for several disorders (National Library of Medicine, 2004). N-heterocycle derivatives have been marketed and approved by the Food and Drug Administration (FDA) for numerous therapeutic indications. A promising opportunity appears to develop a novel and highly potent compound from this scaffold.
Therefore, the main objective of this review is to discuss the N-heterocycle biological activities such as the anticancer, antimicrobial, and anti-inflammatory activities and computationally predict the pharmacokinetics of the most potent compounds and their possible targets that mediate biological responses, as shown in Figure 1.
REPORTED EXPERIMENTAL STUDIES FOR N-HETEROCYCLES
As potential anticancer agents
Background and current treatment
Cancer is defined as the uncontrolled proliferation of abnormal cells that tend to metastasize to other organs or regions of the body through several mechanisms, including mutation, deoxyribonucleic acid (DNA) lesions, cell division, and cell cycle checkpoints (Ames et al., 1995). It is the leading cause of death worldwide, accounting for an estimated 9.9 million deaths in 2020 (World Health Organization, 2020). Additionally, it is expected that the number of cancer mortality cases will continue to grow, reaching 21.4 million worldwide by 2030, due to changes in population demographics (World Health Organization, 2011). The most common types of cancer worldwide are lung and breast cancer, which contribute to 11.7% and 11.4% of the total cancer cases (World health organization, 2020).
Multiple therapeutic approaches are used to treat cancer, and the selection of therapy mainly depends on the type, stage, and patient comorbidities. Treatment is classified as local treatment (e.g., radiotherapy) or systemic treatment (e.g., chemotherapy) (American Cancer Society, 2021). Moreover, cancer cells tend to acquire resistance, which plays a major role in the failure of chemotherapy by several mechanisms (Gatti and Zunino, 2005; Ramos and Bentires-Alj, 2015). The first mechanism is drug inactivation, which occurs with most of the anticancer medications that require metabolic activation for their therapeutic effect, and platinum analogs are an example of this type of resistance by the formation of the platinum Glutathione (GSH) conjugate (Dasari and Bernard Tchounwou, 2014; Peklak-Scott et al., 2008). Second, drug efflux by P-glycoprotein, known as the multidrug resistance protein, is considered the most prevalent transporter to which the failure in treatment in over 90% of patients is attributed (Alfarouk et al., 2015; Aller et al., 2009). Third, DNA damage repair can reverse drug-induced damage, leading to anticancer drug resistance (Bonanno et al., 2014). Fourth, solid tumors metastasize by an epithelial-mesenchymal transition mechanism (Dudas et al., 2020). The fifth mechanism is drug target alteration that occurs due to mutation or modification of enzyme expression (Housman et al., 2014). The last mechanism is epigenetics, including DNA methylation and histone modification by either methylation or acetylation (Housman et al., 2014). As discussed earlier, cancer is the leading cause of death worldwide, and multiple resistance mechanisms are responsible for preventing therapeutic benefits for cancer patients.
Reported anticancer mechanisms for N-heterocycles
Numerous studies have investigated the oxazole and its related heterocycles, such as the oxadiazole and oxazolone scaffold, as a novel anticancer agent, and herein, we will discuss and summarize reported studies based on proposed mechanisms and important structural features that are essential for anticancer activity. According to Kemnitzer et al. (2009), the authors assessed the apoptosis-inducing activity of several oxadiazole derivatives by evaluating the ability to activate caspase, a protease enzyme that plays an essential role in cell death, using multiple cancer cell lines. The study demonstrated that oxadiazole compound C1 possesses EC50 equal to 0.38 µM against colorectal cancer (DLD-1) and 0.91 µM against breast cancer (T47D). However, C1 has no activity against the non-small cell lung cancer (H-1299) cell line. Furthermore, when C1 is given in combination with paclitaxel using MX-1 human breast cancer cells, it causes 80% growth inhibition with good tolerability. Overall, the authors concluded that C1 has significant anticancer activity against DLD-1 and T47D but has poor activity (EC50 > 10 µM) against H-1299 (Kemnitzer et al., 2009).
An additional study was conducted by Boulos et al. (2011) on oxazole derivatives using doxorubicin (DXR) as a reference drug. The results revealed that oxazole compounds C2 and C3 demonstrated an IC50 equal to 19.3 µg/ml using the breast (MCF7) cancer cell line. Both compounds showed a similar mechanism of action to DXR in which it interferes with DNA replication by inhibiting topoisomerase II, an enzyme responsible for the separation of the DNA double helix, aiding its replication process (Boulos et al., 2011).
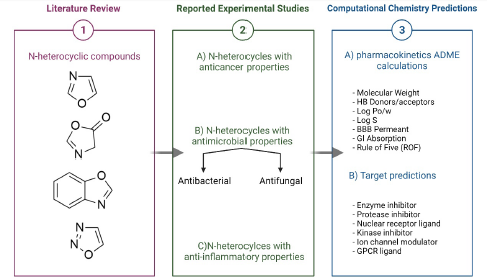 | Figure 1. This article aims to review and discuss the anticancer, antimicrobial, and anti-inflammatory activity for N-heterocycle derivatives then computationally predict the pharmacokinetics and their possible targets that mediate biological responses. [Click here to view] |
In another report, using a human liver cancer cell line (HepG2), Abdelhameid et al. (2020) compared the anticancer activity of sorafenib (SOR), an FDA-approved chemotherapy drug used to treat liver cancer, to several oxazolone derivatives. The study results showed that oxazolone derivatives possess a promising cytotoxic activity via inhibition of vascular endothelial growth factor receptor-2 (VEGF-2) that is responsible for angiogenesis in tumor cells (Pons-Cursach and Casanovas, 2019), as well as suppression of β-tubulin (TUB) polymerization. Nevertheless, both SOR and the oxazolone compound C4 were found to cause morphological changes within cancer cells, increasing the percentage of apoptosis during the cell cycle at the sub-G1 phase and the percentage of 3/7 caspase by induction of p53, a tumor suppressor gene, which activates cell apoptosis (Abdelhameid et al., 2020). Several triazole derivatives have been investigated by El Bourakadi et al. (2020) for their in vitro antiproliferative activity. The reported human cancer cell lines were colorectal adenocarcinoma (HT-29), non-hormone-dependent breast cancer (MDA-MB-231), and mammary gland/breast adenocarcinoma (SKBR3). Compound C5 exhibited significant activity against all the cell lines tested at IC50 values ranging from 1.28 to 7.72 µg/ml. Compound C5 underwent further mechanistic investigation to determine whether the cell growth inhibition was mediated by caspase activation. The HT-29 and MDA-MB-231 cells exhibiting caspase 3/7 activity increased from 6.87% and 6.73% to 21.48% and 39.46%, respectively, which indicates that compound C5 induces caspase activation (El Bourakadi et al., 2020).
A similar study was conducted by Minhas et al. (2006) to assess a series of macrocyclic compounds that contain four to seven oxazole units for their antitumor activity. Oxazole derivatives were evaluated for their ability to stabilize G-quadruplex DNA and induce cell death. Unlike hexaoxazoles, tetraoxazole macrocycle showed little or no binding to G-quadruplex DNA. Hence, the selected hexaoxazole compound C6 binds selectively to G-quadruplex DNA with moderate antitumor activity. Additionally, the reported IC50 values (µM) were 0.5 for murine leukemia (P388) and 0.4 for the human lymphoblastoma (RPMI 8402) (Minhas et al., 2006).
Reported anticancer structural requirements for N-heterocycles
To obtain effective anticancer agents, Savariz et al. (2012) reported that having a good electron-donating substituent is crucial for anticancer activity. The study investigated the in vitro cytotoxic activity of four oxazolone moieties against six cancer cell lines, including glioma (U251), melanoma (UACC-62), breast (MCF7), prostate (PC-3), ovarian (OVCAR-03), and colon (HT-29) cells. The study results demonstrated a potent activity with IC50 < 10 µM against three cancer cell lines, glioma (U251), prostate (PC-3), and ovarian (OVCAR-03), for two out of the four compounds. On the contrary, due to a lack of an electron-donating substituent at the phenyl ring, the other two compounds showed activity only in melanoma (UACC-62). Thus, it is apparent from this study that having a good electron-donating substituent at the phenyl ring at C-1 is important for cytotoxic activity in cancer cells. The most potent compound, C7, with IC50 ≤ 1.50 µM for U251, PC-3, and OVCAR-03 showed promising activity as a novel anticancer agent (Savariz et al., 2012).
Along with the above-discussed study having p-fluorophenyl at the C-2 position equally important for the activity as reported by Kumar et al. (2010), the anticancer activity was determined using a formazan dye [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT)] conversion assay to determine the antitumor activity for 18 oxazole derivatives against 6 human cancer cell lines, prostate (PC-3, DU145, and LnCaP), breast (MCF7 and MDA231), and pancreatic (PaCa2) cells. Five compounds demonstrated an IC50 < 50 µM against numerous cancer cell lines. Compound C8 showed selective and potent cytotoxic activity against PC-3 (42.8), DU143 (31.8), MCF7 (28), and PaCa2 (40.6). The study highlighted that p-fluorophenyl at the C-2 position is necessary for the activity, as seen in C8, which demonstrated promising anticancer activity against all cancer cell lines (Kumar et al., 2010).
Reported anticancer activity for N-heterocycles (miscellaneous)
As summarized in Fadda et al.’s (2020) study, the authors synthesized derivatives that possess a moderate to strong cytotoxic effect against several cancer cell lines. No proposed mechanism or structural requirement has been reported. Moreover, the most potent compound, C9, showed IC50s of 8.9, 9.2, 13.1, and 12.1 µg/ml against liver cancer (HepG2), colon cancer (HTC-116), prostate cancer (PC-3), and breast cancer (MCF7), respectively, suggesting a remarkable anticancer activity for the tested compound (Fadda et al., 2020). In a similar attempt, Sengupta et al. (2008) studied oxadiazole derivatives that possessed anticancer activity against Ehrlich ascites carcinoma (EAC) cells and originated from human breast cancer cells (T47D) by spontaneous passaging. The experiment was performed using Swiss albino mice by measuring the ability of oxadiazole derivatives to inhibit cancer cell growth in ascitic fluid. One of the synthesized oxadiazole compounds, C10, demonstrated the ability to reduce the percentage of tumor weight inhibition (% TWI) by 37.93 and inhibit the tumor cell count (% TCI) by 37.86 (Sengupta et al., 2008). All reviewed studies are summarized in Table 1.
As potential antimicrobial agents
Background and current treatment
Infectious diseases kill 17 million people per year. Approximately 50,000 people, whether adults or children, die every day because of infectious diseases (World Health Organization, 1996). Lower respiratory infections were considered the fourth leading cause of death globally in 2019 by the WHO (2020). Fortunately, an infectious disease can be prevented by a vaccine and treated using the correct antimicrobial drugs. The first antibiotic, penicillin, was discovered by Alexander Fleming in 1928, followed by the major development of different antibiotic classes, which contributed to a significant reduction in the mortality and morbidity of some infectious diseases such as pneumonia and gonorrhea (Silver, 2011).
To cure a patient of infection, the clinician needs to identify the microbe type, and based on the findings, the treatment can be initiated with a specific antibiotic, anti-viral or antifungal (Standing up to infectious disease, 2019). However, antimicrobial therapy may fail due to multiple causes (Cunha and Ortega, 1995). Among the leading causes is antimicrobial resistance (AMR), which is the most challenging one, affecting the quality of life and economy (Septimus, 2018). Furthermore, statistics indicate that, by the year 2050, there will be a rise in AMR that will lead to 10 million people dying every year, and the global economic cost would be up to 100 trillion USD (Review on Antimicrobial Resistance, 2014). Additionally, AMR extends hospital stays, additional doctor visits, and high costs, which pose a high risk of the occurrence of chronic diseases (Centers for Disease Control and Prevention, 2020).
The microorganism can develop resistance through numerous mechanisms, such as destruction of drugs by enzymes, pumps, or changing of the entryways (Centers for Disease Control and Prevention, 2020). Moreover, the most common examples of AMR are modifications of the bacterial structure and membrane, which inhibit the medication that acts on the membrane to attach and produce actions such as penicillin (beta-lactam antibiotic) (Capita and Alonso-Calleja, 2013). The second mechanism is enzymatic inactivation, which more commonly happens to beta-lactam medications as the bacteria produce a lactamase enzyme that degrades the medication, thus yielding to the inactivation of the drugs (Capita and Alonso-Calleja, 2013). The third mechanism is efflux pumps, which transport the drugs outside the cell, thus reducing the intracellular drug concentration (Alanis, 2005). The fourth mechanism is target remodeling. As some drugs bind to the ribonucleic acid (RNA) to produce activity, such as fluoroquinolones, mutation of the binding site will decrease the affinity and antibacterial effect (Alanis, 2005). Lastly, through the development of new metabolic pathways. For example, sulfonamide and trimethoprim interfere with the bacterial folic acid synthesis, so the bacteria form changes in the target enzyme. Thus, reducing their affinity.
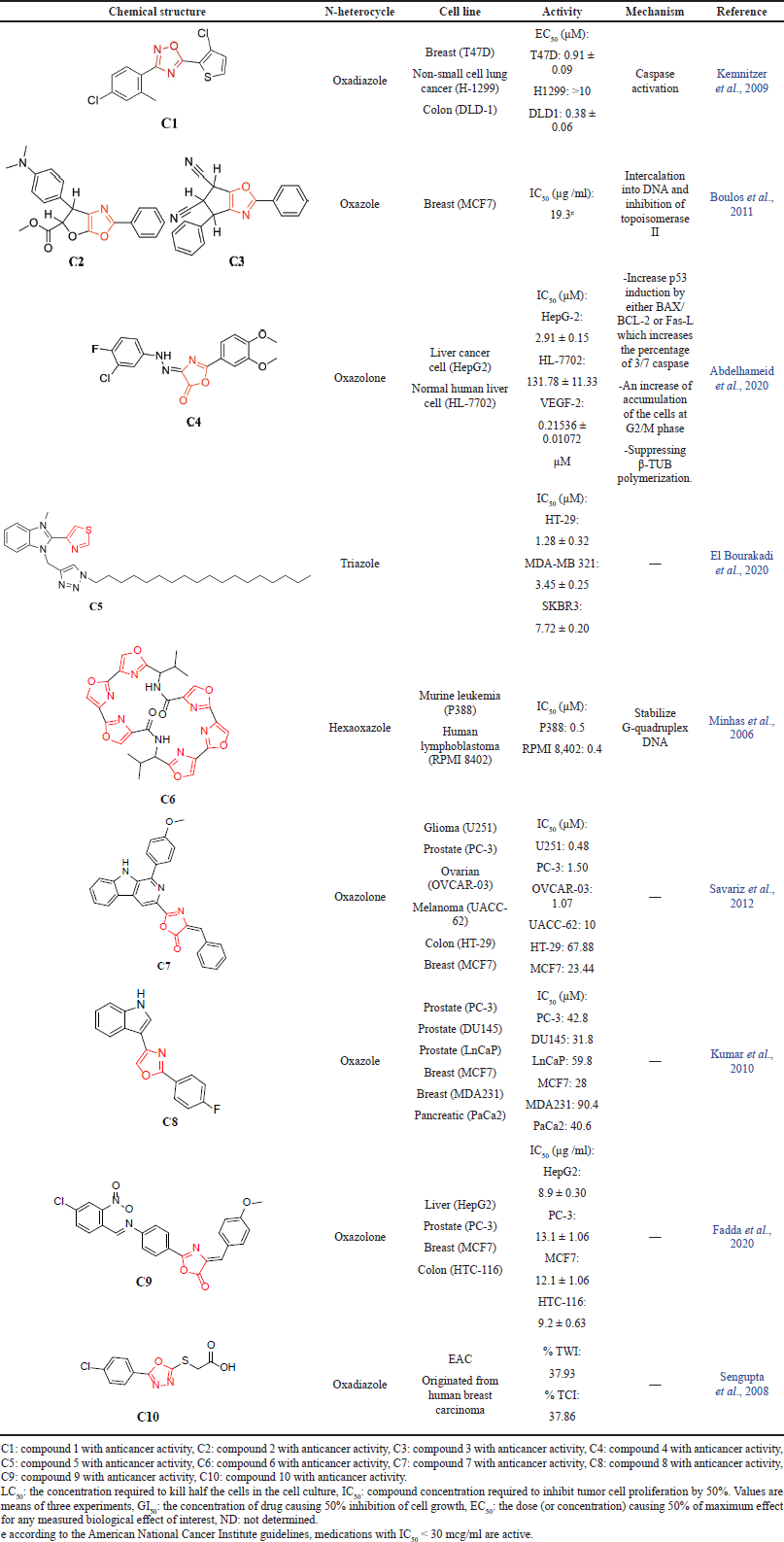 | Table 1. The reported anticancer activity for N-heterocycles. [Click here to view] |
N-heterocycles have been reported to possess antimicrobial activity that could overcome some of the discussed challenges. Thus, herein, we will discuss and review several articles based on structural requirements for antimicrobial activity against multiple organisms such as Staphylococcus aureus Bacillus subtilis, Pseudomonas aeruginosa, Candida albicans, and Aspergillus niger.
Reported antibacterial structural requirements for N-heterocycles
Olomola et al. (2018) synthesized oxazolone derivatives and screened them against several Gram-positive and Gram-negative bacteria, including S. aureus, Bacillus cereus, Streptococcus pneumonia, Enterococcus faecalis, Corynebacterium pyogenes, P. aeruginosa, Proteus vulgaris, Klebsiella pneumoniae, Escherichia coli, and Shigella sp. Using streptomycin and ampicillin as control drugs, Olomola et al. (2018) found that M1 exhibits a higher minimum inhibitory concentration (MIC) in E. coli and S. pneumonia relative to ampicillin which was due to the presence of the bromo group in the phenyl ring. Moreover, the authors proposed that this derivative demonstrates its activity via bacterial cell wall disruption. However, the exact mechanism was not specified (Olomola et al., 2018). Moreover, Rawat and Shukla (2016) investigated the activity of the oxazole compound M2 as an antimicrobial agent. Ampicillin was used as a reference drug against the S. aureus, B. subtilis, P. aeruginosa, and E. coli bacterial strains. Using the zone of inhibition, compound M2 demonstrated antibacterial activity with a zone of inhibition greater than 15 mm, and the authors concluded that the presence of an electron-withdrawing group at the para position is essential for the antibacterial activity (Rawat and Shukla, 2016).
An additional study conducted by Shukla et al. (2016) assessed the antimicrobial effect of oxadiazoles while using ciprofloxacin and fluconazole as reference drugs. The authors highlighted the importance of the electron-donating group for antibacterial activity. The tested bacteria strains were S. aureus, E. faecalis, E. coli, and P. aeruginosa, and the antibacterial activity of ciprofloxacin (MIC) was between 62.5 and 125 μg/ml, while the oxadiazole derivative M3 values were between 15.62 and 125 μg/ml against the same bacterial strains (Shukla et al., 2016). One study investigated 13 oxazolone derivatives, and the results indicated that the oxazolone derivative M4 had an MIC value of 166.4 µg/50 µl against B. subtilis and 160 µg/50 µl against E. coli. Pasha et al. (2007) highlighted that the halogen derivatives of substituted oxazolones appear to have a significant impact on antibacterial activity. Additionally, Andrejevi? et al. (2020) have assessed the antibacterial activity using two Gram-positive, S. aureus and Listeria monocytogenes, and two Gram-negative, P. aeruginosa and E. coli, species. In this study, they investigated the antimicrobial activity of zinc (II) complexes with different N-heterocycles. Consider that zinc shows a significant reduction in child mortality in the treatment of infections causing uncontrollable diarrhea in Asian and African countries (Larson et al., 2009). Considerable zones of inhibition for M5 have been detected for all organisms except for P. aeruginosa (Andrejevi? et al., 2020).
A recent piece of evidence added by Arshad (2018) revealed the antibacterial activity of oxazole derivatives on the Gram-positive bacteria S. aureus and Staphylococcus epidermidis as well as the Gram-negative bacteria E. coli and Proteus mirabilis. It is worth mentioning that the author did not report any structural requirements nor mechanisms for activity. However, the author reported that derivative M6 exhibited significant antibacterial activity ≤24.32 against the four bacteria strains which were measured by the zone of inhibition (mm) (Arshad, 2018). A similar investigation was conducted by Singh et al. (2017) on a few oxazolone derivatives, which were found to possess antimicrobial activity against the bacteria E. coli, P. aeruginosa, S. aureus, and Streptococcus pyogenes, along with the fungi C. albicans, A. niger, and Aspergillus clavatus. Compound M7 exhibited high activity against the four bacterial species with an MIC value equal to or below 125 μg/ml comparable to the antibacterial drugs ampicillin (MIC value 100–250 μg/ml) and chloramphenicol (MIC is 50 μg/ml) (Singh et al., 2017).
In another study done by Naganagowda et al. (2011) the authors synthesized 23 oxazolone and imidazolone derivatives and tested for their antibacterial activity against Gram-negative bacteria P. aeruginosa and E. coli and Gram-positive bacteria (S. aureus and B. subtilis). The study results showed that compound M8 had a zone of inhibition (mm) of less than 20, suggesting N-heterocycle derivatives hold great promise as active antimicrobial candidates (Naganagowda et al., 2011). A similar study conducted by Gadhe et al. (2010) found that compound M9 has a similar zone of inhibition (in mm) to ampicillin sodium against B. subtilis and S. aureus (Gram-positive bacteria) and E. coli and Salmonella typhi (Gram-negative bacteria). All the examined studies are compiled in Table 2a.
Reported antifungal activity for N-heterocycles
A considerable amount of literature has been published on N-heterocycle activity as a novel antifungal agent. However, little to no detailed information was found about the structural requirements for this type of activity. For example, Andrejevi? et al. (2020) evaluated the antifungal activity of M5 against two Candida strains, C. albicans and C. parapsilosis. The author reported that M5 shows inhibition zones (mm) equal to 3 and 7 for C. albicans and C. parapsilosis, respectively (Andrejevi? et al., 2020). In a similar attempt, Gadhe et al. (2010) explored the antifungal properties of oxazole derivatives, and their analogs were evaluated against C. albicans and A. niger using clotrimazole as a reference drug. The study results showed that M9 had a zone of inhibition (mm) equal to 30 and 21 against C. albicans and A. niger (Gadhe et al., 2010).
In Singh et al.’s (2017) study, the authors determined the antifungal activity of the oxazole compound M10 against C. albicans and A. niger. This compound showed a MIC of under 500 μg/ml and was compared with the antifungals nystatin (MIC 100 μg/ml) and griseofulvin (MIC 100–500 μg/ml). However, there was barely any reported activity shown against A. clavatus fungi (Singh et al., 2017). Compound M11 was synthesized by Shukla et al. (2016) and showed promising antifungal activity against two fungal strains, C. albicans and A. niger. The synthesized oxadiazole agent (MIC range from 31.25 to 62.5 μg/ml) demonstrated higher potency relative to fluconazole (MIC values between 62.5 and 125 μg/ml) (Shukla et al., 2016).
In a similar attempt Rawat and Shukla (2016), compared the antifungal activity of ketoconazole with oxazole derivatives. Compound M12 was reported to be the most potent among the other derivatives with antifungal efficacy against C. albicans and A. niger fungal strains. Furthermore, the action was measured using the zone of inhibition. Rawat and Shukla (2016) concluded that compounds with a zone of inhibition greater than 15 mm exhibit strong antifungal activity against microbes. The activities of the compounds in the discussed studies are outlined in Table 2b.
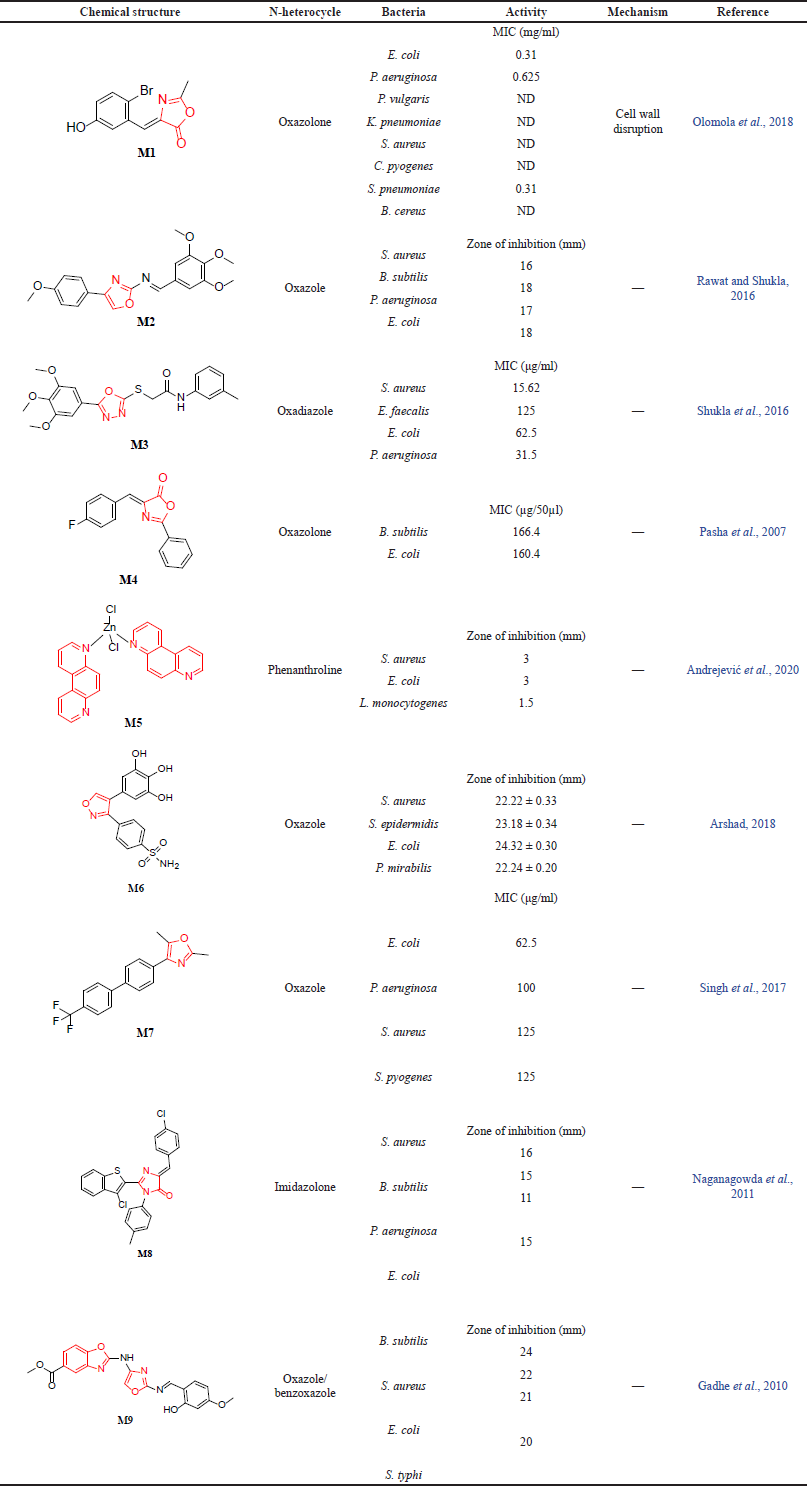 | Table 2a. The reported antibacterial activity of N-heterocycles. [Click here to view] |
As potential anti-inflammatory agents
Background and current treatment
Inflammation reflects the immune system’s response to a foreign object, which could manifest as redness, heat, swelling, pain, and loss of function (Stone et al., 2021). Inflammatory diseases include a wide range of disorders such as diabetes, cardiovascular diseases, chronic respiratory diseases, cancer, arthritis, and joint diseases (Desai et al., 2012). The prevalence of arthritis affects approximately 350 million people worldwide and 43 million people in the United States. It was reported that inflammatory diseases might increase mortality in a situation in which three out of five people die from chronic inflammatory diseases (Pahwa et al., 2020). Inflammation is classified into two types: acute inflammation and systemic chronic inflammation. Acute inflammation is triggered by pathogen-associated molecular patterns that are functional components of the microorganisms, such as peptidoglycan, double-stranded RNA, and lipopolysaccharide (Akira et al., 2006; Janeway Jr and Medzhitov, 2002). Besides, acute inflammation is triggered by damage-associated molecular patterns which are related to tissue destruction or damage (Rubartelli and Lotze, 2007).
As for the treatment of inflammation, numerous choices are available depending on the inflammation type and disease. For example, nonsteroidal anti-inflammatory drugs (NSAIDs) and cyclooxygenase (COX) inhibitors are used to relieve pain caused by arthritis or a musculoskeletal injury (Crofford, 2013; Mathew et al., 2011; Wongrakpanich et al., 2018). In comparison, corticosteroids are used for a variety of conditions such as systemic lupus, asthma, and sarcoidosis (Barnes, 2006; Grutters and Van den Bosch, 2006; Stojan and Petri, 2013). Approved drugs, such as NSAIDs, are commonly prescribed but can exhibit nephrotoxicity and gastrointestinal (GI) side effects.
Reported anti-inflammatory mechanisms and structural requirements for N-heterocycles
A lot of research was conducted to discover novel anti-inflammatories with minimal side effects. Moreover, the unique anti-inflammatory properties of the N-heterocycle scaffold have been an object of research since the 1980s. Herein, we reviewed and summarized the recent advances and updates regarding the anti-inflammatory properties of the N-heterocycle scaffold.
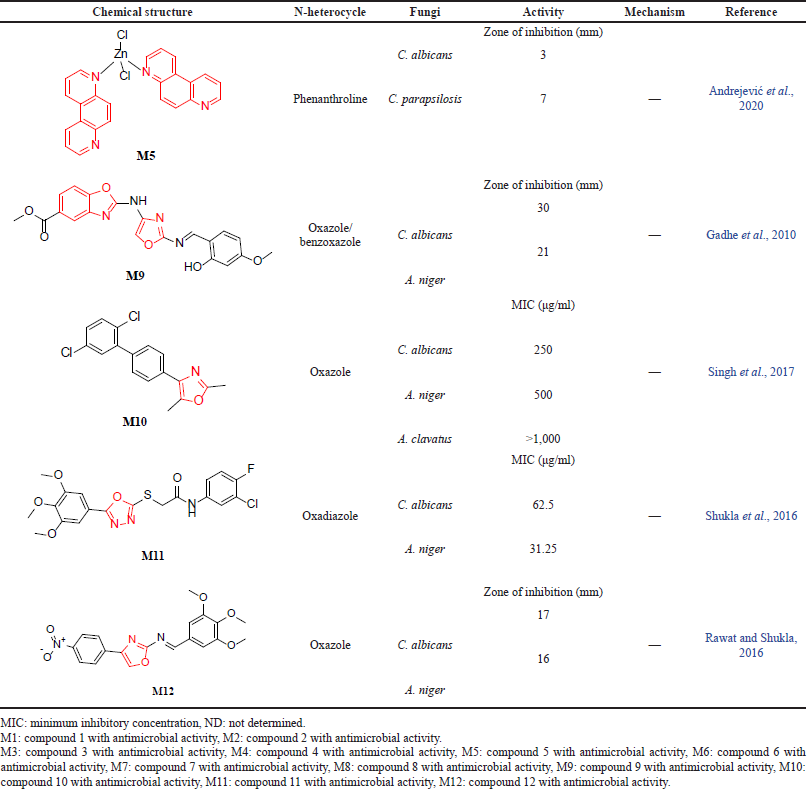 | Table 2b. The reported antifungal activity of N-heterocycles. [Click here to view] |
The study conducted by Mohamed et al. (2017) designed and synthesized a series of oxazolones to evaluate the inhibitory activity against both the COX-1 and COX-2 enzymes. Using IC50 (µmol) as a measurement tool and celecoxib as a reference drug, seven oxazolone derivatives have been evaluated, and the results showed higher inhibitory activity against the COX-2 than COX-1 enzymes. Compound I1 was associated with a lower IC50 against COX-2 relative to the reference drug, suggesting a better inhibitory activity that could overcome the GI side effects (Mohamed et al., 2017). In another attempt to investigate the anti-inflammatory activity of oxadiazole by inhibition of the COX enzyme, Bezerra et al. (2005) reported the ability of 1,2,4-oxadiazole derivatives to reduce carrageenan-induced edema in mice. The test was conducted with the use of aspirin and ibuprofen as standard drugs in comparison to oxadiazole derivatives. The results suggested that compounds I2 and I3 had a proportional anti-inflammatory effect with aspirin and ibuprofen in the inhibition of the COX enzyme. Further analysis showed the presence of a decapentyl group at the C-5 position enhances the anti-inflammatory activity (Bezerra et al., 2005).
Additionally, Gökhan-Kelekçi et al. (2009) assessed the anti-inflammatory activity for 12 benzoxazolone derivatives. Using indomethacin as a reference compound and a carrageenan-induced hind paw edema model as a screening tool, the authors reported a significant positive correlation between bearing no substituent at the phenyl ring and expressing significant activity at the 50 and 100 mg/kg doses in compound I4 when compared to indomethacin. The proposed mechanism is via inhibition of either the COX-1 and/or COX-2 enzymes (Gökhan-Kelekçi et al., 2009). In a recent study conducted by Abd El-Hameed et al. (2021) that investigated several pyridazinone derivatives, the author found that compound I5 can inhibit both the COX and lipoxygenase (LOX) enzymes. The dual inhibition of COX/LOX shows a higher anti-inflammation efficacy and lower incidence of vascular adverse events that could occur in potent COX-2 inhibitors (Abd El-Hameed et al., 2021). In a study conducted by Husain and Priyanka Ahuja (2009) to evaluate the biological activity of oxadiazole compounds as anti-inflammatory agents, the authors tested carrageenan-induced paw edema in rats and compared the activity of the compounds in percent of inhibition with that of indomethacin. Among the 17 oxadiazoles, compound I6 was shown to have the highest anti-inflammatory efficacy, with 56.2% inhibition of inflammation (Husain and Priyanka Ahuja, 2009).
Zheng et al. (2015) synthesized 10 compounds and examined their anti-inflammatory activity using a carrageenan-induced rat paw edema test and aspirin as a reference drug. Interestingly, compound I7 demonstrated anti-inflammatory activity, equal to 56.6%, which showed higher potency relative to aspirin. The study concluded that benzoxazolone derivatives have remarkable anti-inflammatory activity as highlighted (Zheng et al., 2015). A study conducted by Narayana et al. (2005) tested the anti-inflammatory activity of 1,3,4-oxadiazole. The results showed that derivative I8 possesses a higher percentage of edema inhibition (71.1%) than indomethacin (69.2%). Thus, derivative I8 possesses a promising anti-inflammatory activity that could be further developed and optimized to discover novel treatments for inflammatory-based diseases (Narayana et al., 2005). All the related measurements of the discussed studies are summarized in Table 3, and all the proposed mechanisms of N-heterocycles for the three activities are shown in Figure 2.
COMPUTATIONAL CHEMISTRY PREDICTIONS
Pharmacokinetics (ADME) properties of N-heterocycles
The most potent N-heterocycle derivatives that were discussed above were selected and evaluated for their physicochemical properties. Moreover, all the generated SMILES for the chemical structures are summarized in Tables T1S–T3S. The values were measured and calculated using the SwissADME web server, which is a tool that gives access to computer models (e.g., the ESOL model) that can predict the physicochemical properties, pharmacokinetics, and drug-likeness (Daina et al., 2017). Each compound has been drawn by using ChemAxon’s Marvin JS, which enables the user to draw a 2D chemical structure. After drawing completion, the system starts the calculations and runs the computation. The results are shown in Table 4–6.
Molecular weight (MW)
MW can have a significant role in determining solubility, penetration, absorption, and distribution of compounds (Gleeson, 2008). Aside from compounds C5, C6, and I5, all of the measurements fall within the recommended range (150–500 g/mol) as recommended in the SwissADME web server (Daina et al., 2017).
Hydrogen bond-donor/acceptor (HB-donor/acceptor)
An increase in the number of HB-donors and/or acceptors can suppress the permeability of the molecule to the cell membrane (Desai et al., 2012). According to Lipinski et al. (2001) [rule of five (ROF)], HB-D should be ≤5, and HB-A should be ≤10 to predict drugs with high absorption ability. Except for C6, all the compounds follow this rule.
Lipophilicity log Po/w (WLOGP)
LogPo/w [(log P method developed by Wildman and Crippen (WLOGP)] plays a major role with topological polar surface area in classifying compounds into blood–brain barrier (BBB) permeant and GI absorption in the BOILED egg model in SwissADME. It is stated that compounds with log P ranging from +0.4 to +6.0 can penetrate the BBB. Thus, activity on the central nervous system (CNS) can be predicted. All of the anticancer compounds have a Log Po/w less than or equal to 5, except C5. Compounds M5, M7, and M8 of the antimicrobials, as well as I2 and I3 of the anti-inflammatory compounds, fall outside this range.
Solubility log S (SILICOS-IT)
Solubility can be affected by MW, hydrogen bonding, and lipophilicity. As the MW decreases, the solubility increases. According to SwissADME, the classification of compounds that possess Log S > −4 is considered water-soluble. However, compounds with Log S < −6 are moderately soluble (Daina et al., 2017). Therefore, C10, M1, and I7 are highly soluble compounds, while the rest of the compounds were found to be moderate to poorly soluble.
BBB permeability
For CNS activity, BBB permeability is essential. Besides, lipophilicity, MW, and hydrogen bonds may influence brain permeation positively (Di, 2008). As demonstrated in Table 4–6, the compounds capable of BBB penetration are C2, C3, C8, M1, M2, M4, I1, and I4.
GI absorption
Oral absorption is highly affected by solubility, permeability, and MW (Di, 2008). Because of their poor solubility, compounds C5, C6, M5, M6, M7, M9, M11, I2, and I3 demonstrated poor GI absorption.
Rule of five
The ROF, which is also called Lipinski’s rule, is a set of structural characteristic guidelines for drug-like structures (MW ≤ 500, Log P ≤ 5, HB-D ≤ 5, and HB-A ≤ 10) (Di, 2008). All the compounds follow the ROF with zero violations except C5 violated MW > 500, Moriguchi octanol-water partition coefficient (MLOGP) > 4.15, C6 violated MW > 500, NorO > 10, and M8, I2, I3, I5, I8 have one violation, which is MLOGP > 4.15.
Target predictions of N-heterocycles
Using the Molinspiration web server (Molinspiration software), Table 7–9 summarize the bioactivity scores of the most potent anticancer, antimicrobial, and anti-inflammatory N-heterocycle derivatives to predict the probability of the compounds being bioactive at a specific target. As reported in the web server, the higher the bioactivity score (positive value), the more likely it will be active at that target (Molinspiration software).
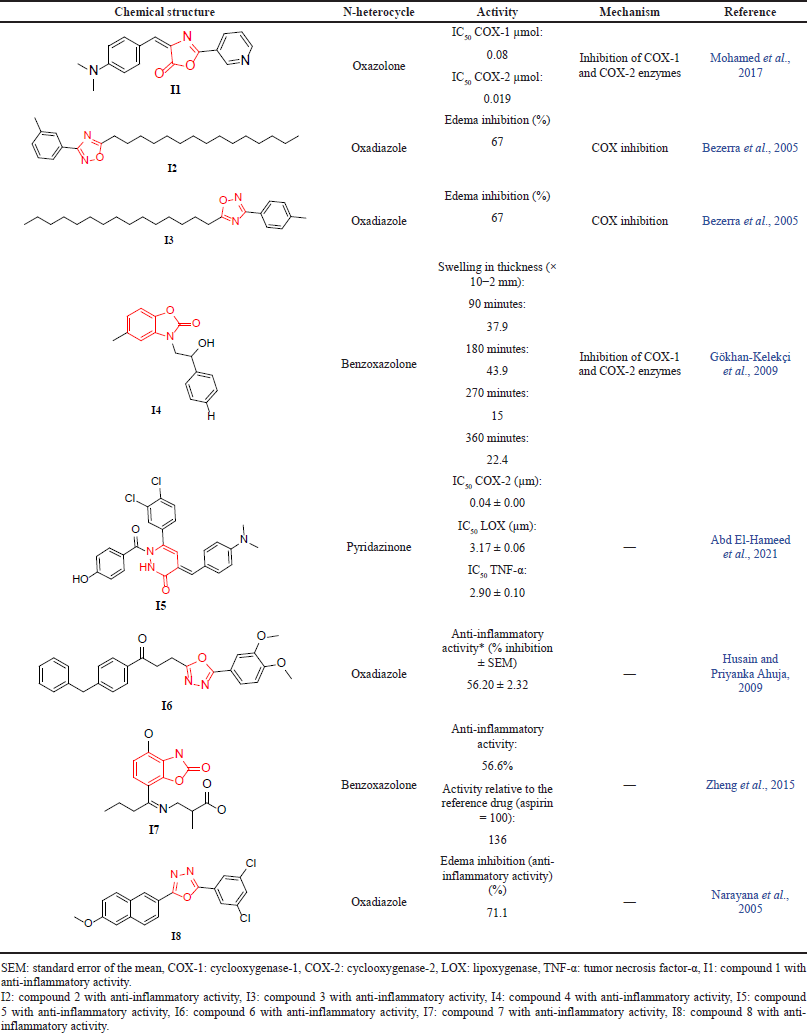 | Table 3. The reported anti-inflammatory activity of N-heterocycles. [Click here to view] |
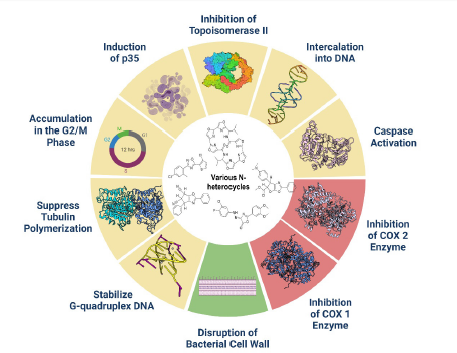 | Figure 2. This figure highlight all the reported mechanisms of N-heterocycle derivatives, it works on several sites on cancer cell lines such as DNA itself and cell cycle. Moreover, it disrupts the bacterial cell wall to produce anti-microbial activity. Furthermore, it works on COX-1 and/or COX-2 for anti-inflammatory activity. [Click here to view] |
G Protein-coupled receptor (GPCR) ligands
GPCRs are the largest superfamily of membrane receptors (Trzaskowski et al., 2012). The GPCR plays a role in the enhancement of phagocyte migration to infected cells so that the phagocytes can easily kill the invading bacteria and fungi and reduce inflammation (Sun and Richard, 2012). As shown in Table 7, C5, C6, and C8 demonstrated positive values for the GPCR target protein, which could potentially mediate anticancer activity. Moreover, among 11 antimicrobial compounds, M6, M7, and M10 possess a positive value, which indicates their good activity at this target, while in the anti-inflammatory compounds the highest positive value is I7, with a score of 0.28, followed by I2 and I3.
Ion channel modulators
Modulators enrich the movement of ions through the cell membrane. Additionally, ion channels such as K+ and Ca2+ are essential for cell proliferation (Paramashivam et al., 2015). Of the anticancer compounds, none were active at this target. However, M6, M7, and M10 are the best antimicrobial derivatives that could serve as ion channel modulators. Moreover, only compound I7 showed considerable ion channel activity.
Kinase inhibitors (KIs)
KI subfamilies facilitate signaling pathways and regulate the invasion, adhesion, and migration of particles and immune cells to cell membranes to reduce the risk of invading microbes (Cheng et al., 2017). Moreover, the protein kinase raises proinflammatory cytokine expression, which causes inflammation. Thus, compounds with a small MW act as KIs by targeting kinase and downregulating its gene level or blocking (adenosine triphosphate)-kinase binding to reduce inflammation (Negi et al., 2021; Patterson et al., 2014). For the anticancer compounds, C8 exhibited remarkable cytotoxic activity against the liver cell line (IC50 2.91 for HepG2) and has been reported to inhibit VEGF-2, which is one of the tyrosine kinases enzymes (Abdelhameid et al., 2020). The antimicrobial compounds that revealed high KI activity are M5, M6, M7, and M10, while anti-inflammatory derivatives, compounds I5 and I8, demonstrated positive values as KIs.
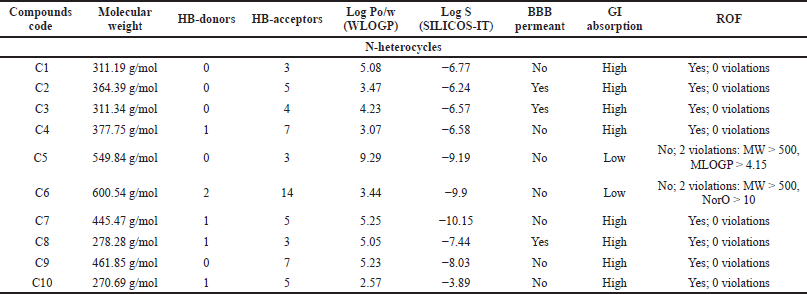 | Table 4. The pharmacokinetics ADME properties of N-heterocycles with anticancer activity. [Click here to view] |
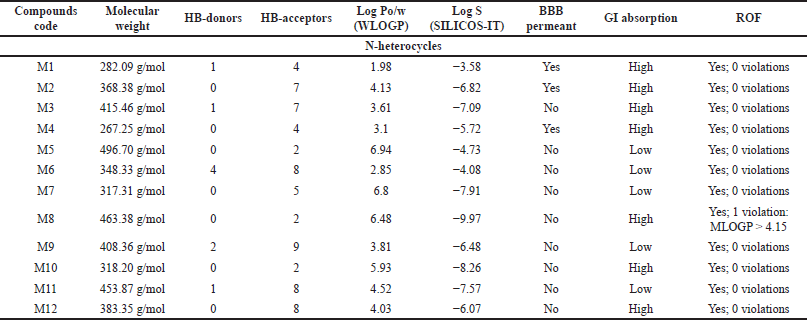 | Table 5. The pharmacokinetics ADME properties of N-heterocycles with antimicrobial activity. [Click here to view] |
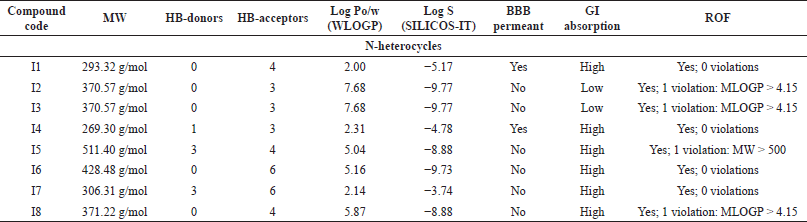 | Table 6. The pharmacokinetics ADME properties of N-heterocycles with anti-inflammatory activity. [Click here to view] |
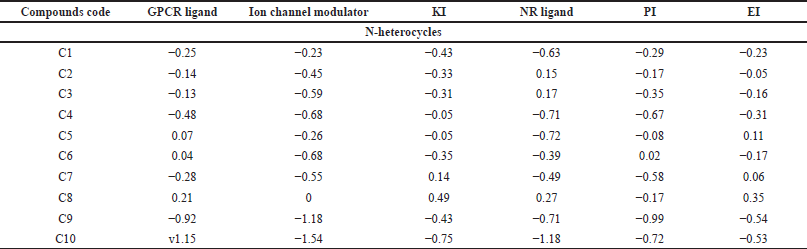 | Table 7. The target predictions of N-heterocycles with anticancer activity. [Click here to view] |
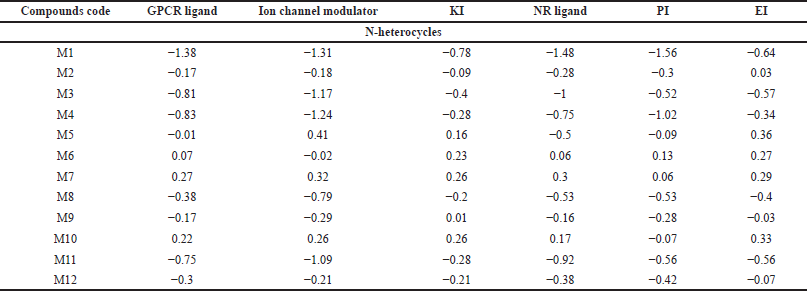 | Table 8. The target predictions of N-heterocycles with antimicrobial activity. [Click here to view] |
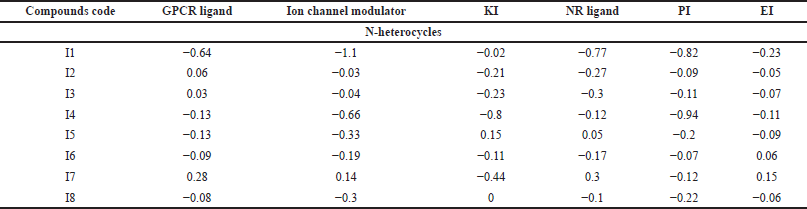 | Table 9. Target predictions of N-heterocycles with anti-inflammatory properties. [Click here to view] |
Nuclear receptor (NR) ligands
The NR is one of the major receptor groups because it covers both intra- and extracellular signals to regulate biological functions such as enhancement of macrophage survival by inhibiting bacterial-induced apoptosis decreasing proinflammatory cytokines and chemokines production and reducing inflammation. Moreover, agonists and antagonists of several NRs are commonly utilized as anticancer agents (Bourguet et al., 2000; Leopold Wager et al., 2019). As shown in Table 1, C2, C3, and C8 exhibited moderate activity with values equal to 0.15, 0.17, and 0.27, respectively. Compounds M7 and M10 demonstrated the highest activity, equal to 0.30 and 0.17, respectively, among the antimicrobial derivatives. Moreover, for the anti-inflammatory I7, the highest value as a NR ligand was 0.30.
Protease inhibitors (PIs)
In Europe, proteasome inhibitors are the backbone of one of the hematologic cancers, multiple myeloma. Because of the proteasome inhibitor resistance, multiple patients were left without active therapy (Driessen et al., 2016; Toscani et al., 2021). Moreover, PIs act against infections by reducing the presence of amino acids inside microorganisms. PIs make channels in the cell membrane and take out cellular content, which results in cell death (Shamsi and Fatima, 2016). Furthermore, they reduce inflammation by inhibiting proinflammatory cytokine production (Fei et al., 2017). C6, which showed activity on leukemia and lymphoblastoma (see Table 1), exhibited positive bioactivity as a PI. While compound M6 shows promising results in inhibiting the protease enzyme, compound M7 follows suit. In contrast, none of the anti-inflammatory derivatives demonstrated a positive bioactivity score as a PI.
Enzyme inhibitors (EIs)
Topoisomerase II, which is one of the important enzymes that regulate DNA replication, is a reported target for C4 and C5 (Boulos et al., 2011). Surprisingly, these two compounds had predicted values of −0.05 and −0.16, respectively, as EIs. Moreover, compounds C5, C7, and C8 exhibited positive values, suggesting enzymes are potential targets for these derivatives. Furthermore, bacteria produce certain enzymes as one way of resisting mechanisms, so targeting enzymes might be a promising approach to overcome bacterial drug resistance. As shown in Table 8, compound M5 (0.36) had the highest value, followed by M10 (0.33), M7 (0.29), and M6 (0.27). Moreover, COX enzyme inhibition is a crucial mechanism of anti-inflammatory compounds. In Table 9, half of the studied compounds were reported to have activity on COX-1 and/or COX-2. Interestingly, I6 and I7 have not been reported in their original studies as COX-1 and/or COX-2 inhibitors (Husain and Priyanka Ahuja, 2009; Zheng et al., 2015). However, both reveal the highest activity, equal to 0.06 and 0.15, respectively.
CONCLUSION AND FUTURE DIRECTION
The discussed studies examined the anticancer activity of a variety of N-heterocyclic compounds, which showed encouraging results against breast, liver, prostate, ovarian, and colorectal cell lines. Most of the studies focused on specific cancer cell lines, and further research could be performed to examine the cytotoxic activity against lung cancer and other types of cell lines that were not covered here. In addition to the fact that most of the studies assessed the efficacy in vitro without further confirmation of efficacy using in vivo models, only two studies investigated the cytotoxic activity against hematological malignancies (melanoma and leukemia).
Moreover, the reported studies demonstrated significant antimicrobial activity. However, the lack of certain important information, such as the mechanism of action, may affect the prediction of the microbe’s mechanism of resistance, thus affecting the efficacy of the developed agents. Future studies could be performed on the mechanism of action of the N-heterocycles as antimicrobial agents. Furthermore, several studies confirmed the possibility of N-heterocycle-containing compounds to attenuate inflammation with a similar mechanism to NSAIDs via COX enzyme inhibition and with lower toxicity (Bezerra et al., 2005; Gökhan-Kelekçi et al., 2009; Mohamed et al., 2017). The discussed studies suggest that the N-heterocycle-derived compounds possess promising medicinal properties that could be utilized for future drug discovery and the development of novel drug entities.
ACKNOWLEDGMENTS
The authors acknowledge financial support from the King Abdullah International Medical Research Center (KAIMRC), Ministry of National Guard Health Affairs, Riyadh, Kingdom of Saudi Arabia. Grant # (SP20.444.R). The authors want to express their sincerest gratitude to the College of Pharmacy (COP) at King Saud bin Abdulaziz University for Health Sciences (KSAU-HS) for their continued support.
List of Abbreviations
AMR: Antimicrobial resistance, BBB: Blood–brain barrier, C1: Compound 1 with anticancer activity, C2: Compound 2 with anticancer activity, C3: Compound 3 with anticancer activity, C4: Compound 4 with anticancer activity, C5: Compound 5 with anticancer activity, C6: Compound 6 with anticancer activity, C7: Compound 7 with anticancer activity, C8: Compound 8 with anticancer activity, C9: Compound 9 with anticancer activity, C10: Compound 10 with anticancer activity, COX: Cyclooxygenase, DNA: Deoxyribonucleic acid, EAC: Ehrlich ascites carcinoma, EC50: Dose (or concentration) causing 50% of maximum effect for any measured biological effect of interest, FDA: Food and Drug Administration, GI50: Concentration of drug causing 50% inhibition of cell growth, GPCR: G protein-coupled receptor, HB-acceptor: Hydrogen bond-acceptor, HB-donor: Hydrogen bond-donor, I1: Compound 1 with anti-inflammatory activity, I2: Compound 2 with anti-inflammatory activity, I3: Compound 3 with anti-inflammatory activity, I4: Compound 4 with anti-inflammatory activity, I5: Compound 5 with anti-inflammatory activity, I6: Compound 6 with anti-inflammatory activity, I7: Compound 7 with anti-inflammatory activity, I8: Compound 8 with anti-inflammatory activity, IC50: Compound concentration required to inhibit tumor cell proliferation by 50%, LC50: Concentration required to kill half the cells in the cell culture, LOX: Lipoxygenase, M1: Compound 1 with antimicrobial activity, M2: Compound 2 with antimicrobial activity, M3: Compound 3 with antimicrobial activity, M4: Compound 4 with antimicrobial activity, M5: Compound 5 with antimicrobial activity, M6: Compound 6 with antimicrobial activity, M7: Compound 7 with antimicrobial activity, M8: Compound 8 with antimicrobial activity, M9: Compound 9 with antimicrobial activity, M10: Compound 10 with antimicrobial activity, M11: Compound 11 with antimicrobial activity, M12: Compound 12 with antimicrobial activity, MIC: Minimum inhibitory concentration, NSAIDs: Nonsteroidal anti-inflammatory drugs, RNA: Ribonucleic acid, ROF: Rule of five, % TWI: Percentage of tumor weight inhibition, % TCI: Percentage of tumor cell count inhibition,VEGF-2: Vascular endothelial growth factor receptor-2.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher’s website along with the published article.
REFERENCES
Abd El-Hameed RH, Mahgoub S, El-Shanbaky HM, Mohamed MS, Ali SA. Utility of novel 2-furanones in synthesis of other heterocyclic compounds having anti-inflammatory activity with dual COX2/LOX inhibition. J Enzyme Inhib Med Chem, 2021; 36(1):977–86; doi:10.1080/14756366.2021.1908277 CrossRef
Abdelhameid MK, Zaki I, Mohammed MR, Mohamed KO. Design, synthesis, and cytotoxic screening of novel azole derivatives on hepatocellular carcinoma (HepG2 cells). Bioorg Chem, 2020; 101:103995; doi:10.1016/j.bioorg.2020.103995 CrossRef
Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell, 2006; 124(4):783–801; doi:10.1016/j.cell.2006.02.015 CrossRef
Alanis AJ. Resistance to antibiotics: are we in the post-antibiotic era? Arch Med Res, 2005; 36(6):697–705; doi:10.1016/j.arcmed.2005.06.009 CrossRef
Alfarouk KO, Stock CM, Taylor S, Walsh M, Muddathir AK, Verduzco D, Bashir AH, Mohammed OY, Elhassan GO, Harguindey S, Reshkin SJ. Resistance to cancer chemotherapy: failure in drug response from ADME to P-gp. Cancer Cell Int, 2015; 15(1):1–13; doi:10.1186/s12935-015-0221-1 CrossRef
Aller SG, Yu J, Ward A, Weng Y, Chittaboina S, Zhuo R, Harrell PM, Trinh YT, Zhang Q, Urbatsch IL, Chang G. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science, 2009; 323(5922):1718–22; doi:10.1126/science.1168750 CrossRef
American Cancer Society. Treatment types. 2021. Available via https://shortest.link/2nBw
Ames BN, Gold LS, Willett WC. The causes and prevention of cancer. Proc Natl Acad Sci USA, 1995; 92(12):5258–65; doi:10.1073/pnas.92.12.5258 CrossRef
Andrejevi? TP, War?ajtis B, Gliši? B?, Vojnovic S, Mojicevic M, Stevanovi? NL, Nikodinovic-Runic J, Rychlewska U, Djuran MI. Zinc(II) complexes with aromatic nitrogen-containing heterocycles as antifungal agents: synergistic activity with clinically used drug nystatin. J Inorg Biochem, 2020; 208:111089; doi:10.1016/j.jinorgbio.2020.111089 CrossRef
Arshad M. Synthesis, characterization, and antimicrobial assessment of some computationally bioactive 1, 2-oxazole derivatives. Russ J Gen Chem, 2018; 88(9):1886–91. CrossRef
Barnes PJ. How corticosteroids control inflammation: quintiles prize lecture 2005. Br J Pharmacol, 2006; 148(3):245–54; doi:10.1038/sj.bjp.0706736 CrossRef
Bezerra NM, De Oliveira SP, Srivastava RM, Da Silva JR. Synthesis of 3-aryl-5-decapentyl-1, 2, 4-oxadiazoles possessing antiinflammatory and antitumor properties. Il Farmaco, 2005; 60(11–12):955–60; doi:10.1016/j.farmac.2005.08.003 CrossRef
Bonanno L, Favaretto A, Rosell R. Platinum drugs and DNA repair mechanisms in lung cancer. Anticancer Res, 2014; 34(1):493–501.
Boulos LS, Ewies EF, Fahmy AF. Synthesis of new bisphosphonate and bisphosphonic acid derivatives and heterocyclic and dialkylcarbamoyl oxazolone derivatives with anticancer and antischistosomal activity. Z Naturforsch B, 2011; 66(10):1056–68. CrossRef
Bourguet W, Germain P, Gronemeyer H. Nuclear receptor ligand-binding domains: three-dimensional structures, molecular interactions and pharmacological implications. Trends Pharmacol Sci, 2000; 21(10):381–8; doi:10.1016/S0165-6147(00)01548-0 CrossRef
Capita R, Alonso-Calleja C. Antibiotic-resistant bacteria: a challenge for the food industry. Crit Rev Food Sci Nutr, 2013; 53(1):11–48; doi:10.1080/10408398.2010.519837 CrossRef
Centers for disease control and prevention. Antibiotic/antimicrobial resistance (AR/AMR). 2020. Available via https://shortest.link/2usm
Cheng Y, Schorey JS, Zhang CC, Tan X. Protein kinase inhibitors as potential antimicrobial drugs against tuberculosis, malaria and HIV. Curr Pharm Design, 2017; 23(29):4369–89; doi:10.2174/1381612823666170612122429 CrossRef
Crofford LJ. Use of NSAIDs in treating patients with arthritis. Arthritis Res Ther, 2013; 15(3):1–10; doi:10.1186/ar4174 CrossRef
Cunha BA, Ortega AM. Antibiotic failure. Med Clin North Am, 1995; 79(3):663–72; doi:10.1016/s0025-7125(16)30062-1 CrossRef
Daina A, Michielin O, Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep, 2017; 7(1):42717; doi:10.1038/srep42717 CrossRef
Dasari S, Bernard Tchounwou P. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol, 2014; 740:364–78; doi:10.1016/j.ejphar.2014.07.025 CrossRef
Desai PV, Raub TJ, Blanco MJ. How hydrogen bonds impact P-glycoprotein transport and permeability. Bioorg Med Chem Lett, 2012; 22(21):6540–8; doi:10.1016/j.bmcl.2012.08.059 CrossRef
Di L, Kerns EH. Preface. Drug-like properties: concepts, structure design and methods. Academic Press, San Diego, CA, pp xviii–xix, 2008. CrossRef
Driessen C, Kraus M, Joerger M, Rosing H, Bader J, Hitz F, Berset C, Xyrafas A, Hawle H, Berthod G, Overkleeft HS, Sessa C, Huitema A, Pabst T, von Moos R, Hess D, Mey UJ. Treatment with the HIV protease inhibitor nelfinavir triggers the unfolded protein response and may overcome proteasome inhibitor resistance of multiple myeloma in combination with bortezomib: a phase I trial (SAKK 65/08). Haematologica, 2016; 101(3):346–55; doi:10.3324/haematol.2015.135780 CrossRef
Dudas J, Ladanyi A, Ingruber J, Steinbichler TB, Riechelmann H. Epithelial to mesenchymal transition: a mechanism that fuels cancer radio/chemoresistance. Cells, 2020; 9(2):428; doi:10.3390/cells9020428 CrossRef
El Bourakadi K, Mekhzoum ME, Saby C, Morjani H, Chakchak H, Merghoub N, Bouhfid R. Synthesis, characterization and in vitro anticancer activity of thiabendazole-derived 1,2,3-triazole derivatives. N J Chem, 2020; 44(28):12099–106; doi:10.1039/C9NJ05685H CrossRef
Fadda AA, Mohammed RM, Tawfik EH, Hammouda MA. Synthesis and anticancer activity of new 2-aryl-4-(4-methoxybenzylidene)-5-oxazolone scaffolds. Bionterface Res Appl Chem, 2020; 11:8096–109. CrossRef
Fei R, Zhang H, Zhong S, Xue B, Gao Y, Zhou X. Anti-inflammatory activity of a thermophilic serine protease inhibitor from extremophile Pyrobaculum neutrophilum. Eur J Inflammation, 2017; 15(3):143–51. CrossRef
Gadhe DCN, Gudipati R, Ampati S, Manda S. Synthesis of some novel methyl2 (2 (arylideneamino) oxazol4 ylamino) benzoxazole5carboxylate derivatives as antimicrobial agents. Int J Chem, 2010; 1(2):1–6.
Gatti L, Zunino F. Overview of tumor cell chemoresistance mechanisms. Methods Mol Med, 2005; 111:127–48; doi:10.1385/1-59259-889-7:127 CrossRef
Gleeson MP. Generation of a set of simple, interpretable ADMET rules of thumb. J Med Chem, 2008; 51(4):817–34; doi:10.1021/jm701122q CrossRef
Gökhan-Kelekçi N, Köksal M, Ünüvar S, Aktay G, Erdo?an H. Synthesis and characterization of some new 2 (3H)-benzoxazolones with analgesic and antiinflammatory activities. J Enzyme Inhib Med Chem, 2009; 24(1):29–37. CrossRef
Grutters J, Van den Bosch J. Corticosteroid treatment in sarcoidosis. Eur Respir J, 2006; 28(3):627–36; doi:10.1183/09031936.06.00105805 CrossRef
Housman G, Byler S, Heerboth S, Lapinska K, Longacre M, Snyder N, Sarkar S. Drug resistance in cancer: an overview. Cancers, 2014; 6(3):1769–92; doi:10.3390/cancers6031769 CrossRef
Husain A, Priyanka Ahuja S. Synthesis and biological evaluation of β-aroylpropionic acid based 1, 3, 4-oxadiazoles. Indian J Pharm Sci, 2009; 71(1):62; doi:10.4103/0250-474X.51963 CrossRef
Janeway Jr CA, Medzhitov R. Innate immune recognition. Ann Rev Immunol, 2002; 20(1):197–216; doi:10.1146/annurev.immunol.20.083001.084359 CrossRef
Kemnitzer W, Kuemmerle J, Zhang HZ, Kasibhatla S, Tseng B, Drewe J, Cai SX. Discovery of 3-aryl-5-aryl-1, 2, 4-oxadiazoles as a new series of apoptosis inducers. 2. Identification of more aqueous soluble analogs as potential anticancer agents. Bioorg Med Chem Lett, 2009; 19(15):4410–5; doi:10.1016/j.bmcl.2009.05.052 CrossRef
Khalil I, Rønn AM, Alifrangis M, Gabar HA, Satti GM, Bygbjerg IC. Dihydrofolate reductase and dihydropteroate synthase genotypes associated with in vitro resistance of Plasmodium falciparum to pyrimethamine, trimethoprim, sulfadoxine, and sulfamethoxazole. Am J Trop Med Hyg, 2003; 68(5):586–9; doi:10.4269/ajtmh.2003.68.586 CrossRef
Kumar D, Kumar NM, Sundaree S, Johnson EO, Shah K. An expeditious synthesis and anticancer activity of novel 4-(3′-indolyl) oxazoles. Eur J Med Chem, 2010; 45(3):1244–9; doi:10.1016/j.ejmech.2009 CrossRef
Larson CP, Saha UR, Nazrul H. Impact monitoring of the national scale up of zinc treatment for childhood diarrhea in Bangladesh: repeat ecologic surveys. PLoS Med, 2009; 6(11):e1000175; doi:10.1371/journal.pmed.1000175 CrossRef
Leopold Wager CM, Arnett E, Schlesinger LS. Macrophage nuclear receptors: emerging key players in infectious diseases. PLoS Pathog, 2019; 15(3):e1007585; doi:10.1371/journal.ppat.1007585 CrossRef
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev, 2001; 46(1–3):3–26; doi:10.1016/s0169-409x(00)00129-0 CrossRef
Mathew ST, Devi SG, Prasanth V, Vinod B. Efficacy and safety of COX-2 inhibitors in the clinical management of arthritis: mini review. ISRN Pharmacol, 2011; 2011; doi:10.5402/2011/480291 CrossRef
Minhas GS, Pilch DS, Kerrigan JE, LaVoie EJ, Rice JE. Synthesis and G-quadruplex stabilizing properties of a series of oxazole-containing macrocycles. Bioorg Med Chem Lett, 2006; 16(15):3891–5; doi:10.1016/j.bmcl.2006.05.038 CrossRef
Mohamed LW, El-Badry OM, El-Ansary AK, Ismael A. Design synthesis of novel oxazolone triazinone derivatives and their biological evaluation as COX-2 inhibitors. Bioorg Chem, 2017; 72:308–14. CrossRef
Molinspiration software. Free web tools for cheminformatics community. Available via https://www.molinspiration.com/
Naganagowda G, Thamyongkit P, Petsom A. Synthesis and antimicrobial activity of oxazolone, imidazolone and triazine derivatives containing benzothiophene. J Korean Chem Soc, 2011; 55(5):794–804. CrossRef
Narayana B, Vijaya Raj KK, Ashalatha BV, Kumari NS. Synthesis of some new 2-(6-methoxy-2-naphthyl)-5-aryl-1, 3, 4-oxadiazoles as possible non-steroidal anti-inflammatory and analgesic agents. Arch Pharm (Weinheim), 2005; 338(8):373–7; doi:10.1002/ardp.200500974 CrossRef
National Library of Medicine. PubChem [Internet]. National Center for Biotechnology Information, Bethesda, MD, 2004.
Negi P, Cheke RS, Patil VM. Recent advances in pharmacological diversification of Src family kinase inhibitors. Egypt J Med Human Genet, 2021; 22(1):52; doi:10.1186/s43042-021-00172-x CrossRef
Olomola T, Akinboye A, Olasunkanmi O, Olasunkanmi L. Synthesis, antimicrobial activities and computational studies of some oxazolone derivatives. IJS, 2018; 20(1):1–14; doi:10.4314/ijs.v20i1.1 CrossRef
Pahwa RGA, Bansal P, Jialal I. Chronic inflammation. 2020. Available via https://www.ncbi.nlm.nih.gov/books/NBK493173/#article-19530.r5
Paramashivam SK, Elayaperumal K, Bhagavan Natarajan B, Devi Ramamoorthy M, Balasubramanian S, Dhiraviam KN. In silico pharmacokinetic and molecular docking studies of small molecules derived from Indigofera aspalathoides Vahl targeting receptor tyrosine kinases. Bioinformation, 2015; 11(2):73; doi:10.6026/97320630011073 CrossRef
Pasha M, Jayashankara V, Venugopala K, Rao GK. Zinc oxide (ZnO): an efficient catalyst for the synthesis of 4- arylmethylidene- 2- phenyl 5 (4H)-oxazolones having antimicrobial activity. J Toxicol Pharmacol, 2007; 2(3):264–70; doi:10.3923/jpt.2007.264.270 CrossRef
Patterson H, Nibbs R, McInnes I, Siebert S. Protein kinase inhibitors in the treatment of inflammatory and autoimmune diseases. Clin Exp Immunol, 2014; 176(1):1–10; doi:10.1111/cei.12248 CrossRef
Peklak-Scott C, Smitherman PK, Townsend AJ, Morrow CS. Role of glutathione S-transferase P1-1 in the cellular detoxification of cisplatin. Mol Cancer Ther, 2008; 7(10):3247–55; doi:10.1158/1535-7163 CrossRef
Pons-Cursach R, Casanovas O. Mechanisms of anti-angiogenic therapy. In: Marmé D (ed.). Tumor angiogenesis: a key target for cancer therapy, Springer International Publishing, Cham, Switzerland, pp 183–208, 2019. CrossRef
Ramos P, Bentires-Alj M. Mechanism-based cancer therapy: resistance to therapy, therapy for resistance. Oncogene, 2015; 34(28):3617–26; doi:10.1038/onc.2014.314 CrossRef
Rawat BS, Shukla SK. Synthesis and evaluation of some new thiazole/oxazole derivatives for their biological activities. World J Pharm Pharm Sci, 2016; 5(8):1473–82.
Review on Antimicrobial Resistance. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. Review on Antimicrobial Resistance, London, UK, 2014.
Rubartelli A, Lotze MT. Inside, outside, upside down: damage-associated molecular-pattern molecules (DAMPs) and redox. Trends Immunol, 2007; 28(10):429–36; doi:10.1016/j.it.2007.08.004 CrossRef
Savariz FC, Foglio MA, De Carvalho JE, Ruiz AL, Duarte MC, Da Rosa MF, Meyer E, Sarragiotto MH. Synthesis and evaluation of new β-carboline-3-(4-benzylidene)-4H-oxazol-5-one derivatives as antitumor agents. Molecules, 2012; 17(5):6100–13; doi:10.3390/molecules17056100 CrossRef
Sengupta P, Dash DK, Yeligar VC. Evaluation of anticancer activity of some 1, 3, 4-oxadiazole derivatives. Indian J Chem Section B, 2008;47: 460–2.
Septimus EJ. Antimicrobial resistance: an antimicrobial/diagnostic stewardship and infection prevention approach. Med Clin North Am, 2018; 102(5):819–29; doi:10.1016/j.mcna.2018.04.005 CrossRef
Shamsi TN, Fatima S. Protease inhibitors as ad-hoc antibiotics. Open Pharm Sci J, 2016; 3(1); doi:10.2174/1874844901603010131 CrossRef
Shukla MB, Mahyavanshi JB, Parmar KA. Synthesis and antimicrobial activities of various N-phenyl-2-{[5-(3, 4, 5-trimethoxyphenyl)-1, 3, 4-oxadiazol-2-yl] sulfanyl} acetamides. Indian J Chem Section B, 2016; 55:374–80.
Silver LL. Challenges of antibacterial discovery. Clin Microbiol Rev, 2011; 24(1):71–109; doi:10.1128/CMR.00030-10 CrossRef
Singh RK, Bhatt A, Chauhan PK, Kant R. Design, synthesis and biological evaluation of some novel biphenyl substituted oxazole derivatives. Chem Biol Interface, 2017; 7(1):32–9.
Standing up to infectious disease. Nat Microbiol, 2019; 4(1):1; doi:10.1038/s41564-018-0331-3. CrossRef
Stojan G, Petri M. Atherosclerosis in systemic lupus erythematosus. J Cardiovasc Pharmacol, 2013; 62(3):255; doi:10.1097/FJC.0b013e31829dd857 CrossRef
Stone WL, Basit H, Burns B. Pathology, inflammation. StatPearls Publishing, Treasure Island, FL, 2021.
Sun L, Richard DY. Role of G protein-coupled receptors in inflammation. Acta Pharmacol Sin, 2012; 33(3):342–50; doi:10.1038/aps.2011.200 CrossRef
Toscani D, Craviotto L, Giuliani N. The role of proteasome inhibitors in multiple myeloma bone disease and bone metastasis: effects on osteoblasts and osteocytes. Appl Sci, 2021; 11(10):4642. CrossRef
Trzaskowski B, Latek D, Yuan S, Ghoshdastider U, Debinski A, Filipek S. Action of molecular switches in GPCRs—theoretical and experimental studies. Curr Med Chem, 2012; 19(8):1090–109; doi:10.2174/092986712799320556 CrossRef
Vo CV, Bode JW. Synthesis of saturated N-heterocycles. J Org Chem, 2014; 79(7):2809–15; doi:10.1021/jo5001252 CrossRef
WHO. WHO reveals leading causes of death and disability worldwide: 2000–2019. 2020. Available via https://bit.ly/2Tn1oCz
Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami J. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis, 2018; 9(1):143–50; doi:10.14336/ad.2017.0306 CrossRef
World Cancer Research Fund International. Global cancer statistics for the most common cancers. 2018. Available via https://bit.ly/3w7YmQ2
World Health Organization. Infectious diseases kill over 17 million people a year: WHO warns of global crisis. 1996. Available via https://bit.ly/3612sia
World Health Organization. Global status report on noncommunicable diseases 2010. 2011. Available via https://www.who.int/nmh/publications/ncd_report_full_en.pdf
World Health Organization. Cancer today. 2020. Available via https://gco.iarc.fr/today/home
Zheng G, Chen T, Peng X, Long S. Synthesis, anti-inflammatory, and analgesic activities of derivatives of 4-Hydroxy-2-benzoxazolone. J Chem, 2015; 2015:1–5. CrossRef
SUPPLEMENTARY FILE
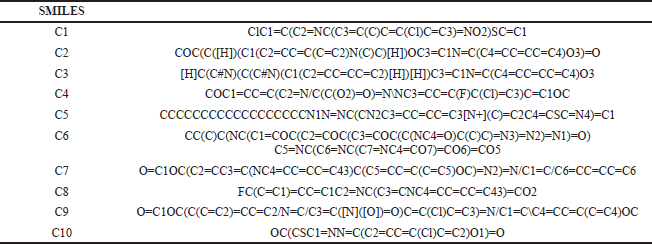 | T1S. SMILES of the compounds with anticancer activity. [Click here to view] |
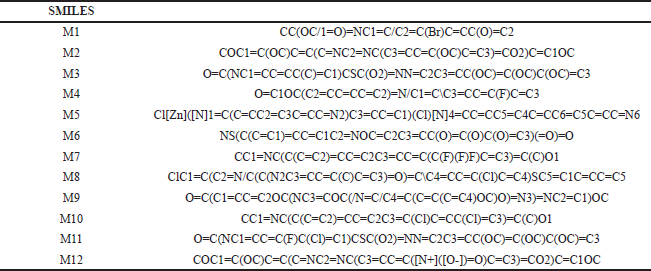 | T2S. SMILES of the compounds with antimicrobial activity. [Click here to view] |
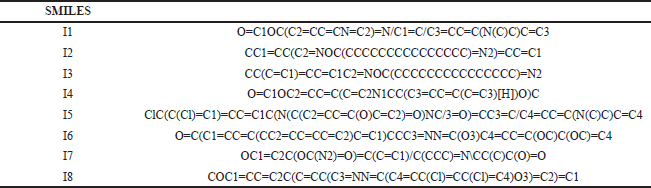 | T3S. SMILES of the compounds with anti-inflammatory activity. [Click here to view] |