INTRODUCTION
Counterfeit medicines (CFMs) are of lower quality than their originals and are fraudulently manufactured with fake packaging and usually no or a wrong active pharmaceutical ingredient (Lai and Zaichkowsky, 1999). They also can be generic and branded products, and according to the World Health Organization (WHO) classification, there are six categories of CFMs including products without active ingredients (32%), products with wrong ingredients (21.4%), products without or with insufficient active ingredients (20.2%), products with fake packaging (15.6%), products with a high level of impurities or contaminants (8.5%), and/or copies of the original product (1%).
Recently, the trade in counterfeiting has developed into an extensive threat to public health and the pharmaceutical industry (Bird, 2007). The WHO estimated that the global trade in CFMs is experiencing continuous growth (WHO, 2007). Up to 10% of the drugs worldwide may be counterfeits (Gibson, 2004; Pincock, 2003); 50% of them involved antimicrobial drugs, and 78% were from developing countries. Moreover, 59% of cases with available information on the quality of drugs were fraudulent, and only 7% had the standard concentration of the active drug (WHO, 1999, 2000). However, reporting of counterfeit drugs within the WHO is only 15% (Cockburn, 2005; WHO, 2014).
The literature review revealed that CFMs are available worldwide and are most prevalent in developing countries due to weak medicine regulation, control, and enforcement (Alsultan, 2010; Dondorp et al., 2004; Nayyar, 2012; Noun et al., 2021; WHO, 2017). Unfortunately, the illegal trade in counterfeits has now extended even to the herbal drugs which are mostly used in the developing countries (Mullaicharam, 2011). Additionally, advanced computer technologies and the widespread use of the internet have also led to the rapid increase of CFMs which result in regulatory systems weakness and lack of awareness among health workers and the public (Akunyili, 2004; Bansal, 2013; Buckley and Gostin, 2013; Koh et al., 2003; Siva, 2010).
Sudan is a vast African country surrounded by a number of other African countries (e.g., Kenya and Uganda). Most of these African countries suffer from the circulation of such drugs. To combat this problem, these countries have put strategies to control this problem through enforcing strong close and carrying out continuous postmarketing surveillance studies.
The role of pharmaceutical quality control and good manufacturing practices is to assess the quality of active pharmaceutical ingredients and excipients (European commission, 1998). Even though the National Medicine Regulatory Authorities have been available in all the developing countries, most of them are not completely operational, while the rest are at various degrees of foundation.
Many challenges facing authorities, healthcare providers, and National Quality Control Laboratories regarding counterfeit and substandard drugs include that they are difficult to trace, their spreading cannot be controlled or stopped, their detection, quantification, and control need well-equipped labs and well-trained trustful personnel, and some expired legitimate drugs can be remarked with a false new expiry date. In addition to that, counterfeiters aim to avoid raising suspicion about the origin and the quality of their products. Also, they take measures that make them slip past the authorities’ control and ultimately deceive consumers.
Despite all of that, gaps still exist in the current literature including documentation of pharmacists’ knowledge regarding counterfeit medications, strategies used by pharmacists to handle counterfeit medications delivered to their practice setting, and how patients are educated about counterfeit medications. Therefore, this study aimed to reveal the extent of the counterfeit practice in Khartoum locality, Sudan, and to assess the public’s experience, attitude, and knowledge about CFMs.
METHODS
Setting and study population
This is a cross-sectional population-based study, conducted in Khartoum city, Sudan, between December 2019 and April 2020. The inclusion criteria were any individual 18 years old and above, willing to participate in the study.
Sampling procedure
A total of 386 participants was obtained upon sample size for public (respondents/participants) calculation using the following equation (Sharon, 2010):
,
where n is sample size; Z is a value from the normal distribution related to 0.05 precision; p is proportion of interest; q is 1-p; e is precision level (0.05).
The convenience sampling technique was then used to select the participants included in the study (Tansey, 2007), after signing a written informed consent to guarantee privacy and confidentiality.
Questionnaire design and validation
The questionnaire was initially written in English and was translated into Arabic. Different types of questions were used: multiple-choice, close-ended, and open-ended. It consisted of general information and demographics, medicine use, awareness of CFM, attitude toward CFM, and recommendations on measures to be taken to control the illicit trade in counterfeit drugs.
The questionnaire was piloted on 20 members of the public for content validation. It was then simplified and shortened to consume 12–15 minutes. Finally, the questionnaire was assessed through experts in the field of pharmacy before data collection.
Data collection
The authors and assistants (surveyors) administered the questionnaire through face-to-face interviews for the population in hospitals, pharmacies, and private clinics. The collected data were checked for completeness, manually scored, and finally coded before the analysis.
Statistical analysis
Data were entered and analyzed using Excel 2016. Descriptive analysis was performed for the questionnaire where frequencies, percentages, and graphical representation were reported for all categorical variables. Fisher’s exact test and Spearman’s correlation were then used for testing the association between variables and the relation of awareness to attitude, respectively. p < 0.05 was considered to be statistically significant.
The scoring system for awareness was as follows: relevant questions were selected; the correct answer was given 1 and the incorrect answer 0. There were a total of 10 points for the awareness classified as 1–3 having a low level of awareness, 4–7 moderate level, and 8–10 high level. Concerning attitude, the total score was 16. Those with a total score of 1–5 were classified as having a poor attitude, 6–11 as a fair attitude, and 12–16 as a good attitude.
Ethical requirements
The ethical clearance (FPEC-03-2019) was obtained from the Ethical Committee of the Faculty of Pharmacy, University of Khartoum. The dates and times when the questionnaires were administered were all documented.
With respect to respondents’ autonomy and anonymity, all of them signed a written informed consent and were guaranteed privacy and confidentiality.
RESULTS
Public awareness and attitude
Demographic characteristics
A total of 386 respondents participated in this study; the mean age was 34 years (18–80 years old). About 218 (56%) were males, and 194 (50%) were university graduates. Obtained data are summarized in Table 1.
Awareness about CFMs
Most of the respondents [222 (58%)] had heard the term “counterfeit medicine” before, while 151 (39%) did not know. Social media was mentioned by the majority of the respondents (112) as the main source of awareness, followed by pharmacy (68), TV (37), and billboards (4). Regarding CFM quality, 73% considered it with worse quality, 22% of the same quality, and 5% of better quality.
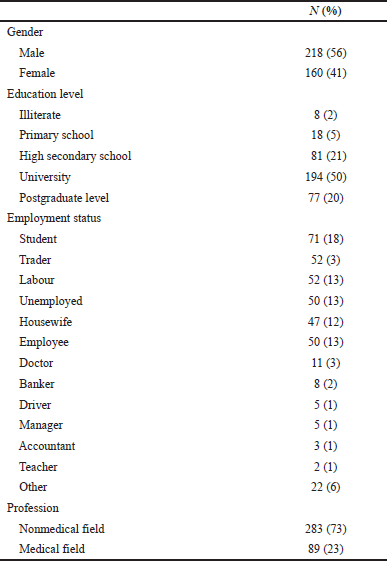 | Table 1. Sociodemographic characteristics of the participants (n = 386). [Click here to view] |
of questions on how respondents dealt with suspected CFMs showed that 57 (15%) of the respondents reported being suspicious of different medicines to be counterfeited, such as insulin injections and atorvastatin. Their reasons behind being suspicious and their action toward that are summarized in Table 2.
The respondents were further asked about how to avoid buying CFMs. 271 of them (70%, N = 473) answered by getting the medicine from a trustworthy pharmacist while 66 reported buying only medicines manufactured outside Sudan
CFMs characteristics, origin, and availability
The respondents reported different characteristics to distinguish the CFMs. A total response of 642 was obtained. 56% of them mentioned the side effects as the main characteristic to identify the CFMs. 52% mentioned those with less effect followed by less price (32%) and different packaging (30%).
In regard to the source of CFMs, 229 (66%) of the respondents reported Egypt as the main origin of CFMs. Also, when the respondents were asked about whom is responsible for CFMs’ availability in Sudan, 329 (85%) mentioned manufacturers followed by distribution companies [259 (67%)] (Table 3).
Measures to reduce CFMs
Most respondents, 316 (82%), reported that counterfeit drugs would be successfully combated through education and sensitizing the public’s awareness (83%) against CFMs. 312 of the respondents (81% of the total responses) reported campaigns conducted by the Ministry of Health for enriching their awareness and 282 (73%) through social media while 229 (59%) believe that the better way to be educated is from their pharmacists through brochures (Table 4). The respondents also reported punishment [155 (40%)] and regulation of medicines entry [180 (54%)] as a major role of the regulatory authorities to combat the CFMs problem.
Using the scoring system, the obtained results indicated a fair level of awareness in 80% of the respondents, while the good and poor levels of awareness were represented by 2% and 18% of the respondents, respectively.
Public views towards CFMs
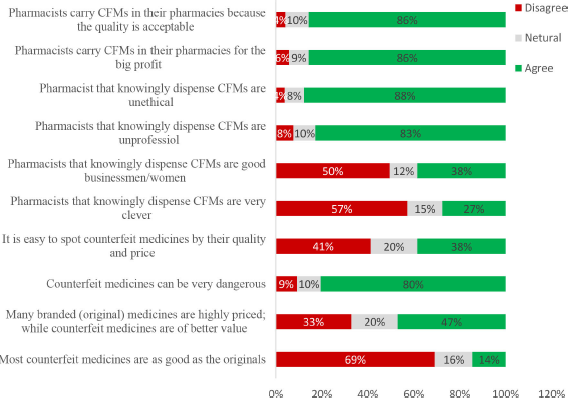
Public attitude was assessed using statements describing the quality, risk, experiences, and CFMs’ price. Obtained data are shown in Figure 1 as “agree,” “neutral,” and “disagree” based on the respondents’ views.
Regarding the overall attitude, a good attitude was found in 68% of the respondents, while poor and fair attitudes were shown in 2% and 30% of the respondents, respectively. Fisher’s exact test revealed no significant relation to the tested variables.
Fisher’s exact test revealed a significant association between the attitude, gender, and TV (p value = 0·011 and 0·003, respectively), while only social media was found to be significantly related to awareness (p value = 0·002). However, sociodemographic characteristics, profession, and education level were not borne out to be significantly associated with the awareness and attitude.
Spearman’s correlation is a bivariate correlation measure suitable for the analysis of data that is not normally distributed. The correlation is measured between −100% and 100%; the negative indicates a negative relationship that is as one increases the other decreases. This test was thus applied to assess the correlation between awareness and attitude. A positive correlation of 16.9% was obtained with statistical significance (p value = 0·001) indicating increasing the awareness will have a positive effect on the attitude.
DISCUSSION
CFMs are a deadly and growing problem. According to the WHO, as many as 1 in 10 medical products circulating in the developing countries is substandard or falsified. The problem is particularly common within Africa. Of all the reported fake drugs to the WHO between 2013 and 2017, 42% of them came from the African region. In 2019, the WHO raised alerts for this problem in Niger, Cameron, Kenya, and Uganda (WHO, 2017). The Food and Drug Administration (FDA) has also developed a reporting system for suspicion about CFMs through MedWatch (FDA, 2019). Although the issue is widely acknowledged, its complexities are poorly understood especially in Africa as no official data is available.
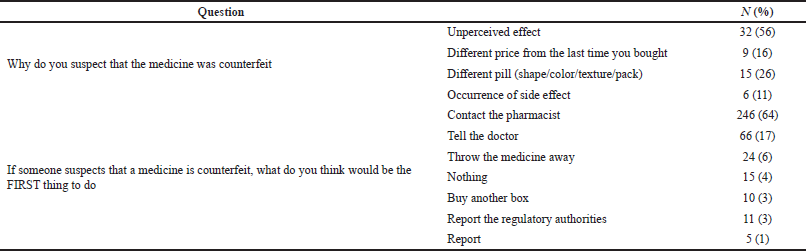 | Table 2. Reason and action toward suspected medicines. [Click here to view] |
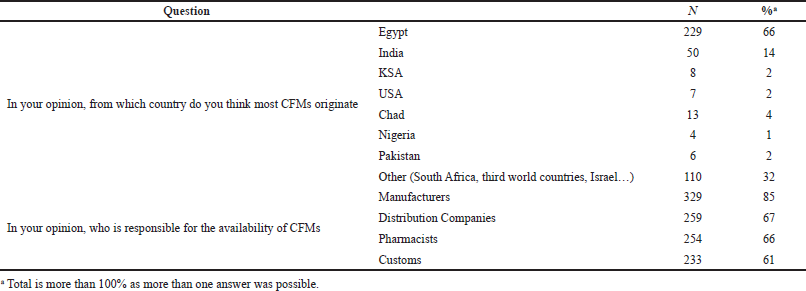 | Table 3. CFMs source and who is responsible for availability. [Click here to view] |
Clearly, there is work to be done from a structural perspective. The WHO is working with the African Union to improve health coverage across Africa. Governments must use the available technology to create more visible supply chains.
To the best of our knowledge, this is the first study to evaluate CFMs in Sudan. The public’s awareness, experience, and view toward CFMs have been explored in Sudan and assessed in order to come up with substantial measures and recommendations to help in combating the widely spread CFMs issue.
In our present study, the mean age of participants was found to be 34 years old, with the acute condition indication reported by a high percentage of respondents rather than chronic indication. Different education levels exist in the sample with the highest percentage for the university level.
The majority of the participants reported that they prefer to buy their medicines from the same pharmacy due to knowledgeable pharmacists and medicines’ availability. It was also indicated that restriction to a certain brand name is attributed to a high perceived effect for medicines.
In regard to the awareness about CFMs, 222 (58%) of the respondents reported that they are aware of the term compared to 93.4% reported in Sholy’s (2018) study. Social media was the most mentioned source for the awareness in contrast to TV in a Lebanese study, considering Egypt and India being the main origin of this problem.
The WHO provided information for consumers when suspecting a medicine is counterfeited to first talk to the pharmacist from whom they bought the medicine or contact the healthcare professional for medical advice (WHO, 2019). An FDA voluntary reporting system named MedWatch is also available for consumers and healthcare professionals (FDA, 2019). In our study, about three-quarters of the respondents reported that the quality of CFMs is less than the authentic ones, which is expected, since CFMs are defined to contain wrong or less active pharmaceutical ingredients than the stated. Different classes of medications are mentioned as a case of suspicion due to the unperceived effect being mentioned as the first reason. Although some of the respondents did nothing or threw the medicine away indicating that they were not educated by a responsible authority on how to deal with suspicion of counterfeit, the majority of them responded in accordance with the WHO guides by either contacting the pharmacists or telling the doctor. The recognition of counterfeit/fake drugs by the public is thus expected to have a great impact on decreasing the purchase of such drugs in the market.
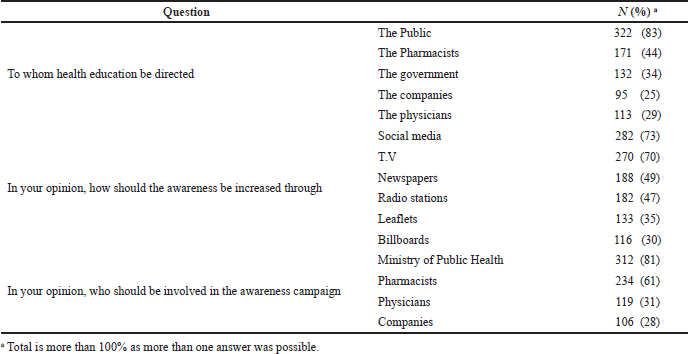 | Table 4. Awareness source and campaigns. [Click here to view] |
 | Figure 1. Respondents’ views and experience toward CFMs (N = 384). [Click here to view] |
A substantial number of respondents reported distinguishing between genuine and CFMs and buying their medicines from trustworthy pharmacists in order to protect themselves. The percentage of respondents who chose “causing side effects” and “producing less effect” as characteristics for CFMs were more than those with “less price” and “different packaging.” This might be due to their lack of knowledge about the packaging materials and their deep concern with medicine’s effect and risk rather than cost and packaging.
One major contributing factor to the prevalence of CFMs in a country is the lack of knowledge and awareness of the society (Alfadl, 2018; Fadlallah, 2016). This was agreed on by 316 (82%) of the respondents suggesting that education will have a vital role in combating the problem. Social media and TV were established as the main sources of information and education which is similar to the Cotonou study that referred to television followed by radio as convincing sources of information (Abdoulaye et al., 2006). Respondents reported that the education should be directed to different parties of the community such as pharmacists, companies, and regulatory authorities and must be carried out by different bodies such as the Ministry of Health through workshops and campaigns conducted at least twice a year.
Moreover, the weak control on chain supply is an additional factor for the spread of CFMs which has been reported by different studies (Marucheck et al., 2011 Taylor, 2011). Respondents believe that manufacturers were responsible for the availability of CFMs.
Thus, counterfeit drugs will be successfully combated through strict regulation and different punishment strategies to handle the offenders (regulation specifically, control of entry port, and QC).
With respect to the attitude of respondents toward pharmacists’ dealing with CFMs, although 38% of them agreed that they are businessmen/women, the majority believed that they are unprofessional and unethical and dealing with CFMs for the profit and easy money. A high percentage of the respondents (86%) agreed that pharmacists carry CFMs in their pharmacies since the quality is acceptable and many branded (original) medicines are highly priced and not available while CFMs are of better value. These results are contrary to their response about the quality of CFMs compared to authentic ones, indicating at this point that respondents’ attitude does not match their awareness. Although different studies reported that people realized that counterfeits were inferior to originals (Commuri, 2009; Matos et al., 2007; Prendergast et al., 2002), their superior prices might compensate for the lower quality and efficacy (Ang et al., 2001). This could be the case for the members of the public with a low socioeconomic status. Alfadl et al. (2012), examined the influence of certain factors on consumers’ behavior regarding CFMs in Sudan and found that motivation and subjective norms were positively and significantly related to the purchase intent of CFM, but not the attitude (Alfadl et al., 2012). Additionally, the findings suggested that Sudanese consumers might be motivated to buy CFMs when medicines are inaccessible and/or unaffordable, and these were considered the main contributors to buying CFMs in developing countries. Therefore, controlling the cost of medicine needs to be considered and evaluated by pharmaceutical companies and health authorities.
38% of the respondents believed it is not easy to spot CFMs by the price and quality. Previous studies reported that patients were confused and unsure if “generic brands” of medicines were in fact counterfeits (d’Astous and Gargouri, 2001). Many studies also reported that lack of patients’ knowledge about CFMs led them to have more negative attitudes toward generic medicines (Bang et al., 2000; Marcketti and Shelley, 2009). Therefore, education and awareness are important as generics of essential medicines have a global public health benefit, because they are less expensive and more accessible to people (Newton et al., 2011). Consequently, respondents of the public awareness survey reported that the best way to avoid buying CFMs was going to a trustworthy pharmacist.
Although there is a positive correlation between awareness and attitude, sociodemographic characteristics, profession, and education level were not borne out to be significantly associated with the awareness and attitude. This result is inconsistent with the Mhando et al. (2016) and Sholy (2018) results, where they reported that education and profession have a significant effect on awareness and attitude (Sholy, 2018; Mhando et al, 2016)
The limitation of the present study is that the population may not appear diverse enough, as respondents were reported to be mainly from Khartoum city. Additionally, the cross-sectional design of the study did not allow generalization of the findings to all community pharmacists in Sudan. Further studies are recommended in other cities and rural areas of Sudan to assess the awareness and attitude toward CFMs. Despite this, our study would be the groundwork for future studies in CFMs as community involvement is a neglected issue in the fight against the illicit trade in counterfeit drugs.
CONCLUSION
The present study concluded that most of the respondents were aware of CFMs with a positive attitude toward them. Social media was reported as the main source of information by the respondents. No significant correlation between variables was reported. Thus, conducting educational campaigns, emphasizing the risks and consequences associated with CFMs, in addition to addressing the public demands for CFMs to strengthen laws and regulation and increase public and other healthcare professionals’ awareness toward CFMs, is highly needed.
ACKNOWLEDGMENTS
The authors acknowledge the Faculty of Mathematical Sciences, University of Khartoum, Sudan, for the sample calculation and statistical analysis. The authors are also thankful to Dr. Bashir Y. for revising the questionnaire and drafts and the surveyors, Mr. Mohamed A., Ms. Roaa E., Mr. Khalid M., Ms. Sara O., Ms. Rana E., Ms. Ayat I., who helped in the data collection and entry. The authors acknowledge Medrxiv.org for publishing the manuscript as “pre-print” with the following link: https://www.medrxiv.org/content/10.1101/2020.09.28.20201400v1
LIST OF ABBREVIATIONS
CFMs Counterfeit medicines
FDA Food and Drug Administration
WHO World Health Organization
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
FUNDING
This research is fully funded by the Ministry of Higher Education and Scientific Research, Sudan, through Grants for Research Projects.
DATA AVAILABILITY
All data supporting this article is available within the article and its supplementary files.
REFERENCES
Abdoulaye I, Chastanier H, Azondekon A, Dansou A, Bruneton C. Evaluation of public awareness campaigns on counterfeit medicines in Cotonou, Benin. Med Trop, 2006; 666:615–18.
Akunyili D. Fake and counterfeit drugs in the health sector: the role of medical doctors. Ann Ibadan Postgrad Med, 2004; 2(2):19–23. CrossRef
Alfadl A, Ibrahim M, Hassali M. Consumer behaviour towards counterfeit drugs in a developing country. J Pharma Health Serv Res, 2012; 3(3):165–72. CrossRef
Alfadl A. General public and community pharmacists perception on counterfeit medicines: a preliminary cross-sectional study in Qatar. J Clin Diag Res. 2018; 12(1):IC01–6. CrossRef
Alsultan M. A descriptive study on medications brought by Pilgrims During Hajj Seasons 2005 and 2006 in Saudi Arabia. World App Sci J, 2010; 10(12):1401–6.
Ang S, Sim Cheng P, Lim E, Kuan Tambyah S. Spot the difference: consumer responses towards counterfeits. J Consum Market, 2001; 18(3):219–35. CrossRef
Bang H, Ellinger A, Hadjimarcou J, Traichal P. Consumer concern, knowledge, belief, and attitude toward renewable energy: An application of the reasoned action theory. Psychol Market, 2000; 17(6):449–68. CrossRef
Bansal D, Malla S, Gudala K, Tiwari P. Anti-counterfeit technologies: a pharmaceutical industry perspective. Sci Pharma, 2013; 81:1–13. CrossRef
Bird R. Counterfeit drugs: a global consumer perspective. Wake Forest Intellect Prop Law J, 2007; 8(3):387–406. CrossRef
Buckley G, Gostin L. Causes of falsified and substandard drugs, 2013. National Academies Press (US); Washington (DC): 2013 May 20 National Academies Press (US); Washington (DC): 2013 May 20
Cockburn R, Newton P, Agyarko E, Akunyili, White N, Counterfeit drugs: threat to public health. Global Forum on Pharmaceutical Anticounterfeiting, Geneva, Switzerland, 2002. CrossRef
Commuri S. The impact of counterfeiting on genuine-item consumers’ brand relationships. J Market, 2009; 73(3):86–98.
d’Astous A, Gargouri E. Consumer evaluations of brand imitations. Eur J Market, 2001; 35(1/2):153–67. CrossRef
Dondorp A, Newton P, Mayxay M, Van Damme W, Smithuis F, Yeung S, White N. Fake antimalarials in Southeast Asia are a major impediment to malaria control: multinational cross-sectional survey on the prevalence of fake antimalarials. Trop Med Int Health, 2004; 9(12):1241–46. CrossRef
European Commission. EudraLex. Good Manufacturing Practice (GMP) Guidelines, Brussels, Belgium, vol 4, 1998.
Fadlallah R. Strategies and Systems-Level Interventions to Combat or Prevent Drug Counterfeiting: A Systematic Review of Evidence Beyond Effectiveness. Pharmaceutical medicine 2016; 5: 263-76. CrossRef
FDA, 2019. Available via https://www.fda.gov/drugs/counterfeit-medicine/what-should-i-do-if-i-believe-i-have-received-or-taken-counterfeit-medicine-information-consumers
Gibson L. Drug regulators study global treaty to tackle counterfeit drugs. BMJ, 2004; 328:486; doi:10.1136/bmj.328.7438.486 CrossRef
Koh R, Schuster E, Chackrabarti I, Bellman A. Securing the pharmaceutical supply chain, White Paper, Auto-ID Labs, Massachusetts Institute of Technology, Cambridge, MA, 2003.
Lai Y, Zaichkowsky L. Brand imitation: do the Chinese have different views? Asia Pac J Manag, 1999; 16(2):179–92
Marcketti S, Shelley M C. Consumer concern, knowledge and attitude towards counterfeit apparel products. Int J Consum Stud, 2009; 33(3):327–37. CrossRef
Ann Marucheck, Noel Greis, Carlos Mena, Linning Cai, et al. Mhando L, Jande MB, Liwa A, Mwita S, Marwa KJ Product safety and security in the global supply chain: issues, challenges and research opportunities. J Oper Manag, 2011; 297(2016):707–20. CrossRef
Matos C, Ituassu C, Rossi C. Consumer attitudes toward counterfeits: a review and extension. J Consum Market 2007; 24(1):36–47. CrossRef
Mhando L, Jande MB, Liwa A, Mwita S, Marwa KJ. Public awareness and identification of counterfeit drugs in tanzania: a view on antimalarial drugs. Adv Public Health, 2016; 8.
Mullaicharam A. Counterfeit herbal medicine. Int J Nutr Pharmacol Neurol Dis, 2011; 2:97–102. CrossRef
Nayyar G, Breman J, Newton P, Herrington J. Poor-quality antimalarial drugs in southeast Asia and sub-Saharan Africa. Lancet Infect Dis, 2012; 12:488–9. CrossRef
Newton P, Amin A, Bird C, Passmore P, Dukes G, Tomson G, White N. The primacy of public health considerations in defining poor quality medicines. PLoS Med, 2011; 8(12):e1001139. CrossRef
Noun M, Nasr L, Khan I, Arafat B, Assi S. Knowledge and perspectives of the public towards the prevalence and harm associated with counterfeit medicines in Lebanon. Emerg Trends Drug Addict Health. 2021; 1:100019. CrossRef
Pincock S. WHO tries to tackle problem of counterfeit medicines in Asia. BMJ, 2003; 327:1126. CrossRef
Prendergast G, Chuen L, Phau I. Understanding consumer demand for non-deceptive pirated brands. Market Intell Plann, 2002; 20(7):405–16. CrossRef
Sharon L. Sampling design and analysis. 2nd edition, Chapman and Hall/CRC Press, Boca Raton, FL, 2010.
Sholy L. Public awareness, experiences and views about counterfeit medicines in Lebanon. Journal of pharmaceutical health Services Research Volume9, Issue2, June 2018 Pages 161-169. CrossRef
Siva N. Tackling the booming trade in counterfeit drugs. Lancet, 2010; 376:1725–26. CrossRef
Tansey O. Process tracing and elite interviewing: a case for non-probability sampling. PS Polit Sci Polit, 2007; 40:765–72. CrossRef
Taylor P. UK gang jailed for counterfeit medicines plot. Secur Pharma, 2011. Available via http://www.securingpharma.com/uk-gang-jailed-for-counterfeit-medicines-plot/s40/a869/ (Accessed 12 April 2015).
WHO, 2019. Available via https://ww.who.int/news-room/fact-sheets/details/substandard-and-falsified-medical-products
WHO. Counterfeit and sub-standard drugs in Myanmar and Vietnam. WHO, Geneva, Switzerland, 1999
World Health Organization. Counterfeit drug reports: 1999-October 2000, 2000. Available via www.who.int/medicines /services/counterfeit/overview/en/1
World Health Organization. How big is the problem of counterfeit medicines? 2017. Available via http://www.who.int/medicines/services/counterfeit/faqs/12/en/index.htm
WHO. Medicines. General information on counterfeit medicines. Available via http://www.who.int/medicines/services/counterfeit/overview/en/.
World Health Organization. Summary of WHO counterfeit drug database as of April 1999, unpublished paper of the WHO Division of Drug Management and Policies. WHO, Geneva, Switzerland, 1999.
World Health Organization. 2014. What encourages counterfeiting of drugs? 2014. Available via http://www.who.int/medicines/services/counterfeit/faqs/15/en/.