INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is described as excessive fat accumulation in the liver cell without alcohol intake. It can also be caused due to viral hepatitis or drug-induced autoimmune disease (Marcuccilli and Chonchol, 2016). These secondary causes include medication side effects, specific endocrine conditions, and hepatitis C virus infection. The severity of the disease ranges from mild steatosis to non-alcoholic steatohepatitis (NASH), which can further progress to cirrhosis and hepatocellular carcinoma (HCC) (Lau and Wong, 2018). NAFLD can also be called metabolic-associated fatty liver disorder due to its close association with obese, type 2 diabetes mellitus (T2DM) reported as “Metabolic Syndrome” (MetS). The absence of appropriate and sensitive non-invasive testing for NAFLD makes accurate diagnosis difficult. When the level of a liver enzyme rises in overweight or obese people with no identified cause of liver disease, or when imaging studies suggest hepatic steatosis, it is commonly diagnosed on a presumptive basis (Salt, 2004).
The prevalence of NAFLD globally is estimated to be 25% (Mundi et al., 2020). NAFLD patients mostly remain asymptomatic, and they are diagnosed at the fibrosis or cirrhosis stage. People with central obesity are more likely to have NAFLD, including insulin resistance (IR) or T2DM, hypertension, excessive abdominal fat, and dyslipidemia. This group of chronic diseases is associated with an increased risk of cardiovascular disease and is referred to as the “MetS.” Nearly 70% to 80% of patients have MetS or IR. Some patients experience vague right upper quadrant pain, high alanine aminotransferase (ALT), and hepatomegaly as risk factors for MetS (Hossain et al., 2016). It is also thought to be a hepatic symptom of MetS (Gottlieb and Canbay, 2019).
If untreated, NAFLD progresses to hepatic inflammation and early fibrosis, indicating the onset of NASH. End-stage NAFLD symptoms include liver inflammation, fibrosis, cirrhosis, and increased risk of HCC (Eng and Estall, 2021). The present review is carried out by compiling literature from 1990 to 2021 concerning the traditional uses, phytochemistry, pharmacological activities, and toxicological aspects of various herbal plants and their role in treating NAFLD. Pieces of literature were collected from multiple online search engines, viz. Google Scholar, PubMed, Semantic Scholar, and Science Direct. Keyword combinations undertaken for the searches were NAFLD, molecular mechanism or epidemiology, dietary factors, or herbal plants.
Pathogenesis of NAFLD
In white adipose tissue (WAT), impaired insulin signaling raises lipolysis that generates fatty acid (FA). The results in substrate overload and further increased hepatic IR, with enhanced de novo lipogenesis as well as triglycerides (TG) accumulation (Buzzetti et al., 2016). Around 60% of FA for hepatic TG accumulation in NAFLD patients is derived from WAT-generated FA. 15% obtained from the diet and 25% from increased de novo lipogenesis, which is controlled through carbohydrate response element-binding protein and peroxisome proliferator-activated receptor δ (PPAR δ) (Buzzetti et al., 2014).
According to the two-hit theory, NAFLD consists of two stages of liver injury, such as intrahepatic lipid build-up and inflammatory development to NASH. During the first hepatic theory, fructose metabolism elevates intrahepatic lipid and de novo lipogenesis which inhibits mitochondrial β-oxidation of long-chain FAs. According to the second hit theory, fructose generates reactive oxygen species (ROS) that must be quenched by liver anti-oxidants and promotes protein fructosylation due to its five-membered furanose ring molecular instability. Many persons with NASH have vitamin deficiencies and a lack of anti-oxidant capacity to inhibit the formation of ROS, resulting in necroinflammation shown in Figure 1 (Lim et al., 2010).
HERBAL PLANTS IN NAFLD
Plants with active ingredients, pharmacological and toxicological effects, and their involvement in reducing the etiology of fatty liver disease are represented in Tables 1 and 3.
Phyllanthus niruri Linn.
Phyllanthus niruri is a herbal plant inhabited in Southeast Asian countries of the family Phyllanthaceae. It can treat numerous liver disorders, especially hepatitis and jaundice. Current studies show that it exhibits hepatoprotective properties on hepatitis-induced rats and is abundant in phenolic compounds and flavonoids, which is essential for a substantial anti-oxidant property. It will lower fat accumulation in the liver by lowering serum FA, decreasing IR, inhibiting α-glucosidase, cholesterol micellization, and pancreatic lipase that leads to a low amount of free fatty acid (FFA) and glucose which results in a de novo lipogenesis process with less fat accumulation in the liver. It minimizes hepatic fibrosis by reducing Malondialdehyde which is responsible for stimulating the hepatic stellate cell (Zarzour et al., 2017).
Phyllanthus niruri reveals its antiangiogenic effect against NAFLD. Additionally, it regulates adipocytokine secretion by improving insulin signaling inside the liver, which leads to lowering of fat accumulation as well as inflammation and down-regulation of [solute carrier family 10 (sodium/bile acid cotransporter) member 2] (SLC10A2), PPARγ and collagen alpha type I genes (Zarzour et al., 2018).
Phyllanthus emblica Linn.
The fruit of P. emblica, a member of the Phyllanthaceae family shows a variety of biological activities such as anti-inflammatory, anti-oxidative, anti-microbial, hypolipidemic, and hepatoprotective activities. It is also observed that the fruit exhibits a hepatoprotective effect exerted by ellagic acid, flavonoids, gallic acid, vitamin C, and it could be linked to antioxidant and anti-inflammatory activities (Kirschweng et al., 2018). Some observation shows that water extract of P. emblica (WEPE) minimizes adipose tissue weights of peritoneal and epididymal fat. This helps ameliorate steatosis in the liver of high-fat diet (HFD) induced rats, increase anti-oxidant enzyme activity and deplete lipid peroxidation. Thus, WEPE possibly shortens the further formation of NASH (Tung et al., 2018).
WEPE helps ameliorate steatosis in the liver of HFD induced rats by increasing PPAR-α in the liver. It is also observed that in the liver tissues, the WEPE fruit decreases the messenger ribonucleic acid (mRNA) of sterol regulatory element-binding protein -1c (SREBP-1c), and in peritoneal fat pads of HFD-induced rats, it enhances the mRNA of adiponectin (Huang et al., 2017).
Phyllanthus urinaria Linn.
Phyllanthus urinaria, a member of the Phyllanthaceae family is found to have minimized necroinflammation and hepatic steatosis but has never been tested in NASH patients. In NASH patients, it is better to enhance liver histology compared with placebo. It is also found to alleviate oxidative stress and liver fat in NASH patients. It also suppresses c-Jun N-terminal kinase (JNK), cytochrome P450-2E1 (CYP2E1), interleukin-6 (IL-6), nuclear factor-kappa B (NFκB), hepatic lipid peroxides, tumor necrosis factor-α (TNF-α). It decreases the transcriptional activity of cytosine-cytosine-adenosine-adenosine-thymidine (CCAAT) and intensifies lipolytic CYP450 expression (Wong et al., 2013).
Allium sativum Linn.
Garlic is utilized as a medicinal plant in many cultures. It is a member of the Amaryllidaceae family and contains a variety of bioactive chemicals, including diallyl disulfide, S-allyl cysteine, S-methyl cysteine sulfoxide, allicin, ajoene, and SAC sulfoxide. It is also observed that regular garlic intake alleviates cancer and cardiovascular diseases. Several studies show that garlic consumption has a beneficial effect on obesity and IR, identified as a critical driver of NAFLD development. The impact of garlic on dyslipidemia (which is recognized as a significant risk factor of NAFLD) is due to increased adiponectin levels, reducing the enzyme activity involved in liver fat production, lowering intestinal absorption of TGs (Sangouni et al., 2020).
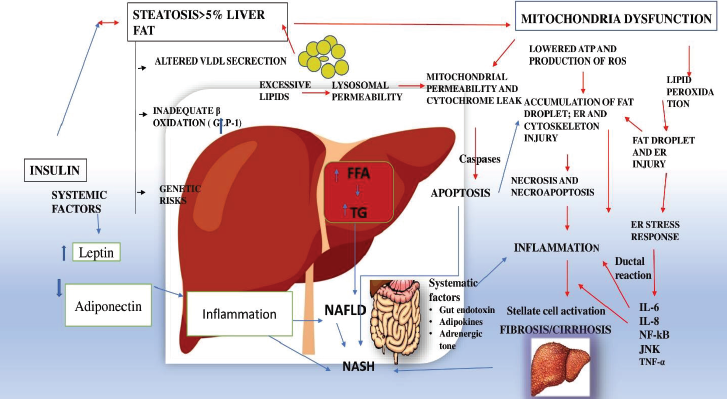 | Figure 1. The figure represents, various factors such as FFA, TG, altered VLDL, inadequate β-oxidation, IR, adiponectin, leptin that plays a pivotal role in the cause of non-alcoholic fatty liver disorder (NAFLD), NASH and fibrosis/cirrhosis. NFκB block apoptosis induced by TNF alpha and mediates with the JNK for the suppression of the cascade leading to inflammation. [Click here to view] |
Myrica rubra (Lour.) Siebold & Zucc
Bayberry is a subtropical tree found in Southeast Asian countries that belongs to the Myricaceae family. It contains a lot of anthocyanins and phenolic acids such as sinapic, ferulic, caffeic acid, and salicylic acid. Their study suggested that specific pathological conditions such as inflammation, oxidative stress, liver steatosis has improved in experimental NASH. Bayberries are high in polyphenols, which function as an anti-inflammatory and anti-oxidant. It is having the capacity to deplete apoptosis in young individuals, lowers plasma biomarkers, and is characterized by oxidative stress and reduced inflammation. Thus, bayberry can prevent and treat the complications formed at the initial stage of NAFLD (Guo et al., 2014).
Curcuma longa Linn.
Curcuma longa, also known as turmeric, is a perennial herb in the family of Zingiberaceae. In Asian cuisine, turmeric is widely used as curry powder and traditionally used as a home remedy for various ailments. Curcumin exhibits a polyphenol structure and is used as renoprotective, anti-oxidant, anti-cancer, and immunomodulatory. It inhibits the synthesis of fatty liver and the development of some unsaturated fatty liver acids such as oleic acid, stearic acid, and linoleic acid; this might help ameliorate hepatic steatosis and inhabits FAs liver disease development. It also enhances oxidative stress levels and helps prevent NAFLD by reducing ROS production and preventing liver damage in steatohepatitis through decreasing cytosolic and nuclear translocation of high mobility group box 1 and NFκB as well as inducing PPAR-γ (Mansour-Ghanaei et al., 2019).
Curcumin also exhibits anti-neoplastic, anti-inflammatory, and anti-bacterial properties (Ghosh, 2019). According to the findings of their study, short-term curcumin supplementation enhances hepatic transaminase levels in NAFLD patients (Panahi et al., 2017). In NASH mice, it prevents the O-GlcNAcylation pathway, which leads to anti-oxidant responses (Lee et al., 2019). Intake of this supplementation improves the glycemic and lipid index (Rahmani et al., 2016).
It is observed that curcumin can treat NAFLD mice induced with a high-fat high fructose diet by enhancing the metabolism of bile acids which is associated with nuclear erythroid 2-related factor 2 (Nrf2)/farnesoid X receptor (FXR)/Liver X receptor alpha pathway regulation and by preventing hepatic lipogenesis. It effectively restores the metabolic capability of the fatty liver by reversing the expression of CYP7A and CYP3A (Yan et al., 2018a, 2018b).
Silybum marianum (Linn.) Gaertn
Silymarin is a milk thistle plant extract from the Asteraceaea family that has been used for centuries as a traditional herbal remedy for liver ailments. It contains isosilybin A and B, silydianin, silybins A and B, silychristin, and polyphenolic compounds, as well as six important flavonolignans. It also possesses anti-fibrotic, anti-inflammatory, and anti-oxidant properties (Kheong et al., 2017). It is found in Asia, Southern Europe, North and South America, North Africa, and South Australia (Camini and Costa, 2020).
The major biologically active component of S. marianum is silybin. It is not dose-dependent and can be used to treat NAFLD patients, notably NASH, compared to hepatoprotective drugs and antimetabolic disorders agents (Zhong et al., 2017). The mechanism of action of silybin is followed by its act of interaction with various tissues. Silybin reacts through deactivating pro-nflammatory signals, which are acquired from activation of NFκB that is involved in the induction of the synthesis of the cytokineslike IL-1, IL-6, granulocyte-macrophage colony-stimulating factor, TNF-α (Federico et al., 2017).
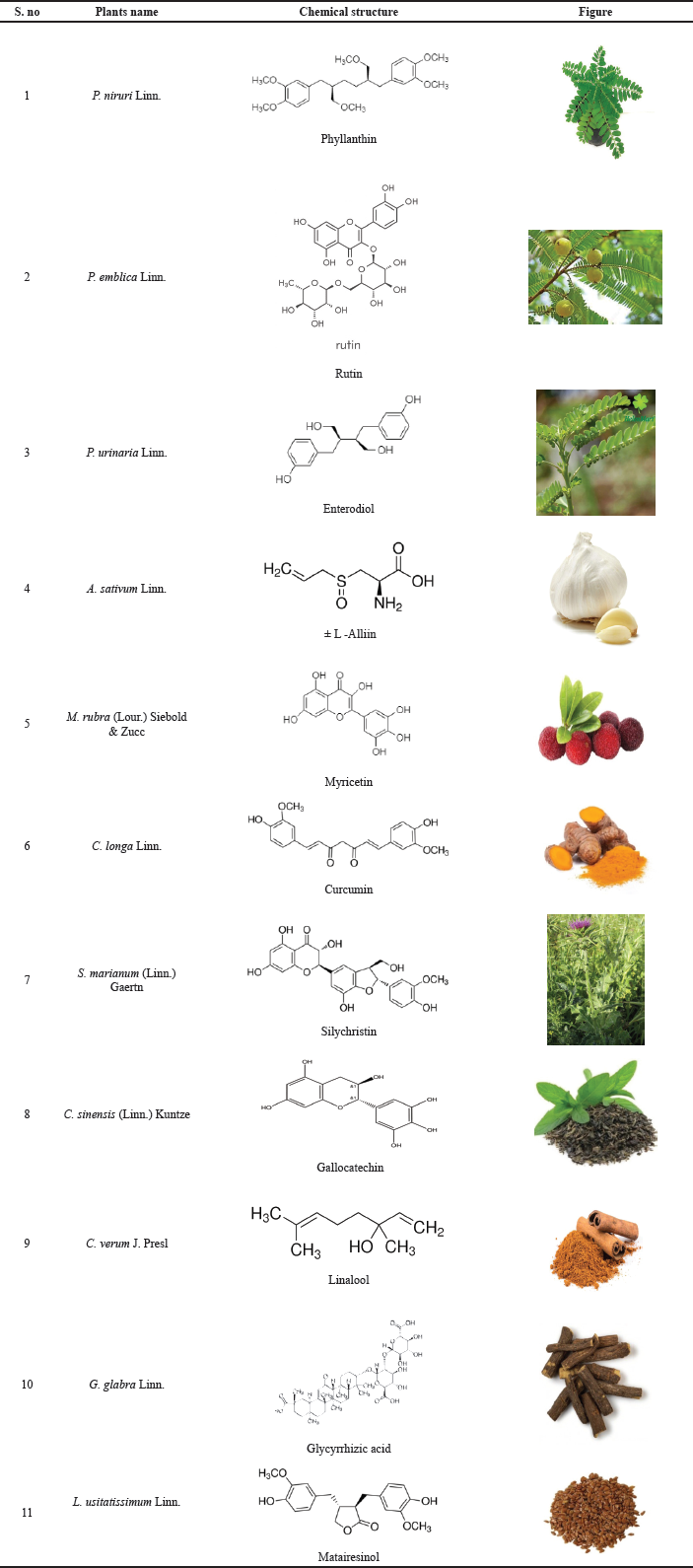 | Table 1. Herbal plants and their active constituent. [Click here to view] |
Silymarin inhibits free radical injury and lipid peroxidation. It is also found that silymarin can block the release of TNF-α by removing hydroxyl radicals and maintaining the average superoxide dismutase level (Navarro et al., 2019).
It is also observed that silymarin is found to shrink inflammation, and treatment can minimize IR and deplete fasting insulin levels. In NAFLD patients, silybum + phospholipids + vitamin E complex is located to enhance liver enzymes plasma level, improve echography score of liver steatosis. NASH is associated with mitochondrial dysfunction. During the process of NASH, the excess of FFA intensifies in hydrogen peroxide (H2O2) mitochondrial production, which in return oxidize mitochondrial membranes and maintain the activity of uncoupling protein-2 and carnitine palmitoyl transferase-1 (Abenavoli and Bellentani, 2013). Some studies found that milk thistle extract exhibits a reducing trend in NASH and numerical reduction in the steatosis score when differentiated with the vehicle group (Pais and Amato, 2014).
Camellia sinensis (Linn.) Kuntze
Green tea (GT), a member of the Theaceae family, is the most commonly found in East Asia. It is derived from the leaves of C. sinensis, which are high in polyphenolic components such epigallocatechin gallate, catechin, and epigallocatechin 3 gallate (EGCG). It is used as a traditional medicine in diabetes, obesity, and cardiovascular diseases. It is also observed that catechins decline lipid peroxidation levels, oxidative stress (Mansour-Ghanaei et al., 2018).
GT minimizes the cholesterol level in the liver by lowering the fat absorption from the gastrointestinal tract and elevating the reabsorption of bile acid. It also regulates liver enzymes levels and reduces lipogenesis activity by preventing the expression of adipose lipogenic genes and hepatic genes (Mahmoodi et al., 2020). It enhances the anti-oxidant activity and reduces the level of expression of TNF-α (Karolczak et al., 2020).
GT extract (GTE) is found to improve gene expression related to lipid oxidation. It also inhibits fatty liver accumulation through activation of adenosine monophosphate kinase (AMPK) and may also increase miR-34a, which is responsible for lowering PPAR- α expression with progression of steatosis. The hepatic lipid accumulation is prevented by increasing the hepatic miR-24, which further increases the insight target-a lipogenesis inhibitor. The primary polyphenol present in GT, EGCG, attunes the many miRNAs present in hepatocytes, which involves the modulation of insulin sensitivity, lipid metabolism, apoptosis, and inflammation. Changes in the expression of miR-122, miR-107, and miR-103 and the induction of plant polyphenols can shrink hepatic steatosis. A complete mapping of the pathway is required to reveal the involvement of miR34a and miR-194 in NAFLD (Torres et al., 2019).
EGCG has been utilized to lessen fat deposition in the liver in HFD fed in mice, which acts as a murine model of NAFLD in humans via specific pathways that include signal transduction, transcriptional, autophagy, and intracellular secondary message pathway (Ushiroda et al., 2019). The risk of NAFLD is ameliorated by the presence of anti-oxidants, lipid-lowering, anti-inflammatory effects, IR, and gut dysbiosis that are present in abundance in GT (Zhou et al., 2019).
Cinnamomum verum J. Presl
Cinnamon is a spice derived from the inner bark of the tree species C. verum, which belongs to the Lauraceae family and has anti-oxidant and insulin sensitizing characteristics. Some of the in vivo and in vitro studies estimated its effect on glucose metabolism by lowering post-prandial intestinal glucose absorption by preventing α-glucosidase and pancreatic α-amylase. It also enhances glycogen synthesis, potentiates insulin receptor activity and insulin release, and inhibits gluconeogenesis. Cinnamon plays a significant role in subsequent ROS and lipid peroxidation, which is considered as a known vital process in the development of NAFLD (Askari et al., 2014).
Chlorella vulgaris Beijerink
Chlorella vulgaris is a freshwater single-celled green eukaryotic microalga in the Chlorellaceae family that can be used as a supplement to treat NAFLD. Some studies state that it is rich in essential FAs and amino acids. It also consists of a reliable amount of fiber and intracellular phytochemicals such as tocopherols, carotenoids, and ubiquinone. This medication can also be used to prevent and manage hypertension, dyslipidemia, weight loss, and hyperglycemia. The supplementation of this can ameliorate IR and fasting serum glucose. In NAFLD patients, it is found to facilitate liver function and inflammatory biomarkers (Ebrahimi-Mameghani et al., 2017).
Glycyrrhiza glabra Linn.
Glycyrrhiza glabra, popularly known as licorice, is a Fabaceae-family herbaceous perennial legume native to Western Asia, North Africa, and Southern Europe. Glycycoumarin (GCM), a compound found in licorice, has been studied for its excellent bioavailability and high efficacy against alcoholic liver disease, nonalcoholic fatty liver, and acetaminophen-induced hepatotoxicity by activating the Nrf2 anti-oxidant system, inducing autophagy, activating AMPK-mediated energy homeostasis, inhibiting oncogenic kinase T lymphokine-activated killer cell. This shows the use of GCM as a hepatoprotective agent (Zhang et al., 2020).
GCM is found to inhibit hepatocyte lipoapoptosis. It is also found to activate impaired autophagy caused by lipid metabolic disorders. GCM reduces endoplasmic reticulum stress-mediated JNK and mitochondrial apoptotic pathway activation during autophagy activation (Zhang et al., 2016a, 2016b). Licorice root can also be used to treat bronchitis, stomach ulcers, viral infections, and sore throat. It is found in various countries like Italy, Russia, China, and Turkey. Glycyrrhizin exhibits antioxidant, immune-modulating activities, and anti-inflammatory properties (Hajiaghamohammadi et al., 2012).
Glycyrrhizin is considered to be a curative treatment of NASH accompanied by cholestasis. It aims for bile acid-mediated meta-inflammation and inhibits the FXR-NLRP3 inflammasome common pathway (Yan et al., 2018a, 2018b).
Diammonium glycyrrhizinate obtained from licorice roots is a glycyrrhizic acid considered a vital bioactive pentacyclic triterpenoid glycoside. It consists of anti-allergic, anti-viral, antitumor, and anti-oxidant properties. Diammonium glycyrrhizinate can be used to treat NAFLD since it can enhance the damage of the liver caused by anti-inflammatory activity in the liver (Li et al., 2018).
Linum usitatissimum Linn.
Flax, commonly known as common flax or linseed, is a flowering plant in the Linaceae family that is high in polyunsaturated fatty acids, especially linoleic acid, phytoestrogenic lignans, insoluble and soluble dietary fibers, anti-oxidants, and proteins. Many studies also found that flaxseeds can decrease the risk of MetS, dyslipidemia, and cardiovascular diseases. It is also found that flaxseed supplementation affects IR. A patient’s flaxseed can minimize TNF-α and high sensitivity c reactive protein (Yari et al., 2016).
ROLE OF PHYTOCONSTITUENTS IN NAFLD
Flavonoids
Flavonoids are prevalent polyphenolic compounds present in nature. They are found to bind with glycosides more than aglycones regularly. Almost all flavonoids possess three rings, namely two aromatic rings and one heterocyclic ring. Based on variations in the C, ring flavonoids are divided into subclasses such as flavonols, flavanones flavones, anthocyanidins chalcones, and isoflavones. They have been proven to have beneficial effects on lipid metabolism, oxidative stress, IR, and inflammation which are considered the most critical pathophysiological pathways in NAFLD represented in Figure 2 and Table 2 (Van De Wier et al., 2017).
Saponins
These are glycoside aglycones of three common terpenoids found in terrestrial plants. Some saponins are antipyretic, anti-cancer, and anti-bacterial. The main active ingredients in Polygala tenuifolia, Platycodon grandiflorus, Panax ginseng, and Glycyrrhiza uralensis are saponins (Liu et al., 2015a, 2015b; Zhang et al., 2016a, 2016b). Saponin extract reversed the elevation in the expression levels of lipogenesis-related genes induced by fast-food diet (FFD), including MLX interacting protein-like and fatty acid synthase (FASN) in regular chow diet fed mice. The expression of FA oxidation-related genes, such as PPAR-α along with carnitine palmitoyltransferase-1, was increased in FFD mice, which improved saponin extract administration (Wang et al., 2021).
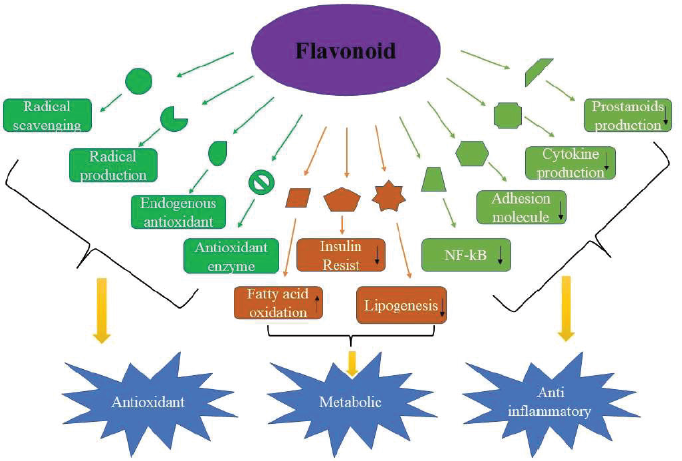 | Figure 2. Flavonoids with different stages of action such as radical scavenging, endogenous antioxidant, increased FA oxidation, decreased production of cytokine, prostanoid, IR and lipogenesis for their role as anti-oxidant, metabolic and anti-inflammatory. [Click here to view] |
 | Table 2. Pathway and its function for flavonoids. [Click here to view] |
Dioscin is considered to be a natural steroidal saponin found in many herbs. It is found to show anti-fungal, anti-hyperlipidemic, and anti-tumor activity (Hsieh et al., 2013). When taken orally, it improves fat accumulation in the liver, lowers blood lipid levels, reduces TG deposition via FASN inhibition, promotes FA beta-oxidation, lowers liver cholesterol, regulates the mitogen-activated protein kinase (MAPK) signaling pathway and autophagy, and decrease oxidative stress and inflammation (Liu et al., 2015a, 2015b).
Alkaloids
This is a class of naturally occurring nitrogenous organic compounds. It has antifungal, antibacterial, antitumor, and analgesic properties (Kukula-Koch et al., 2016;Stegelmeier et al., 2015). It has also been shown to have a significant effect on NAFLD. Berberine an alkaloid is frequently used to treat inflammatory disorders and diarrhea in China (Zhang et al., 2013). Berberine helps combat metabolic problems such as diabetes and obesity (Zhang et al., 2010;Zhou et al., 2011). Berberine can be used as a cholesterol-lowering drug due to the distinct mechanism that distinguishes it from statins (Kong et al., 2004). When rats were administered an HFD that produced hepatic steatosis, berberine restored systemic alterations in gene expression. The lncRNA MRAK052686 and its associated gene Nrf2 are implicated in the pathogenesis of NAFLD in many modules of berberine-regulated genes (Yuan et al., 2015).
Terpenoids
Terpenoids are organic compounds with several hydrocarbon isoprene units and their oxygenated derivatives in their molecular formulae. Aldehydes, alcohols, carboxylic acids, esters, and ketones are examples of oxygenated products. It can be found in abundance in nature and are the primary constituent of various plant essences and pigment resins (Arendt et al., 2016). Terpenoids have a variety of physiological functions, including cough relief, expulsion of wind, induction of sweating, insecticide activity, and pain relief (analgesia) (Choi et al., 2013).
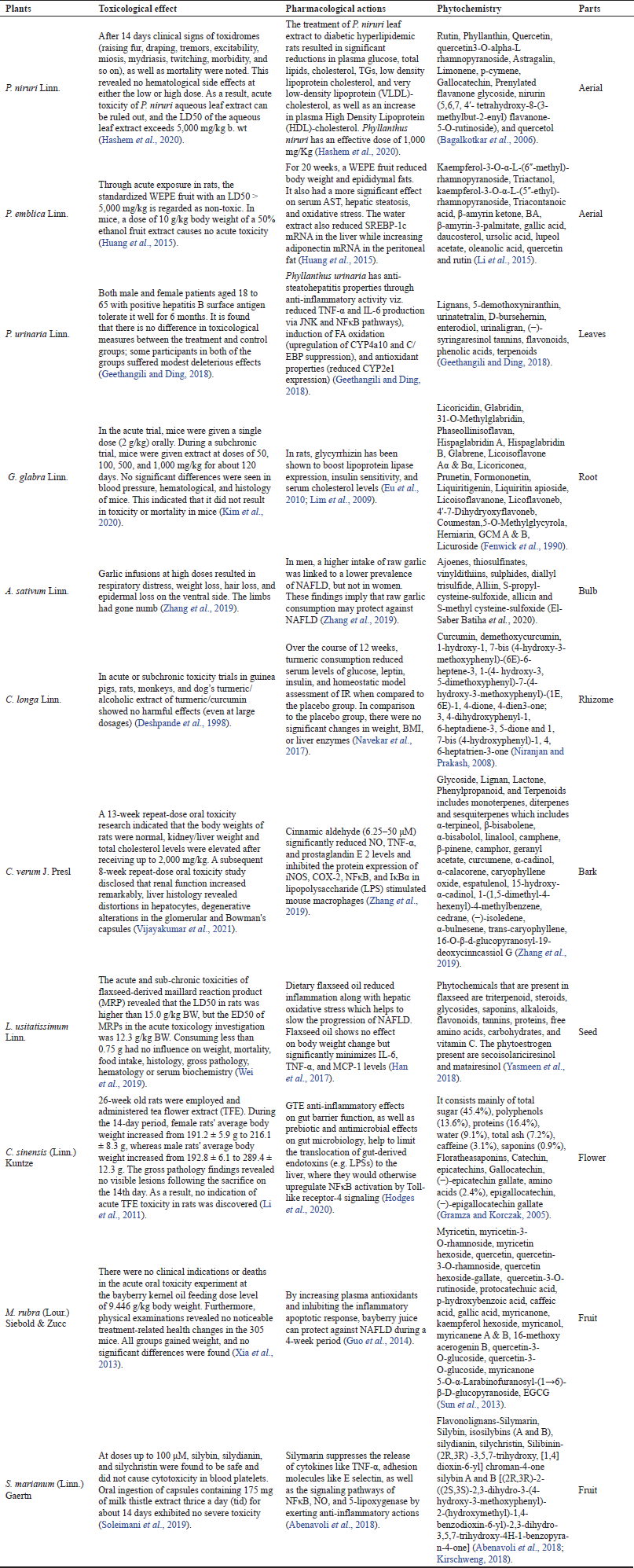 | Table 3. Pharmacological and toxicological effect of various herbal plants. [Click here to view] |
Betulinic Acid (BA) is a pentacyclic lupane type triterpene (3B-hydroxy-lup-20(29) en-28-oic acid). BA can be found in various foods, medicinal herbs, and plants, mainly birch bark. It is non-toxic in mice at concentrations of up to 500 mg/ kg body weight, and the main mechanism for its hepatoprotective qualities is its anti-oxidants, which boost the body’s redox system and lessen liver lipid peroxidation. By reducing hepatic steatosis via the calcium/calmodulin-dependent protein kinase-AMPKSREBP1 signaling pathway, BA efficiently reduces intracellular lipid accumulation in liver cells, limiting fatty liver deposition. It has also altered the way MAPK pathways are controlled. BA therapy inhibits HFD-induced changes in nuclear SREBP 1c activity and hepatic TG accumulation (Quan et al., 2013).
Polyphenols
Polyphenols are considered as a diverse class of plant-derived compounds that consist of various water-soluble antioxidants reported as health-promoting agents. It can be suggested in the treatment of various metabolic disorders. Their bioactive compounds are abundant in fruits, vegetables, and beverages such as coffee, tea, red wine, and dark chocolate (Abenavoli et al., 2017).
Curcumin, the yellow pigment found in the C. longa plant is derived from curry and spices. Its anti-oxidant effects have been extensively researched in the context of liver metabolism (Pan et al., 2014). Curcumin protects the liver by suppressing the expression of NFκB target genes such as monocyte chemotactic protein 1 (MCP-1), cyclooxygenase-2 (COX-2), and intercellular cell adhesion molecule-1. According to Vizzutti et al. (2010), curcumin can reduce alpha-smooth muscle actin levels in NASH mice, along with the formation of ROS and tissue inhibitors of metalloproteinases-1 secreting activated hepatic stellate cells. Curcumin alleviates NAFLD by activating Nrf2, AMPK, and nuclear receptors, altering lipid metabolism and oxidative stress, and inhibiting NLRP3 inflammasome activation and gut microbiota, reducing inflammation and steatosis (Yan et al., 2020).
Resveratrol is a phytoalexin polyphenolic molecule that has been demonstrated to assist in the pathology of NAFLD (Bujanda et al., 2008). Some of the methods used to decrease inflammation include inhibition of pro-inflammatory mediator synthesis and release, modification of eicosanoids synthesis, prevention of kupffer cell adhesion molecules, and inhibition of COX-2, nitric oxide (NO) synthase via inhibitory effects on NFκB or activator protein-1 proteins (Alarcon De La Lastra and Villegas, 2005; Jang et al., 1997).
Chloroquine inhibits autophagy in acute myeloid leukemia-12 (AML-12) cells, which suggests that resveratrol’s actions are likely linked to autophagy activation (Ji et al., 2015). According to the research, resveratrol has also been shown to diminish inflammatory liver damage in methionine-choline deficient diet-induced NASH mice in addition to IL-1, IL-6, ALT, aspartate aminotransferase (AST), and TNF-α levels in the blood that have been associated with autophagy (Shen et al., 2019). Furthermore, resveratrol has been shown to heal liver injury in experimental mice by activating autophagy and reducing NFκB activation (Li et al., 2014), implying that activation of autophagy could be used as an anti-inflammatory method to prevent NAFLD progression (Zhang et al., 2018)
CONCLUSION
The use of herbal medicine becomes vital for treating a variety of human ailments. The studies conducted with a deep understanding of the root cause of the disease have been applied widely to modify the ancient form of treatment to globally accepted medicines. The development of herbal medicine regarding its pathogenesis and pathway of the disease, i.e., for the NAFLD, has been widely studied and provided no prominent data with the background of the disease. There is no Food and Drug Administration approved medicines for NAFLD treatment. There is a wide rush in formulating and developing a drug either in allopathic medicine or the Indian system of medicine for the treatment of NAFLD. Herbal drugs will have a significant role in the future when the pathway of the disease is well studied with herbal plants. When compared to allopathic medicine, herbal medicine is cost-effective as it is readily available and needs only a thorough study for its scientific validation for proof of toxicity and efficacy.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Abenavoli L, Bellentani S. Milk thistle to treat non-alcoholic fatty liver disease: dream or reality? Expert Rev Gastroenterol Hepatol, 2013; 7(8):677–9. CrossRef
Abenavoli L, Izzo AA, Mili? N, Cicala C, Santini A, Capasso R. Milk thistle Silybum marianum: a concise overview on its chemistry, pharmacological, and nutraceutical uses in liver diseases. Phytother Res, 2018; 32(11):2202–13. CrossRef
Abenavoli L, Milic N, Luzza F, Boccuto L, De Lorenzo A. Polyphenols treatment in non-alcoholic fatty liver disease. J Transl Int Med, 2017; 5(3):144. CrossRef
Alarcon De La Lastra C, Villegas I. Resveratrol as an anti-inflammatory and anti-aging agent: mechanisms and clinical implications. Mol Nutr Food Res, 2005; 49(5):405–30. CrossRef
Arendt P, Pollier J, Callewaert N, Goossens A. Synthetic biology for the production of natural and new-to-nature terpenoids in photosynthetic organisms. Plant J, 2016; 87(1):16–37. CrossRef
Askari F, Rashidkhani B, Hekmatdoost A. Cinnamon may have therapeutic benefits on lipid profile, liver enzymes, insulin resistance, and high-sensitivity C-reactive protein in non-alcoholic fatty liver disease patients. Nutr Res, 2014; 34(2):143–8. CrossRef
Bagalkotkar G, Sagineedu SR, Saad MS, Stanslas J. Phytochemicals from Phyllanthus niruri Linn. and their pharmacological properties: a review. J Pharm Pharmacol, 2006; 58(12):1559–70. CrossRef
Bujanda L, Hijona E, Larzabal M, Beraza M, Aldazabal P, García-Urkia N, Sarasqueta C, Cosme A, Irastorza B, González A, Arenas JI. Resveratrol inhibits non-alcoholic fatty liver disease in rats. BMC Gastroenterol, 2008; 8(1):1–8. CrossRef
Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of the non-alcoholic fatty liver disease (NAFLD). Metabolism, 2016; 65(8):1038–48. CrossRef
Buzzetti E, Pinzani M, Tsochatzis EA. The two-hit pathogenesis of the non-alcoholic fatty liver disease (NAFLD). Metabolism, 2014; 62(7):1028–38.
Camini FC, Costa DC. Silymarin: not just another anti-oxidant. J Basic Clin Physiol Pharmacol, 2020; 31(4):1–12. CrossRef
Choi YJ, Park SY, Kim JY, Won KC, Kim BR, Son JK, Lee SH, Kim YW. Combined treatment of Betulinic acid, a PTP1B inhibitor, with Orthosiphon stamineus extract decreases body weight in high-fat-fed mice. J Med Food, 2013; 16(1):2–8. CrossRef
Deshpande SS, Lalitha VS, Ingle AD, Raste AS, Gadre SG, Maru GB. Subchronic oral toxicity of turmeric and ethanolic turmeric extract in female mice and rats. Toxicol Lett, 1998; 95(3):183–93. CrossRef
Ebrahimi-Mameghani M, Sadeghi Z, Farhangi MA, Vaghef-Mehrabany E, Aliashrafi S. Glucose homeostasis, insulin resistance and inflammatory biomarkers in patients with non-alcoholic fatty liver disease: beneficial effects of supplementation with microalgae Chlorella vulgaris: a double-blind placebo-controlled randomized clinical trial. Clin Nutr, 2017; 36(4):1001–6. CrossRef
El-Saber Batiha G, Magdy Beshbishy A, G Wasef L, Elewa YH, A Al-Sagan A, El-Hack A, Mohamed E, Taha AE, M Abd-Elhakim Y, Prasad Devkota H. Chemical constituents and pharmacological activities of garlic (Allium sativum L.): a review. Nutrients, 2020; 12(3):872. CrossRef
Eng JM, Estall JL. Diet-induced models of non-alcoholic fatty liver disease: food for thought on sugar, fat, and cholesterol. Cells, 2021; 10(7):1805. CrossRef
Eu CH, Lim WY, Ton SH, bin Abdul Kadir K. Glycyrrhizic acid improved lipoprotein lipase expression, insulin sensitivity, serum lipid and lipid deposition in high-fat diet-induced obese rats. Lipids Health Dis, 2010; 9(1):1–9. CrossRef
Federico A, Dallio M, Loguercio C. Silymarin/silybin and chronic liver disease: a marriage of many years. Molecules, 2017; 22(2):191. CrossRef
Fenwick G, Lutomski J, Nieman C. Glycyrrhiza glabra L. (Liquorice): composition, uses, and analysis. Food Chem, 1990; 38(2):119–43. CrossRef
Geethangili M, Ding ST. A review of the phytochemistry and pharmacology of Phyllanthus urinaria L. Front Pharmacol, 2018; 9:1109. CrossRef
Ghosh S. Curcumin as a potential therapeutic option for NAFLD and other metabolic diseases: the need for establishing the underlying mechanism (s) of action. Hepatol Int, 2019; 13(3):245–7. CrossRef
Gottlieb A, Canbay A. Why bile acids are so important in nonalcoholic fatty liver disease (NAFLD) progression. Cells, 2019; 8(11):1358. CrossRef
Gramza A, Korczak J. Tea constituents (Camellia sinensis L.) as anti-oxidants in lipid systems. Trends Food Sci Technol, 2005; 16(8):351–8. CrossRef
Guo H, Zhong R, Liu Y, Jiang X, Tang X, Li Z, Xia M, Ling W. Effects of bayberry juice on inflammatory and apoptotic markers in young adults with features of non-alcoholic fatty liver disease. Nutrition, 2014; 30(2):198–203. CrossRef
Hajiaghamohammadi AA, Ziaee A, Samimi R. The efficacy of licorice root extract in decreasing transaminase activities in non-alcoholic fatty liver disease: a randomized controlled clinical trial. Phytother Res, 2012; 26(9):1381–4. CrossRef
Han H, Qiu F, Zhao H, Tang H, Li X, Shi D. Dietary flaxseed oil prevents western-type diet-induced non-alcoholic fatty liver disease in apolipoprotein-E knockout mice. Oxid Med Cell Longev, 2017; 2017:3256241. CrossRef
Hashem M, Hashish E, Atteya M. Alternative approaches to ameliorate nonalcoholic fatty liver disease: Phyllanthus niruri clinicopathological significance; a review. Zagazig Vet J, 2020; 48(4):399–413. CrossRef
Hodges JK, Sasaki GY, Bruno RS. Anti-inflammatory activities of green tea catechins along the gut-liver axis in non-alcoholic fatty liver disease: lessons learned from preclinical and human studies. J Nutr Biochem, 2020; 12:108478. CrossRef
Hossain N, Kanwar P, Mohanty SR. A comprehensive updated review of pharmaceutical and non-pharmaceutical treatment for NAFLD. Gastroenterol Res Pract, 2016; 2016:7109270. CrossRef
Hsieh MJ, Tsai TL, Hsieh YS, Wang CJ, Chiou HL. Dioscininduced autophagy mitigates cell apoptosis through modulation of PI3K/ Akt and ERK and JNK signaling pathways in human lung cancer cell lines. Arch Toxicol, 2013; 87(11):1927–37. CrossRef
Huang CZ, Tung YT, Hsia SM, Wu CH, Yen GC. The hepatoprotective effect of Phyllanthus emblica L. fruit on high fat diet-induced non-alcoholic fatty liver disease (NAFLD) in SD rats. Food Funct, 2017; 8(2):842–50. CrossRef
Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science, 1997; 275(5297):218–20. CrossRef
Ji G, Wang Y, Deng Y, Li X, Jiang Z. Resveratrol ameliorates hepatic steatosis and inflammation in methionine/choline-deficient diet-induced steatohepatitis through regulating autophagy. Lipids Health dis, 2015; 14(1):1–9. CrossRef
Karolczak D, Seget M, Bajerska J, B?aszczyk A, Drzyma?a-Czy? S, Walkowiak J, Marsza?ek A. Green tea extract prevents the development of nonalcoholic liver steatosis in rats fed a high-fat diet. Pol J Pathol, 2020; 70(4):295–303. CrossRef
Kheong CW, Mustapha NR, Mahadeva S. A randomized trial of silymarin for the treatment of non-alcoholic steatohepatitis. Clin Gastroenterol Hepatol, 2017; 15(12):1940–9. CrossRef
Kim HY, Zuo G, Lee SK, Lim SS. Acute and Subchronic toxicity study of a nonpolar extract of licorice roots in mice. Food Sci Nutr, 2020; 8(5):2242–50. CrossRef
Kirschweng B, Tilinger MD, Hégely B, Samu Gy, Tatraaljai D, Foldes E, Pukánszky B. Melt stabilization of PE with natural antioxidants: Comparison of rutin and quercetin. Eur Polym J, 2018; 103:228–37 CrossRef
Kong W,Wei J, Abidi P, Lin M, Inaba S, Li C, Wang Y, Wang Z, Si S, Pan H, Wang S, Wu J, Wang Y, Li Z, Liu J, Jiang JD. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med, 2004; 10:1344–51. CrossRef
Kukula-Koch W, Koch W, Angelis A, Halabalaki M, Aligiannis N. Application of pH-zone refining hydrostatic countercurrent chromatography (hCCC) for the recovery of anti-oxidant phenolics and the isolation of alkaloids from Siberian barberry herb. Food Chem, 2016; 203:394–401. CrossRef
Lau LH, Wong SH. Microbiota, obesity, and NAFLD. Adv Exp Med Biol, 2018; 1061:111–25. CrossRef
Lee DE, Lee SJ, Kim SJ, Lee HS, Kwon OS. Curcumin ameliorates non-alcoholic fatty liver disease through inhibition of O-GlcNAcylation. Nutrients, 2019; 11(11):2702. CrossRef
Li B, Huang GQ, Lu RM, Wei JH, Zhong ZG. Study on the chemical composition of Phyllanthus emblica. Zhong Yao Cai, 2015; 38(2):290–3.
Li B, Jin Y, Xu Y, Wu Y, Xu J, Tu Y. Safety evaluation of tea (Camellia sinensis (L.) O. Kuntze) flower extract: assessment of mutagenicity and acute and subchronic toxicity in rats. J Ethnopharmacol, 2011; 133(2):583–90. CrossRef
Li L, Hai J, Li Z, Zhang Y, Peng H, Li K, Weng X. Resveratrol modulates autophagy and NF-κB activity in a murine model for treating non-alcoholic fatty liver disease. Food Chem Toxicol, 2014; 63:166–73. CrossRef
Li Y, Liu T, Yan C, Xie R, Guo Z, Wang S, Zhang Y, Li Z, Wang B, Cao H. Diammonium glycyrrhizinate protects against non-alcoholic fatty liver disease in mice through modulation of gut microbiota and restoration of the intestinal barrier. Mol Pharm, 2018; 15(9):3860–70. CrossRef
Lim JS, Mietus-Snyder M, Valente A, Schwarz JM, Lustig RH. The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat Rev Gastroenterol Hepatol, 2010; 7(5):251–64. CrossRef
Lim WY, Chia YY, Liong SY, Ton SH, Kadir KA, Husain SN. Lipoprotein lipase expression, serum lipid, and tissue lipid deposition in orally-administered glycyrrhizic acid-treated rats. Lipids Health Dis, 2009; 8(1):31. CrossRef
Liu M, Xu L, Yin L, Qi Y, Xu Y, Han X, Zhao Y, Sun H, Yao J, Lin Y, Liu K. Potent effects of dioscin against obesity in mice. Sci Rep, 2015a; 5(1):7973. CrossRef
Liu X, Yu JL, Liu M, Shu JC, Huang HL. Research progress of bioactivity of steroidal saponins in recent ten years. Zhongguo Zhong Yao Za Zhi, 2015b; 40(13):2518–23.
Mahmoodi M, Hosseini R, Kazemi A, Ofori-Asenso R, Mazidi M, Mazloomi SM. Effects of green tea or green tea catechin on liver enzymes in healthy individuals and people with non-alcoholic fatty liver disease: a systematic review and meta-analysis of randomized clinical trials. Phytother Res, 2020; 34(7):1587–98. CrossRef
Mansour-Ghanaei F, Hadi A, Pourmasoumi M, Joukar F, Golpour S, Najafgholizadeh A. Green tea as a safe alternative approach for non-alcoholic fatty liver treatment: a systematic review and meta-analysis of clinical trials. Phytother Res, 2018; 32(10):1876–84. CrossRef
Mansour-Ghanaei F, Pourmasoumi M, Hadi A, Joukar F. Efficacy of curcumin/turmeric on liver enzymes in patients with non-alcoholic fatty liver disease: a systematic review of randomized controlled trials. Integr Med Res, 2019; 8(1):57–61. CrossRef
Marcuccilli M, Chonchol M. NAFLD, and chronic kidney disease. Int J Mol Sci, 2016; 17(4):562. CrossRef
Mundi MS, Velapati S, Patel J, Kellogg TA, Abu Dayyeh BK, Hurt RT. Evolution of NAFLD and its management. Nutr Clin Pract, 2020; 35(1):72–84. CrossRef
Navarro V, Belle SH, D’Amato M, Adfhal N, Brunt EM, Fried MW, Reddy KR, Wahed AS, Harrison S, Silymarin in NASH and C Hepatitis (SyNCH) Study Group. Silymarin in non-cirrhotics with nonalcoholic steatohepatitis: a randomized, double-blind, placebo-controlled trial. PLoS One, 2019; 14: e0221683. CrossRef
Navekar R, Rafraf M, Ghaffari A, Asghari-Jafarabadi M, Khoshbaten M. Turmeric supplementation improves serum glucose indices and leptin levels in patients with non-alcoholic fatty liver diseases. JAm Coll Nutr, 2017; 36(4):261–7. CrossRef
Niranjan A, Prakash D. Chemical constituents and biological activities of turmeric (Curcuma longa L.)-a review. J Food Sci Technol, 2008; 45(2):109.
Pais P, D’Amato M. In vivo efficacy study of milk thistle extract (ETHIS-094™) in STAM™ model of non-alcoholic steatohepatitis. Drugs R D, 2014; 14(4):291–9. CrossRef
Pan MH, Lai CS, Tsai ML, Ho CT. Chemoprevention of nonalcoholic fatty liver disease by dietary natural compounds. Mol Nutr Food Res, 2014; 58(1):147–71. CrossRef
Panahi Y, Kianpour P, Mohtashami R, Jafari R, Simental-Mendía LE, Sahebkar A. Efficacy and safety of phytosomal curcumin in non-alcoholic fatty liver disease: a randomized controlled trial. Drug Res, 2017; 67(04):244–51. CrossRef
Quan HY, Kim SJ, Jo HK, Kim GW, Chung SH. Betulinic acid alleviates non-alcoholic fatty liver by inhibiting SREBP1 activity via the AMPK– mTOR–SREBP signaling pathway. Biochem Pharmacol, 2013; 85(9):1330–40. CrossRef
Rahmani S, Asgary S, Askari G, Keshvari M, Hatamipour M, Feizi A, Sahebkar A. Treatment of non-alcoholic fatty liver disease with curcumin: a randomized placebo-controlled trial. Phytother Res, 2016; 30(9):1540–8. CrossRef
Salt WB. Non-alcoholic fatty liver disease: a comprehensive review. J Insur Med, 2004; 36(1):27–41.
Sangouni AA, Azar MR, Alizadeh M. Effect of garlic powder supplementation on hepatic steatosis, liver enzymes and lipid profile in patients with non-alcoholic fatty liver disease: a double-blind randomized controlled clinical trial. Br J Nutr, 2020; 124(4):450–6. CrossRef
Shen F, Wang Y, Sun H, Zhang D, Yu F, Yu S, Han H, Wang J, Ba Y, Wang C, Li W. Vitamin D receptor gene polymorphisms are associated with triceps skinfold thickness and body fat percentage but not with body mass index or waist circumference in Han Chinese. Lipids Health Dis, 2019; 18(1):1–8. CrossRef
Soleimani V, Delghandi PS, Moallem SA, Karimi G. Safety and toxicity of silymarin, the major constituent of milk thistle extract: an updated review. Phytother Res, 2019; 33(6):1627–38. CrossRef
Stegelmeier BL, Brown AW, Welch KD. Safety concerns of herbal products and traditional Chinese herbal medicines: dehydropyrrolizidine alkaloids and aristolochic acid. J Appl Toxicol, 2015; 35(12):1433–7. CrossRef
Sun C, Huang H, Xu C, Li X, Chen K. Biological activities of extracts from Chinese bayberry (Myrica rubra Sieb. et Zucc.): a review. Plant Food Hum Nutr, 2013; 68(2):97–106. CrossRef
Torres LF, Cogliati B, Otton R. Green tea prevents NAFLD by modulation of miR-34a and miR-194 expression in a high-fat diet mouse model. Oxid Med Cell Longev, 2019; 2019:4168380. CrossRef
Tung YT, Huang CZ, Lin JH, Yen GC. Effect of Phyllanthus emblica L. fruit on methionine and choline-deficiency diet-induced nonalcoholic steatohepatitis. J Food Drug Anal, 2018; 26(4):1245–52. CrossRef
Ushiroda C, Naito Y, Takagi T, Uchiyama K, Mizushima K, Higashimura Y, Yasukawa Z, Okubo T, Inoue R, Honda A, Matsuzaki Y. Green tea polyphenol (epigallocatechin-3-gallate) improves gut dysbiosis and serum bile acids dysregulation in high-fat diet-fed mice. J Clin Bio Nutr, 2019; 65(1):34–46. CrossRef
Van De Wier B, Koek GH, Bast A, Haenen GR. The potential of flavonoids in the treatment of non-alcoholic fatty liver disease. Crit Rev Food Sci Nutr, 2017; 57(4):834–55. CrossRef
Vijayakumar K, Rengarajan RL, Suganthi N, Prasanna B, Velayuthaprabhu S, Shenbagam M, Vijaya Anand A. Acute toxicity studies and protective effects of Cinnamon cassia bark extract in streptozotocininduced diabetic rats. Drug Chem Toxicol, 2021:1–11. CrossRef
Vizzutti F, Provenzano A, Galastri S, Milani S, Delogu W, Novo E, Caligiuri A, Zamara E, Arena U, Laffi G, Parola M. Curcumin limits the fibrogenic evolution of experimental steatohepatitis. Lab Invest, 2010; 90(1):104–15. CrossRef
Wang F, Park JS, Ma Y, Ma H, Lee YJ, Lee GR, Yoo HS, Hong JT, Roh YS. Ginseng saponin enriched in Rh1 and Rg2 ameliorates non-alcoholic fatty liver disease by inhibiting inflammasome activation. Nutrients, 2021; 13(3):856. CrossRef
Wei CK, Ni ZJ, Thakur K, and Liao AM, Hu F, Huang JH, and Wei ZJ. Acute, genetic, and sub-chronic toxicities of flaxseed derived Maillard reaction products. Food Chem Toxicol, 2019; 131:110580. CrossRef
Wong VW, Wong GL, Chan AW, Chu WC, Choi PC, Chim AM, Yiu KK, Yu J, Chan FK, Chan HL. Treatment of non-alcoholic steatohepatitis with Phyllanthus urinaria: a randomized trial. J Gastroenterol Hepatol, 2013; 28(1):57–62. CrossRef
Xia Q, Pan S, Zheng M, Chen J, Fang Z, Johnson S, Yang Y, Xing J, Lu S. Fatty acid profile, oxidative stability and toxicological safety of bayberry kernel oil. Food Chem Toxico, 2013; 60:92–7. CrossRef
Yamamoto Y, Gaynor RB. Therapeutic potential of inhibition of the NFκB pathway in the treatment of inflammation and cancer. J Clin Invest, 2001; 107(2):135–42. CrossRef
Yan C, Zhang Y, Zhang X, Aa J, Wang G, Xie Y. Curcumin regulates endogenous and exogenous metabolism via Nrf2-FXR-LXR pathway in NAFLD mice. Biomed Pharmacother, 2018a; 105:274–81. CrossRef
Yan T, Wang H, Cao L, Wang Q, Takahashi S, Yagai T, Li G, Krausz KW, Wang G, Gonzalez FJ, Hao H. Glycyrrhizin alleviates nonalcoholic steatohepatitis via modulating bile acids and meta-inflammation. Drug Metab Dispos, 2018b; 46(9):1310-9. CrossRef
Yan T, Yan N, Wang P, Xia Y, Hao H, Wang G, Gonzalez FJ. Herbal drug discovery for the treatment of non-alcoholic fatty liver disease. Acta Pharm Sin B, 2020; 10(1):3–18. CrossRef
Yari Z, Rahimlou M, Eslamparast T, Ebrahimi-Daryani N, Poustchi H, Hekmatdoost A. Flaxseed supplementation in non-alcoholic fatty liver disease: a pilot randomized, open-labeled, controlled study. Int J Food Sci Nutr, 2016; 67(4):461–9. CrossRef
Yasmeen M, Nisar S, Tavallali V, Khalid T. A review of phytochemicals and uses of flaxseed. Int J Chem Biochem Sci, 2018; 13:70–5.
Yuan X, Wang J, Tang X, Li Y, Xia P, Gao X. Berberine ameliorates non-alcoholic fatty liver disease by a global modulation of hepatic mRNA and lncRNA expression profiles. J Transl Med, 2015; 13(1):24. CrossRef
Zarzour A, Hamdan R, Ahmad M, Asmawi M, Kaur G, Saeed MA, Al-Mansoub MA, Saghir SA, Usman NS, Al-Dulaimi DW, Yam MF. Phyllanthus niruri standardized extract alleviates the progression of non-alcoholic fatty liver disease and decreases the atherosclerotic risk in Sprague–Dawley rats. Nutrients, 2017; 9(7):766. CrossRef
Zarzour A, Hamdan R, Alshawsh MA, Asif M, Al-Mansoub MA, Mohamed Z, Ahmad M, Abdul Majid AM, Asmawi M, Kaur G, Al-Dualimi DW. Adipocytokine regulation and antiangiogenic activity underlie the molecular mechanisms of therapeutic effects of Phyllanthus niruri against non-alcoholic fatty liver disease. Nutrients, 2018; 10(8):1057. CrossRef
Zhang C, Fan L, Fan S, Wang J, Luo T, Tang Y, Chen Z, Yu L. Cinnamomum cassia Presl: a review of its traditional uses, phytochemistry, pharmacology, and toxicology. Molecules, 2019; 24(19):3473. CrossRef
Zhang E, Yin S, Song X, Fan L, Hu H. Glycycoumarin inhibits hepatocyte lipoapoptosis through activation of autophagy and inhibition of ER stress/GSK-3-mediated mitochondrial pathway. Sci Rep, 2016a; 6(1):1–2. CrossRef
Zhang E, Yin S, Zhao S, Zhao C, Yan M, Fan L, Hu H. Protective effects of glycycoumarin on liver diseases. Phytother Res, 2020; 34(6):1191–7. CrossRef
Zhang H, Wei J, Xue R, Wu JD, Zhao W, Wang ZZ, Wang SK, Zhou ZX, Song DQ, Wang YM, Pan HN. Berberine lowers blood glucose in type 2 diabetes mellitus patients through increasing insulin receptor expression. Metabolism, 2010; 59(2):285–92. CrossRef
Zhang J, Cao H, Zhang B, Cao H, Xu X, Ruan H, Yi T, Tan L, Qu R, Song G, and Wang B. Berberine potently attenuates intestinal polyp’s growth in ApcMin mice and familial adenomatous polyposis patients through inhibition of Wnt signaling. J Cell Mol Med, 2013; 17(11):1484–93. CrossRef
Zhang L, Yao Z, Ji G. Herbal extracts and natural products in alleviating non-alcoholic fatty liver disease via activating autophagy. Front Pharmacol, 2018; 9:1459. CrossRef
Zhang S, Gu Y, Wang L, Zhang Q, Liu L, Lu M, Meng G, Yao Z, Wu H, Xia Y, Bao X. Association between dietary raw garlic intake and newly diagnosed non-alcoholic fatty liver disease: a population-based study. Eur J Endocrinol, 2019; 181(6):591–602. CrossRef
Zhang ZY, Wu JP, Gao BB, Ren HT, Liu YL, Li XR, Li KP, Xu QM, Yang SL. Two new 28-nor-oleanane-type triterpene saponins from roots of Camellia oleifera and their cytotoxic activity. J Asian Nat Prod Res, 2016b; 18(7):669–76. CrossRef
Zhong S, Fan Y, Yan Q, Fan X, Wu B, Han Y, Zhang Y, Chen Y, Zhang H, Niu J. The therapeutic effect of silymarin in the treatment of nonalcoholic fatty disease: a meta-analysis (PRISMA) of randomized control trials. Medicine, 2017; 96(49):e9061. CrossRef
Zhou J, Ho CT, Long P, Meng Q, Zhang L, Wan X. Preventive efficiency of green tea and its components on non-alcoholic fatty liver disease. J Agric Food Chem, 2019; 67(19):5306–17. CrossRef
Zhou L, Wang X, Yang Y, Wu L, Li F, Zhang R, Yuan G, Wang N, Chen M, Ning G. Berberine attenuates cAMP-induced lipolysis via reducing the inhibition of phosphodiesterase in 3T3-L1 adipocytes. Biochim Biophys Acta, 2011; 1812(4):527–35. CrossRef