INTRODUCTION
Essential oils are commonly used in various industrial sectors such as the food, chemical, cosmetics, and pharmaceutical industries. The high economic value of essential oils has led to the emergence of various strategies to enhance the yield and production process of essential oils by developing new plant cultivars and optimizing techniques, including variations of different plant treatments (Haghighi et al., 2017; Lourenço et al., 2019). In these conditions, the chemical profile of essential oils usually deviates from the standard product quality and develops sustainable quality control methods (Xiong et al., 2019).
Quality control is crucial in the production process before the product is marketed to the general public. All commercial products must pass an industry-mandated quality control test. Due to the content of biosynthetic conditions and the complexity of essential oils, quality control has become highly challenging. Among many essential oil-producing plants, the most well-known plant from the Myrtaceae family (Myrtle family), which consists of 140 genera and about 3,500–5,800 species, is scattered in tropical and subtropical areas (Farag et al., 2018; Frauches et al., 2016; Johnson and Briggs, 1984; Wilson et al., 2001). Some studies have proven that myrtle family extract helps treat gastroenteritis, dysentery, and diabetes (Azevedo et al., 2012). Essential oils from the myrtle family, such as myrtle, guava, cloves, Eucalyptus, and tea tree, are used for medical and commercial purposes (Siddique et al., 2017). The Myrtaceae genera discussed in this review include Eucalyptus, Eugenia, Melaleuca, and Syzygium. The analysis of this essential oil information, both in terms of characterization of the classification model based on chemical composition and other information obtained from the analysis, can control the quality of essential oils in similar studies.
Gas chromatography (GC) combined with mass spectroscopy (MS) or flame ionization detector (FID) is a standard method and is often used to analyze the chemical composition of essential oils from natural products, mainly plants (Farag et al., 2018; Khanh et al., 2020). However, this method has feebleness, namely, the use of large amounts of organic solvents, sample preparation, and analysis time, requiring a relatively long time and high temperatures to cause sample damage (Shi et al., 2020). Recently, vibrational spectroscopy methods have been developed using infrared (IR) spectroscopy, which is more efficient (Baranska et al., 2005, 2006; Schulz et al., 2004, 2005; Schulz and Baranska, 2007; Wang and Sung, 2011).
IR spectroscopy has been successfully used in analyzing the authenticity of herbal medicines (Laasonen et al., 2002), detecting impurities or adding chemicals to the sample (adulteration context) (Dong et al., 2012), measuring a single sample (Schulz and Baranska, 2007), and sample mix (Chan et al., 2007; Escamilla et al., 2013) in the plant matrix. Fourier Transform Infrared Spectroscopy (FTIR) is a measurement technique by collecting IR spectra formed due to vibrations in the atoms in a molecule. In spectrum measurement using FTIR spectroscopy, several techniques for measuring samples are standard: Attenuated Total Reflectance (ATR) (Beasley et al., 2014).
ATR is a sampling technique that is connected to the FTIR device. Today, in many fields, ATR-FTIR spectroscopy is preferred over transmission FTIR spectroscopy (Manheim et al., 2016; Özgenç et al., 2017; Parhizkar et al., 2017; Peets et al., 2017). The ATR-FTIR technique allows a sample to be directly used in solid and liquid form, so there is no need to do sample preparation in advance, and it is a fast technique in the initial steps to characterize samples or materials. Other advantages are that it is easy to prepare samples, there is no need to grind potassium bromide (KBr), a broader spectrum of variation, and negligible difference in particle size (Thompson et al., 2009). Therefore, ATR-FTIR can be an effective, fast, simple, reliable, sensitive, and nondestructive method for identifying and also analyzing essential oils and analyzing physical and chemical parameters in quality control (Bittner et al., 2016).
The absorption spectra of the molecules in the sample usually overlap as spectroscopy presents the output data (IR spectrum) in high dimensional (HD) space. This condition makes it harder for visual and direct interpretation. Hence, the chemometric method is used to facilitate the data interpretation process. In the last few decades, chemometrics has been successfully applied in building regression or classification models using HD data, including the IR spectrum (Gad et al., 2013; Muro et al., 2015). The addition of chemometric techniques to the analysis of the spectrum has been proven effective in displaying specific differences between plant chemotypes (Baranska et al., 2005; Schulz et al., 2004; Schulz and Baranska, 2007). Combining ATR-FTIR spectroscopy with chemometric methods is widely used to classify and quantify various science and technology fields (Stöbener et al., 2019).
This narrative review aims to investigate the role and use of ATR-FTIR and chemometric methods as a rapid analysis for essential oils from Myrtaceae plants. This article provides a comprehensive overview of various literature on FTIR, ATR-FTIR, chemometrics, analysis of essential oils, essential oils from Myrtaceae, application of ATR-FTIR, and chemometrics in essential oils from Myrtaceae plants, and their prospects.
METHODS
This current narrative review of the search literature for this study was accomplished from January to October 2021 using electronic databases such as Scopus, PubMed, Web of Science, Directory Open Access Journal, and Google Scholar. During the literature search, the following keywords were used: “Myrtaceae Plants,” “Essential Oil from Myrtaceae,” “ATR-FTIR Spectroscopy,” and “Chemometric Analysis.”
Fourier Transform Infrared Spectroscopy (FTIR)
IR spectroscopy is a technique that uses the interaction between electromagnetic radiation in the IR region to investigate the scattering, reflection, absorption, or transmission of IR radiation that occurs during the interaction process. From the results of the IR absorbance of a sample, researchers can determine the structure and chemical information (Glassford et al., 2013). IR spectroscopy has been used successfully in authenticating herbal medicines (Laasonen et al., 2002), detecting impurities or adding chemicals to the sample (adulteration context) (Dong et al., 2012), and measuring both a single sample (Schulz and Baranska, 2007) and sample mix (Chan et al., 2007; Escamilla et al., 2013) in the plant matrix.
The IR region is divided into three wavenumber regions: far-IR at 400–10 cm−1, mid-IR at 4,000–400 cm−1, and near-IR at 14,285–4,000 cm−1. This regional difference varies depending on the type of instrumentation used to measure the IR spectrum and depends on the radiation nature (Lin et al., 2009). There are two instruments, namely, the dispersive IR spectroscopy and FTIR instrument. However, dispersive IR spectroscopy has limitations in sample handling and the inability to combine with precise spectral readings and processing to provide valuable quantitative information (Rohman, 2019). FTIR spectroscopy has become more commonly used to replace IR-dispersive instruments' limitations by providing higher energy yields, significantly scanning speeds, multiplexing capabilities, increasing accuracy, and stabilizing wavelength. FTIR spectroscopy is emerging as a technique for identifying, confirming, and quantitative analyzing (van de Voort et al., 2008).
FTIR is a measurement technique by collecting IR spectra formed due to periodic vibrations of the atoms in a molecule. The principle of this technique is to shoot a beam of IR radiation at a sample so that the molecules in the sample absorb energy and vibration occurs. The absorption of this energy can be captured or detected at various wavelengths or frequencies. The difference in the absorbance patterns absorbed by each compound causes the compounds to be quantified and differentiated (Karoui, 2018; Sankari et al., 2010).
FTIR uses an interferometer to separate wavelengths (Karoui, 2018). The most commonly used interferometer is the Michelson interferometer (Karoui et al., 2008). The detector used in FTIR is made of materials that can receive high-speed signals, such as a mercury cadmium telluric detector or a pyroelectric lithium tantalate detector (LiTaO3). There are two kinds of optical configurations in FTIR, namely, single beam and double beam. FTIR spectroscopy reading begins when the energy released from the source passes through the interferometer before the energy passes through the sample, then proceeds to the detector, computer, and reading section. The interferometer radiation source will be divided half by the beam splitter towards the stationary mirror and the mobile mirror, which constantly moves (Karoui, 2018; Karoui et al., 2008). The beam splitter is usually made of KBr coated with germanium (Ge) between the mirrors (Karoui et al., 2008). The beam splitter reunites the two separated lights (Karoui, 2018), producing a constructive/destructive interference pattern caused by the variance between the two beam components' distance traveled. The part of the light that comes back together then reaches the detector (Ismail et al., 1997; Prieto et al., 2017).
In spectrum measurement using FTIR spectroscopy, there are three techniques for measuring samples that are commonly used, namely, ATR, Diffuse Reflectance Infrared Fourier Transform, and Photo Acoustic Spectroscopy. Each of these techniques has different characteristics in the molecular vibration spectrum (Beasley et al., 2014).
Attenuated Total Reflectance-Fourier Transform Infrared Spectroscopy (ATR-FTIR)
ATR is a relatively fast technique in the initial steps of the characterization of samples. Other advantages are that it is easy to prepare samples, there is no need for KBr grinding, a broader spectrum variation, and a negligible difference in particle size (Thompson et al., 2009). The ATR principle is based on the phenomenon of total internal reflection. It measures the changes in IR reflected light internally in interaction with the sample via zinc selenium (ZnSe), crystals, or diamonds. When the sample is placed on the ATR crystal, the sample will absorb energy so that the result is an attenuated IR wave (Gredilla et al., 2016).
ATR-FTIR spectroscopy involves directing the IR light at the interface between IR transparent materials with a high refractive index called the internal reflex element (IRE), for example, a prism of ZnSe, crystal, Ge, or silicon material, and the sample on the IRE. The angle of incidence of the IR beam is greater than the critical angle, which causes total internal reflection to occur. On the reflected surface, evanescent waves are formed, and these waves interact with the sample, attenuating the IR rays coming out of the IRE (Glassford et al., 2013). Vibrational spectroscopy methods such as ATR-IR are fast, noninvasive, and sensitive methods for analyzing physical and chemical parameters in quality control (Bittner et al., 2016). Besides, ATR-FTIR spectroscopy also offers high-quality characteristic information and is relatively inexpensive. The sample used does not require initial preparation, and it is also possible to carry out semiquantitative analysis, apart from qualitative analysis (Bunaciu and Aboul-enein, 2021; Kucharska-ambro?ej and Karpinska, 2020; Peets et al., 2017).
Chemometrics
Spectral overlap in the spectroscopic analysis becomes very likely to happen, so it is necessary to develop a method that involves recognizing a pattern as a filter to identify the part of the spectrum of the sample under study (Nikzad-langerodi et al., 2017). The chemometric method is a practical qualitative and quantitative analysis (Mazivila and Olivieri, 2018). Chemometrics is a mathematical and statistical application in chemical data processing (Rohman, 2019). This method aims to produce valuable information from the data set using optimal sample measurement techniques and produce chemical information from the sample data (Kumar and Sharma, 2018).
The advantage of using chemometrics in the analysis is its ability to analyze multivariate data produced from measuring several variables in the same sample. This data is called multivariate data (Guillén and Cabo, 1997). Advances in chemometric methods can be used to analyze large amounts of complex sample data. In addition, this method also produces significant, accurate, and short-term data (Kumar and Sharma, 2018). Chemometric analysis methods are divided into supervised and unsupervised methods.
Supervised methods
The supervised pattern recognition method has been extensively used in analyzing data with different applications for purposes such as classification, discrimination (differentiating), individualization, and impurity detection (Kumar and Sharma, 2018). The analysis using this method uses prior knowledge-based machine reading (Li et al., 2020). A model is formed based on a sample from a known class. This model can then predict the class of previously unknown samples (Kumar and Sharma, 2018). The objectives of the supervised method can be divided into two; classification and regression.
For classification purposes, sample fingerprint analysis is often performed for qualitative purposes such as differentiation, recognition, and traceability. This classification can be separated based on different approaches: the analysis of discrimination (differences) between classes and class modeling analysis (Oliveri, 2017). Discriminant/difference analysis (“hard modeling”) focuses on problems in many classes (multiclass), which includes Partial Least Squares-Discriminant Analysis (PLS-DA), Linear DA, and k-nearest neighbor (Kumar and Sharma, 2018). For example, the problem includes differences in plant origin, geographic origin, and time of growth (Li et al., 2020).
Class modeling analysis (soft modeling) focuses on one class's problems, and only that class is concerned. For example, in the problem of determining the authenticity and recognition of herbal medicine “A” (target sample), the data set containing fake herbal medicine “A” or samples other than herbal medicine “A” becomes a nontarget sample, preventing it from being analyzed on a regular and comprehensive basis. This condition causes the sample to fail in forming a class. Herbal medicine “A” is the target class in in-class modeling, and herbal medicine “A” that contains counterfeit ingredients or herbal medicine other than herbal medicine “A” is a separate heterogeneous group of the target class (Li et al., 2020).
One example of a class modeling technique is the Soft Independent Modeling Class Analogy (SIMCA) (Kumar and Sharma, 2018). SIMCA combination or modification methods where the method approach is limited to those listed above because it is adjusted to the research needs (Kucharska-ambro?ej and Karpinska, 2020).
For regression analysis purposes, it is generally carried out depending on the properties of the information obtained from the sample fingerprints. Regression techniques that are often used are Partial Least Square Regression, Support Vector Regression, and Artificial Neural Network. In vibrational spectroscopy, quantitative analysis cannot be carried out immediately. However, with multivariate calibration applications, data from vibrational spectroscopy can be indirectly used to measure certain substances (Li et al., 2020). Hence, this regression analysis can be used for rapid screening and quantitative purposes (Ma et al., 2018a, 2018b).
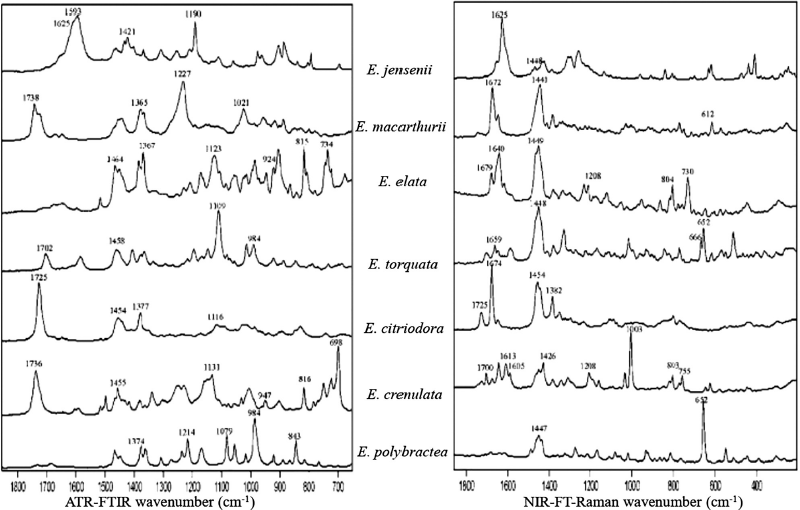 | Figure 1. Essential oils spectra from the leaves of different Eucalyptus species using ATR-FTIR and NIR-FT-Raman spectroscopy (Baranska et al., 2005). [Click here to view] |
Unsupervised methods
The unsupervised method is divided into exploratory analysis and similarity analysis (SA) methods (Kharbach et al., 2020). Exploratory analysis extracts the main fingerprint characteristics and contributes to an unbiased view of the data set. This method can obtain information on cluster trends between groups, the relationship between variables, variables, and the sample (Callao and Ruisanchez, 2018; Yi et al., 2016). The relationship between variables can explain which variables can complement or provide similar information and which variables are important in group differentiation (Callao and Ruisanchez, 2018).
In the exploratory analysis method, the principal component analysis (PCA) and hierarchical cluster analysis (HCA) methods are often used to analyze chemical fingerprints, and the combination of these two methods can complement each other (Kumar and Sharma, 2018; Li et al., 2020). The HCA is an analysis method that produces a cluster hierarchy to classify samples based on the similarity of each fingerprint (Kumar and Sharma, 2018; Li et al., 2018, 2020). The HCA method can be visualized as a dendrogram, a tree diagram showing the sequence of merging or splitting due to differences (Li et al., 2020). The HCA method is used for exploration purposes, especially identifying similarities and differences in the samples under study, which reduces the data dimension and draws the dominant pattern in the complex matrix (Mazivila and Olivieri, 2018). This method is frequently used to investigate the similarity of fingerprints from different plants. Whereas in SA, the similarity parameters are statistically measured to see the similarity or dissimilarity of the various fingerprints obtained (Dai et al., 2019; Kharbach et al., 2020; Zhang et al., 2019; Zhang and Sun, 2019).
Essential oils analysis
Essential oils, also known as aromatic plant essences, are volatile oils responsible for the flavor and aroma of organic materials (Amorati et al., 2013). All plant organs, including buds, flowers, bark or wood, roots, fruits, leaves, twigs, stems, and seeds, synthesize them and store them in epidermic cells, secretory cells, cavities, canals, or glandular trichomes (Bakkali et al., 2008).
Essential oils have a long history of use, which has resulted in a slew of quality control measures. The chromatographic method is frequently used to determine essential oils. Standard methods used to analyze and determine the components of essential oils from plants are GC-FID or GC-MS (d’Acampora Zellner et al., 2010; Farag et al., 2018). This method has the advantages of high accuracy, specificity, and sensitivity. However, unfortunately, it requires an extended analysis time (about 60–90 minutes) (Freitas et al., 2018), a long sample preparation time, large amounts of organic solvents, and high temperatures that impact sample damage (Shi et al., 2020). The addition of chemometric techniques to the analysis of the spectrum effectively displays specific differences between plant chemotypes (Baranska et al., 2005; Schulz et al., 2004; Schulz and Baranska, 2007). Some studies have successfully reported the analysis of essential oils using the GC-MS/GC-FID method with and without chemometric combination, as seen in Table 1.
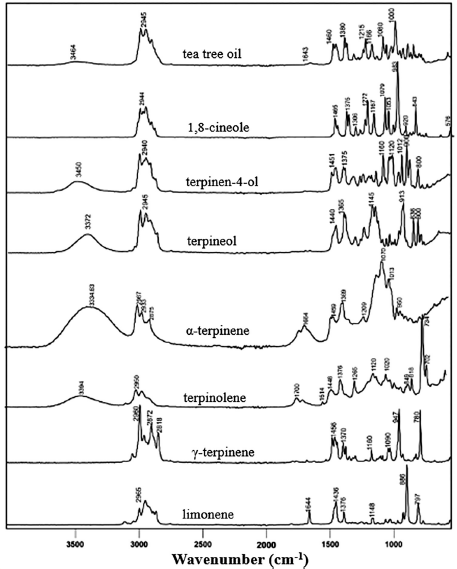 | Figure 2. The ATR-FTIR with MIR spectra of the major compounds and a typical tea tree oil from M. alternifolia (Tankeu et al., 2014). [Click here to view] |
Using the MCR-ALS chemometric technique, the chromatogram peaks of the highly overlapping T. vulgaris (A) and T. serpyllum (B) can be analyzed. The MCR-ALS algorithm can separate the coeluted peaks of groups A and B into pure chromatograms and mass spectra using the MCR-ALS algorithm. A quantitative analysis of the peaks eluted together is impossible without chemometric techniques. The PCA technique was used to identify intrinsic components that play a role in differentiating T. vulgaris and T. serpyllum after the MCR-ALS results were obtained (Asadollahi-Baboli et al., 2015).
The spectrum data obtained from the P. sordidum plant was analyzed using a combination of PCA and CA. Both methods aid in reducing multivariate data and in the presentation of the results. The data for diagram formation is generated using the PCA technique, which plots both the oil sample (an object) and the oil content (variable). At the same time, the CA provides data for the classification tree from which the sample locations (objects) are gathered. By combining these two, researchers determined the relationship between the three P. sordidum populations studied and the essential oil composition that resulted (Brunnel et al., 2016).
The HCA method is a technique that can be used to classify samples (variables) based on the distance between the relationships formed. Samples of C. japonicum and C. setosum, divided into 14 specimens, divided the two plants into four clusters, in which clusters four samples were grouped because they had a similar aroma (Zeng et al., 2016).
The SA technique is a sample analysis technique based on the similarity between each sample and using the correlation coefficient. The SA results in analyzing P. cablin showed that samples taken from Hainan province differed from those from Guangdong and Guangxi provinces. Furthermore, the HCA technique in classifying P. cablin divides the sample into three main clusters based on 12 characteristic peaks from the GC-FID profiles (Yang et al., 2016).
The combination of PCA and HCA is used to classify various Nigella clan species from several regions. The HCA technique shows two clusters, denoted as clusters 1a and 1b. Cluster 1a grouped all N. sativa from different regions into one group. Meanwhile, cluster 1b groups other Nigella species apart from N. sativa. The PCA technique is an additional multivariate tool to understand heterogeneity among different Nigella (different species and regions of origin). The OPLS-DA technique was used to determine whether N. sativa and N. damascena essential oils' profiles are uniquely identified as markers for each species (Farag et al., 2018).
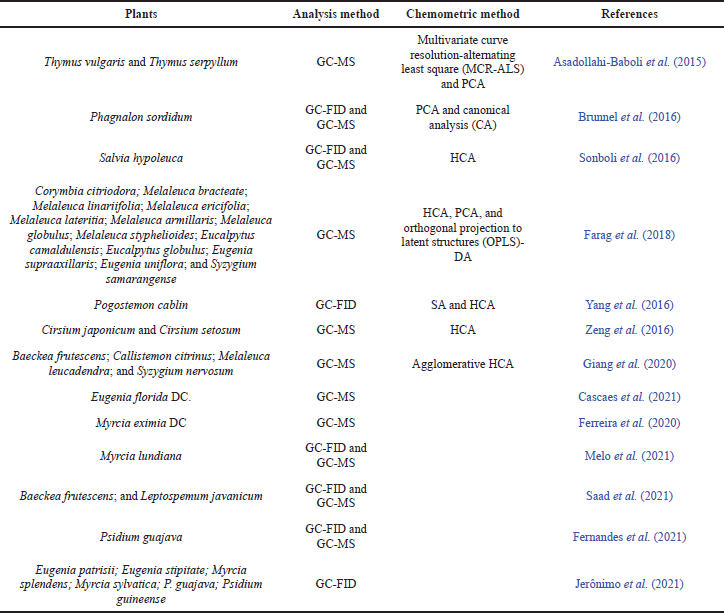 | Table 1. Research on essential oil analysis from Myrtaceae plants using the GC-MS/GC-FID method and chemometric combination. [Click here to view] |
Essential oils from Myrtaceae family
Myrtaceae is a plant family with a wide range of essential oils (Siddique et al., 2020). The Myrtaceae family includes over 140 genera and about 3,500–5,800 species (Frauches et al., 2016). The species from this family are aromatic plants with great agroindustrial potential (Sardi et al., 2017) and are distributed in Australia, Africa, South America, India, Southeast Asia, and other Pacific islands (Thornhill et al., 2015). The Myrtle family consists of various genera, including Eucalyptus, Melaleuca, Eugenia, and Syzygium.
The genus Eucalyptus consists of about 900 species and subspecies (Brooker and Kleining, 2004; Gilles et al., 2010). Eucalyptus originates from Australia and Indonesia and has spread worldwide (Hamdy et al., 2007; Payn et al., 2008). This clan is one of three similar genera called “eucalypts,” including Corymbia and Angophora (Farag et al., 2018). Eucalyptus has been widely used for many purposes and has recently grown worldwide in warm temperatures (Tavares et al., 2019). The essential oil extracted from this plant exhibits antifungal, antibacterial, antiseptic, antioxidant, expectorant, anticancer, and anti-inflammatory properties (Frauches et al., 2016; Ghaffar et al., 2015; Paosen et al., 2017; Sebei et al., 2015; Sharifi-Rad et al., 2017b). In addition, this oil has been traditionally used for treating respiratory ailments, such as nasal congestion, influenza, and the common cold (Sebei et al., 2015; Sharifi-Rad et al., 2017b). The main constituent of Eucalyptus oil is 1,8-cineole (cineole or eucalyptol) (Mohamed et al., 2015; Sebei et al., 2015).
The genus Eugenia is the largest genus in the myrtle family, which includes more than 1,058 species distributed in tropical and subtropical areas (Farag et al., 2018). Consumption of Eugenia fruit is highly recommended because it is rich in nutrients important for health (Seraglio et al., 2018). The fruit is rich in minerals, vitamins, carotene, anthocyanins, phenolic compounds, sugar, and fiber (de Souza et al., 2018; Santos et al., 2015). The bioactive components have properties like antioxidants, antihypertensive, antidiarrheal, anti-inflammatory, antimicrobial, antifungal, antidiabetic, antigenotoxic, antihyperglycemic, antitumor, antimutagenic, antinociceptive, and gastroprotective (da Silva et al., 2018; Dametto et al., 2017; de Souza et al., 2018; Han and Parker, 2017; Moura and Franzener, 2018; Ullah et al., 2018). The main components of essential oils from this family are α-pinene and β-caryophyllene (Agredo, 2017; Mesquita et al., 2017; Pereira et al., 2017). In contrast to other species of this genus, E. uniflora contains selina-1,3,7(11)-trien-8-one and selina-1,3,7(11)-trien-8-one epoxide as the main components in its oil, which has antifungal activity (dos Santos et al., 2018).
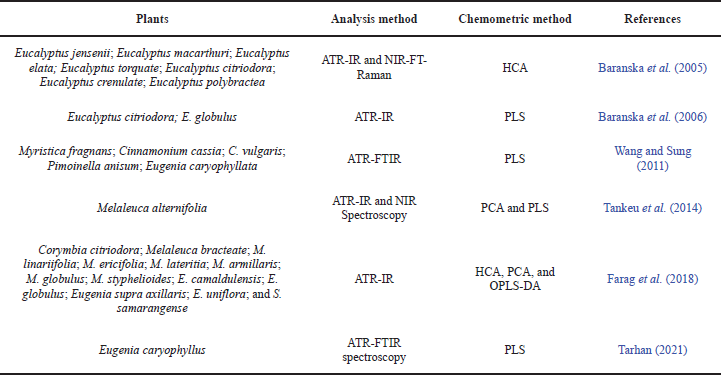 | Table 2. Research on essential oil analysis from Myrtaceae plants using the ATR-FTIR/FTIR-chemometric method. [Click here to view] |
The genus Melaleuca consists of 260 species spread in Australia and Southeast Asia, the Caribbean, and the southern part of the United States (Tran et al., 2013). The essential oil from this family exhibits anti-inflammatory, antibacterial, insecticidal, fungicidal, antiviral, and antioxidant properties (Baldissera et al., 2016; Ebani et al., 2018; Hammer, 2015; Louhibi et al., 2015; Osunsanmi et al., 2016; Padalia et al., 2015; Sharifi-Rad et al., 2017a; Siddique et al., 2015). Research on the chemical composition of the essential oil of this genus has previously been reported, which is 1,8-cineole; eugenol methyl ether; α-pinene; terpinene-4-ol; α-terpinene; terpinolene; and caryophyllene oxide, which is the main component in most of the essential oils of various species in this family (Albouchi et al., 2017; Fall et al., 2017; Kong et al., 2020; Siddique et al., 2017, 2020).
The genus Syzygium includes approximately 1,139 species scattered in tropical and subtropical areas, many of which are medicinal plants in Southeast Asia (Li et al., 2015). This clan belonged to the Eugenia clan until 1972. It was proven that the Syzygium and Eugenia clans were two different genera, seen from the analysis of anatomical data and confirmed by their molecular research (Byng et al., 2015). The extract obtained from this clan has antibacterial, antiviral, antiprotozoal, antifungal, anti-inflammatory, antioxidant, anticancer, and antidiabetic activity (Byng et al., 2015; Ranghoo-Sanmukhiya et al., 2019; Rocchetti et al., 2019; Syama et al., 2018). This genus contains α-pinene, γ-terpinene, caryophyllene oxide, and β-caryophyllene as the main components (Gao et al., 2012; Lee et al., 2016).
Application of ATR-FTIR and chemometric in essential oil from Myrtaceae plants
The ATR-FTIR spectra can be used to identify the functional groups of the molecules in the samples. The basis for the analysis of essential oils lies in the main components contained in the Myrtaceae plants. The spectrum of terpenoid compounds is the main constituent component of essential oils. The spectrum analysis of terpenoid compounds is based on their vibrational spectrum. IR spectra of samples of commercially available terpenoids present in larger quantities of essential oils were recorded in the IR fingerprint region between 1,800 and 400 cm−1. In the IR region, unique and reproducible spectral information is available for compound identification. Some studies have successfully reported the analysis of essential oils using the ATR-FTIR/FTIR-chemometric method, as shown in Table 2.
Baranska et al. (2005) successfully analyzed essential oils of the Myrtaceae family from the Eucalyptus using ATR-FTIR and NIR-FT-Raman spectroscopy and HCA chemometric methods. The combination of the two provided rapid results in chemotaxonomic characterization. Mid-IR is used for wavenumbers between 650 and 4,000 cm−1, while HCA is used for wavenumbers 1,400–1,750 cm−1 (Baranska et al., 2005). The main components of essential oils are recognizable by both vibrational spectroscopy techniques based on the spectral information of pure terpenoids. The spectroscopic analysis is based on straps of individual volatiles, so it is possible to distinguish between different essential oil profiles of several Eucalyptus species, as shown in Figure 1. The presented spectroscopic data has been shown to correlate with the data obtained by GC analysis (Baranska et al., 2005).
Baranska et al. (2006) also analyzed commercially available terpenoid compounds present in Eucalyptus essential oil in larger quantities. Citronellal, β-citronellol, and 1,8-cineole were the main components found in Eucalyptus oil. Citronellal and citronellol dominated the IR spectrum of E. citriodora oil, while 1,8-Cineole dominated the IR spectrum of E. globulus oil. The difference between E. globulus oil obtained from China and Australia can only be seen through a visual spectrum examination due to the slight difference in the two compositions. The HCA chemometric method was used to find specific spectral variations between E. globulus oil obtained from China and Australia. The spectra collection of E. citriodora oil was classified because it contained a large amount of 1.8 cineole (Baranska et al., 2006).
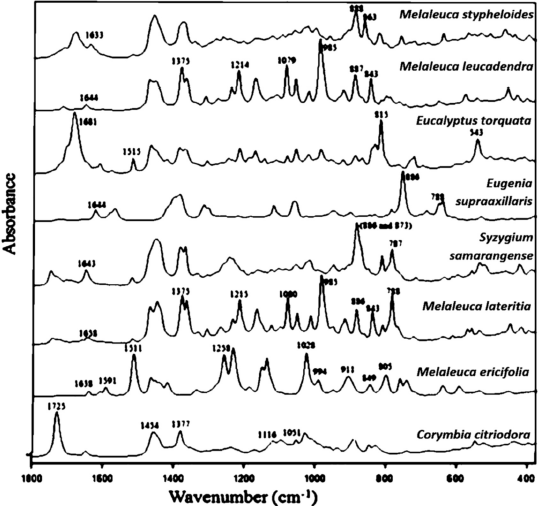 | Figure 3. ATR-FTIR spectra of essential oils from the Myrtaceae family (Farag et al., 2018). [Click here to view] |
Tankeu et al. (2014) successfully analyzed tea tree oil from M. alternifolia using ATR-FTIR spectroscopy in the mid-IR area with a wave number of 4,000–550 cm−1 and in the near-IR area with a wave number of 10,000–4,000 cm−1. This study used samples of tea tree oil products from various cultivation sites in South Africa. The main components of M. alternifolia oil were 1,8-cineole; α-terpineol; terpinene-4-ol; α-terpinene; γ-terpinene; terpinolene; and limonene. The IR spectrum of the tea tree oil (product sample) indicated a slight deviation in the absorbance of the product sample component and the pure sample component, as shown in Figure 2. This was most likely due to the presence of other components in the sample.
Analysis using the PCA and PLS chemometric methods was also carried out. A PCA was performed on centralized spectral data to identify clusters and trends in the data. A PLS regression analysis was carried out on mid-IR and near-IR spectra data to develop a calibration model based on GC quantitative reference values for selected essential oil content. The GC-MS/FID analysis method was also compared. This regression model successfully predicted the contents of an unknown tea tree oil sample's seven main components. Mid-IR data provided better results when compared to near-IR in terms of sample prediction. The mid-IR data results in the calibration model were more similar to the GC reference values than the near-IR data results (Tankeu et al., 2014).
Farag et al. (2018) has also successfully analyzed essential oils from the Myrtaceae family using ATR-FTIR spectroscopy and chemometric methods. The initial step was to isolate the essential oils contained in the leaves of 14 species of the Myrtaceae family (Melaleuca, Eucalyptus, Syzygium, and Eugenia) using the hydrodistillation method. The isolated essential oils were then measured using ATR-FTIR spectroscopy in the mid-IR region between 375 and 4,000 cm−1. The results of the IR spectrum obtained were then further processed using HCA, which was carried out in the range of wave numbers 3,651–2,645 and 1,800–375 cm−1. The ATR-FTIR spectroscopy analysis obtained various spectra of essential oils from the Myrtaceae family (w) (Farag et al., 2018).
Another study conducted by Tarhan (2021) succeeded in analyzing eugenol, eugenyl acetate, and caryophyllene inside clove essential oil (E. caryophyllus) using ATR-FTIR and quantifying it using PLS. The main components of essential oils were recognized by both vibrational spectroscopy techniques based on the spectral information of pure terpenoids. The spectroscopic analysis was based on straps of individual volatiles, so it was possible to distinguish between different essential oil profiles of several Eucalyptus species. The presented spectroscopic data has been shown to correlate with the data obtained by GC analysis. The detailed spectral analysis of the examined oils was based on their vibrational spectra. The functional group of eugenol contains a double bond and a strong band, as shown in the 1,640–1,500 cm−1. Other characteristic peaks of eugenol at 1,637 and 1,610 cm−1 were due to the C = C expansion and contraction of the aromatic moiety. Peaks were seen at 1,430, 1,264, 1,233, 1,204, 1,119, 912, 816, and 794 cm−1. This condition might be due to CH2 deformation vibration, expansion, and contraction vibration of the CO bond of the hydroxyl-bonded carbon. Ether and alcohol group C–O vibration, C–C stretching vibration, N–CH2–OCH3, C–H vibration, CH2 and C–H ring deformation, and coupling vibration-caryophyllene exhibited high intensity in the spectral range of 3,000–2,800 cm−1 due to the aliphatic peak (Tarhan, 2021).
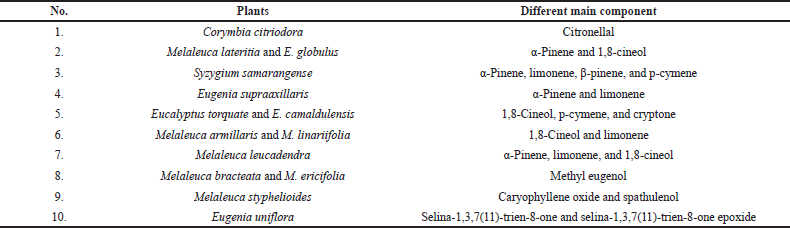 | Table 3. The different main components of essential oils from some plants of the Myrtaceae family (Farag et al., 2018). [Click here to view] |
This obtained spectrum showed several wavenumber spectra that can be used to differentiate between citronellol (1,454, 1,377, and 1,051 cm−1); citronellal (1,725, 1,454, 1,377, and 1,116 cm−1); methyl eugenol (1,638, 1,606, 1,591, 1,511, 1,258, 1,028, 994, 911, 849, and 805 cm−1); α-pinene (1,658, 886, and 788 cm−1); β-pinene (1,641 and 873 cm−1); limonene (1,644 and 886 cm−1); 1,8-cineole (1,375, 1,214,1,079, 983, and 843 cm−1); p-cymene (1,515, 813, and 541cm−1); and caryophyllene oxide (1,626, 1,457, 1,365, 888, and 866 cm−1) (Farag et al., 2018). These wavenumber spectra indicated conformity with those reported in previous publications regarding the interpretation of essential oils' vibrational spectrum isolated from Myrtaceae (Baranska et al., 2005; Wang and Sung, 2011). Comparing the wavenumber spectrum between the essential oils studied and their main components showed that the different main components in each of the essential oils studied dominate the vibration spectrum produced by each essential oil (as shown in Table 3). Some components of essential oil, in small amounts, do not significantly affect the ATR-FTIR spectrum (Farag et al., 2018).
Analysis of a sample will always be associated with its application in product quality control. Due to the content of biosynthetic conditions and the complexity of essential oils, quality control has become highly challenging. Classification based on plant chemical content does not always correspond to taxonomic classification, but this is not a problem because essential oil quality control is based on the main components of the oil, whether bioactive or industrial. Vibrational spectroscopy is nondestructive, fast, with minimal sample preparation and a minimum number of analytes. In this study, the ATR-FTIR spectrum produced fingerprints for each essential oil, showing the characteristic profile of the main chemical component. The chemometric method analysis has also succeeded in quickly classifying essential oils based on their chemical composition. This concluded that ATR-FTIR is a fast, efficient, and nondestructive method that can be applied to control essential oils' quality based on their chemical composition (Farag et al., 2018).
Perspective and future direction
This review discussed the spectroscopic potential of ATR-FTIR in research on analyzing essential oil samples from the Myrtaceae family, including the genera Eucalyptus, Eugenia, Melaleuca, and Syzygium. Although GC-MS and GC-FID are essential oil analysis methods that are very commonly used, these methods have disadvantages in need for sample preparation, a long analysis time, large amounts of organic solvents, and high temperatures that cause the possibility of sample defects.
The ATR-FTIR spectroscopy method is an alternative method that is faster, easier, more efficient, and nondestructive and does not even require sample preparation because this method can be used on solid or liquid samples. As the overlapping ATR-FTIR spectrum can be a constraint in the analysis, another method is needed to assist the analysis process, namely, chemometrics. The chemometric method is an analytical method that applies mathematical and statistical techniques to analyze samples.
Chemometric methods are divided into many types, such as PCA, HCA, PLS-DA, SA, and others, where the use of this method is tailored to the needs of the analysis to be carried out and the data presented. HCA has been shown to classify the types of essential oils of the Myrtaceae family based on their chemical composition. Even though they have the same species, essential oils from different areas can be differentiated using this HCA method. Multivariate calibration applications such as PLS can indirectly assist in measuring certain substances.
From the above discussion, it can be seen that the use of a combination of ATR-FTIR spectroscopic methods and chemometric methods has the potential to enhance efficiency in obtaining basic sample information that can also be applied in the field of quality control. Due to the content of biosynthetic conditions and the complexity of essential oils, quality control has become highly challenging. For this reason, the use of this method is appropriate when viewed in terms of the benefits that have been previously described.
In addition to obtaining basic information from pure samples for quality control, ATR-FTIR spectroscopy can also be used to control finished essential oils on the market without the need for sample preparation or analyte separation due to the advantages of this method, which can analyze samples in solid or liquid form. Hence, the combination of ATR-FTIR and chemometric methods provides a reliable method for adulteration detection, analysis of changes in compound structure, analysis of patterns and degradation products, estimate expiration date, and measurement substance.
Its application can also extend to various fields, such as the health sector and the forensic field. Further development will increase the application of more reliable and efficient analysis tools in the sample analysis process to obtain more sample information. This combination of ATR-FTIR spectroscopy and chemometric methods will remain a robust, nondestructive, efficient, simple, sensitive, and fast technique for analyzing various substances in various fields until the present and possibly beyond.
CONCLUSION
The Total reflectance total attenuation IR Fourier transform (ATR-FTIR) spectroscopy method is an alternative that is faster, simpler, more efficient, nondestructive, and even requires no sample preparation as it can be used on solid or liquid samples. Chemometric methods are divided into several categories, such as principal components analysis, HCA, PLS analysis, SA, and others, where the use of this method is tailored to the needs of the analysis performed and the data presented. The ATR-FTIR and chemometrics are recommended in future research in the analysis of essential oil samples from the Myrtaceae family. The combination of ATR-FTIR spectroscopy and chemical measurement provides a powerful, nondestructive, efficient, simple, and fast analysis method for a small sample. In addition to obtaining basic information from purified samples for quality control, the ATR-FTIR spectrometer can also be used to monitor finished essential oils on the market without the need for sample preparation or separation of analytes because of this method's ability to analyze samples in both solid and liquid states.
ACKNOWLEDGMENT
This research was supported by the Indonesian Ministry of Research, Technology and Higher Education and the Directorate of Research and Community Engagement, Universitas Indonesia. This research was funded by the Directorate of Research and Community Engagement, Universitas Indonesia, via “Program Publikasi Terindeks Internasional (PUTI) Q3 2020, grant number NKB-1810/UN2.RST/HKP.05.00/2020”.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Agredo LES. Caracterização dos compostos voláteis e avaliação das propriedades antioxidantes e antimicrobianas de óleo essencial e extrato de uvaia obtido com CO2 supercrítico. Universidade estadual de campinas faculdade, São Paulo, Brasil, 2017.
Albouchi F, Sifaoui I, Reyes-Batlle M, Lopez-Arencibia A, Pineri JE, Lorenzo-Morales J, Abderrabba M. Chemical composition and anti-acanthamoeba activity of Melaleuca styphelioides essential oil. Experiment Parasitol, 2017; 183:104–8. CrossRef
Amorati R, Foti MC, Valgimigli L. Antioxidant activity of essential oils. J Agric Food Chem, 2013; 61(46):10835–47. CrossRef
Asadollahi-Baboli M, Aghakhani A, Bikdeloo V. Application of polyamide nanofibers, SPME/GC-MS, and chemometrics for comprehensive analysis of volatiles in Thymus vulgaris L. and Thymus serpyllum L. Food Anal Meth, 2015; 9:528–36. CrossRef
Azevedo PR, Silva LCN, Silva AG, Macedo AJ, Araújo JM, Silva MV. Antimicrobial activity and phytochemical screening of branches, fruits, and leaves of Eugenia brejoensis. Scientia Plena, 2012; 8(5):1–4. CrossRef
Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effect of essential oils: a review. Food Chem Toxicol, 2008; 46(2):446–75. CrossRef
Baldissera MD, Souza CF, Júnior GB, de Vargas AC, Boligon AA, de Campos MMA, Stefani LM, Baldisserotto B. Melaleuca alternifolia essential oil enhances the non-specific immune system and prevents oxidative damage in rhamdia quelen experimentally infected by Aeromonas hydrophila: effects on cholinergic and purinergic systems in liver tissue. Fish Shellfish Immunol, 2016; 8(2017):1–8. CrossRef
Baranska M, Schulz H, Reitzenstein S, Uhlemann U, Strehle MA, Kru H, Quilitzsch R, Foley W, Popp J. Vibrational spectroscopic studies to acquire a quality control method of Eucalyptus essential oils. Biopolymers, 2005; 78(5):237–48. CrossRef
Baranska M, Schulz H, Walter A, Rosch P, Quilitzsch R, Losing G, Popp J. Investigation of Eucalyptus essential oil by using vibrational spectroscopy methods. Vibrat Spectroscop, 2006; 42:341–45. CrossRef
Beasley MM, Bartelink EJ, Taylor L, Miller RM. Comparison of transmission FTIR, ATR, and DRIFT spectra: implications for assessment of bone bioapatite diagenesis. J Archaeol Sci, 2014; 46:16–22. CrossRef
Bittner LK, Schönbichler SA, Schmutzler M, Lutz OMD, Huck CW. Vibrational spectroscopic methods for the overall quality analysis of washing powders. Talanta, 2016; 148:329–35. CrossRef
Brooker MIH, Kleining DA. Field guide to Eucalypts: North Australia, volume 3. 2nd edition, Blooming Books, Melbourne, Australia, 2004.
Brunnel M, Vitrac C, Costa K, Mzali F, Vitrac X, Muselli A. Essential oil composition of Phagnalon sordidum (L.) from corsica, chemical variability and antimicrobial activity. Chem Biodivers, 2016; 13:299–308. CrossRef
Bunaciu AA, Aboul-enein HY. Adulterated drug analysis using FTIR spectroscopy. Appl Spectroscop Rev, 2021; 56(5):1–15. CrossRef
Byng JW, Florens FBV, Baider C. Syzygium pyneei (Myrtaceae), a new critically endangered endemic species from Mauritius. PhytoKeys, 2015; 66:61–6. CrossRef
Callao MP, Ruisanchez I. An overview of multivariate qualitative methods for food fraud detection. Food Control, 2018; 86:283–93. CrossRef
Cascaes M, Perc S, de Oliveira MS. Oils from leaves of two specimens of Eugenia florida DC. Molecules, 2021; 26(19):1–12.
Chan CO, Chu CC, Mok DKW, Chau FF. Analysis of berberine and total alkaloid content in cortex Phellodendri by near infrared spectroscopy (NIRS) compared with high-performance liquid chromatography coupled with ultra-visible spectrometric detection. Analytica Chimica Acta, 2007; 592:121–31. CrossRef
d’Acampora Zellner B, Bicci C, Dugo P, Mondello L. Analysis of essential oils. In: Basser KHC, Buchbauer G (eds.). Handbook of essential oils: science, technology, and applications, CRC Press, Boca Raton, FL, pp 151–83, 2010. CrossRef
da Silva C, Prasniewski A, Calegari MA, de Lima VA, Oldoni TLC. Determination of total phenolic compounds and antioxidant activity of ethanolic extracts of propolis using ATR – FTIR spectroscopy and chemometrics. Food Anal Meth, 2018; 11:2013–21. CrossRef
Dai T, Yang F, Liu J, Sun G. Evaluation of the quality consistency of Zhenju Jiangya tablets by systematic quantified fingerprint method in combination with antioxidant activity and three compounds analyses. Microchem J, 2019; 150:104175. CrossRef
Dametto AC, Agustoni D, Moreira TF, Plaza CV, Prieto AM, Silva TGA, Souza FO, Boralle N, Sorbo JM, Silva DHS, Soares CP. Chemical composition and in vitro chemoprevention assessment of Eugenia jambolana Lam. (Myrtaceae) fruits and leaves. J Funct Foods, 2017; 36:490–502. CrossRef
de Souza AM, de Oliveira CF, de Oliveira VB, Betim FCM, Miguel OG, Miguel MD. Traditional uses, phytochemistry and antimicrobial activities of Eugenia species – a review authors the Eugenia genus. Planta Med, 2018; 18:1232–48. CrossRef
Dong WJ, Ni YN, Kokot S. Quantitative analysis of two adulterants in Cynanchum stauntonii by near-infrared spectorscopy combined with multi-variate calibrations. Chem Papers, 2012; 66(12):1083–91. CrossRef
dos Santos JFS, Rocha JE, Bezerra CF, do Nascimento Silva MK, de Matos YMLS, de Freitas TS, dos Santos ATL, da Cruz RP, Machado AJT, Rodrigues THS, de Brito ES, Sales DL, de Oliveira Almeida W, da Costa JGM, Coutinho HDM, Moraiz-Braga MFB. Chemical composition, antifungal activity and potential anti-virulence evaluation of the Eugenia uniflora essential oil against Candida spp. Food Chem, 2018; 261:233–39. CrossRef
Ebani VV, Najar B, Bertelloni F, Pistelli L, Mancianti F, Nardoni S. Chemical composition and in vitro antimicrobial efficacy of sixteen essential oils against Escherichia coli and Aspergillus fumigatus isolated from poultry. Vet Sci, 2018; 5:62–75. CrossRef
Escamilla MN, Sanz FR, Li H, Schönbichler SA, Yang B, Bonn GK, Huck CW. Rapid determination of baicalin and total baicalein content in Scutellariae radix by ATR-IR and NIR spectroscopy. Talanta, 2013; 114:304–10. CrossRef
Fall R, Ngom S, Sall D, Sembene M, Samb A. Chemical characterization of essential oil from the leaves of Callistemon viminalis (D.R.) and Melaleuca leucadendron (Linn.). Asian Pac J Trop Biomed, 2017; 7(4):347–51. CrossRef
Farag, NF, El-ahmady SH, Abdelrahman EH, Naumann A, Schulz H, Azzam SM, El-kashoury EA. Characterization of essential oils from Myrtaceae species using ATR-IR vibrational spectroscopy coupled to chemometrics. Ind Crops Prod, 2018; 124:870–77. CrossRef
Fernandes CC, Rezende JL, Silva EAJ, Silva FG, Stenico L. Crotti AEM, Esperandim VR, Santiago MB, Martins CHG, Miranda MLD. Chemical composition and biological activities of essential oil from flowers of Psidium guajava (Myrtaceae). Braz J Biol, 2021; 81(3):728–36. CrossRef
Ferreira OO, da Cruz JN, de Jesus Pereira Franco C, Silva SG, da Costa WA, de Oliveira MS, de Aguiar Andrade EH. First report on yield and chemical composition of essential oil extracted from Myrcia eximia DC (Myrtaceae) from the Brazilian Amazon. Molecules, 2020; 25(4):783. CrossRef
Frauches NS, Oliveira T, Bekman C, Largueza D, Teodoro AJ. Brazilian Myrtaceae fruits?: a review of anticancer properties. Brit J Pharm Res, 2016; 12(1):1–15. CrossRef
Freitas JVB, Filho EGA, Silva LMA, Zocolo GJ, de Brito ES, Gramosa NV. Chemometric analysis of NMR and GC datasets for chemotype characterization of essential oils from different species of Ocimum. Talanta, 2018; 180:329–36. CrossRef
Gad HA, El-ahmady SH, Abou-shoer MI, Al-Azizi MM. Application of chemometrics in authentication of herbal medicines: a review. Phytochem Anal, 2013; 24:1–24. CrossRef
Gao Y, Hu Q, Li X. Chemical composition and antioxidant activity of essential oil from Syzygium samarangense (BL.) Merr. et Perry Flower-Bud. Spatula, 2012; 2: 23–33. CrossRef
Ghaffar A, Yameen M, Kiran S, Kamal S, Jalal F, Munir B, Saleem S, Rafiq N, Ahmad A, Saba I, Jabbar A. Chemical composition and in-vitro evaluation of the antimicrobial and antioxidant activities of essential oils extracted from seven Eucalyptus species. Molecules, 2015; 20:20487–98. CrossRef
Giang NT, Huong LT, Satyal P, Tai TA, Dai DN, Hung NH, Ngoc NTB, Setzer WN. Mosquito larvicidal activity, antimicrobial activity, and chemical compositions of essential oils from four species of Myrtaceae from central Vietnam. Plants, 2020; 9(4):544. CrossRef
Gilles M, Zhao J, An M, Agboola S. Chemical composition and antimicrobial properties of essential oils of three Australian Eucalyptus species. Food Chem, 2010; 119(2):731–37. CrossRef
Glassford SE, Byrne B, Kazarian SG. Recent applications of ATR FTIR spectroscopy and imaging to proteins. Biochim Biophys Acta; 2013; 1834(12):2849–58. CrossRef
Gredilla A, Fdez-ortiz De Vallejuelo S, Elejoste N, De Diego A, Madariaga JM. Non-destructive spectroscopy combined with chemometrics as a tool for green chemical analysis of environmental samples: a review. Trends Anal Chem, 2016; 76:30–9. CrossRef
Guillén MD, Cabo N. Characterization of edible oils and lard by fourier transform infrared spectroscopy. Relationships between composition and frequency of concrete bands in the fingerprint region. JAOCS, 1997; 74(10):1281–86. CrossRef
Haghighi TM, Saharkhiz MJ, Khosravi AR, Fard FR, Moein M. Essential oil content and composition of vitex pseudo-negundo in Iran varies with ecotype and plant organ. Ind Crops Prod, 2017; 109:53–9. CrossRef
Hamdy RS, Abd El-Ghani MM, Youssef TL, El-Sayed, M. The floristic composition of some historical botanical gardens in the Metropolitan of Cairo, Egypt. Afr J Agri Res, 2007; 2(11):610–48.
Hammer KA. Treatment of acne with tea tree oil (Melaleuca) products: a review of efficacy, tolerability and potential modes of action. Int J Antimicrob Agent, 2015; 45(2):106–10. CrossRef
Han X, Parker TL. Antiinflammatory activity of clove (Eugenia caryophyllata) essential oil in human dermal fibroblasts. Pharm Biol, 2017; 55(1):1619–22. CrossRef
Ismail, AA, van de Voort FR, Sedman J. Fourier transform infrared spectroscopy: principles and applications. Tech Instrument Anal Chem, 1997; 18:93–139. CrossRef
Jerônimo LB, da Costa JS, Pinto LC, Montenegro RC, Setzer WN, Mourão RHV, da Silva JKR, Maia JGS, Figueiredo PLB. Antioxidant and cytotoxic activities of Myrtaceae essential oils rich in terpenoids from Brazil. Nat Prod Commun, 2021; 16(2):1–13. CrossRef
Johnson LAS, Briggs BG. Myrtales and Myrtaceae - a phylogenetic analysis. Ann Miss Bot Gard, 1984; 71(3):700–56. CrossRef
Karoui R. Spectroscopic technique?: mid-infrared (MIR) and Fourier Transform Mid-Infrared (FT-MIR) spectroscopies. 2nd edition. In: Sun D-W (ed.). Modern techniques for food authentication, Elsevier Inc., London, UK, 2018. CrossRef
Karoui R, Pierna JAF, Dufour E. Spectroscopic technique: mid-infrared (MIR) and fourier transform mid-infrared (FT-MIR) spectroscopies. 1st edition. In: Sun D-W (ed.). Modern techniques for food authentication, Elsevier Inc, London, UK, 2008.
Khanh TH, Ban PH, Hoi TM. Constituents of essential oils from the leaf, fruit, and flower of Decaspermum parviflorum (Lam.) J Scott Arch Pharm Pract, 2020; 11(1):88–92.
Kharbach M, Marmouzi I, El M, Bouklouze A, Vander Y. Recent advances in untargeted and targeted approaches applied in herbal-extracts and essential-oils fingerprinting - a review. J Pharm Biomed Anal, 2020; 177:112849. CrossRef
Kong Q, An P, Xu Z, Zhang R, Qi J, Ren X. New insights into the alleviating role of Melaleuca alternifolia oil on metabolites pathway disorder of grapes caused by Aspergillus niger, verified by corresponding key genes expression. Food Chem, 2020; 327:127083. CrossRef
Kucharska-ambro?ej K, Karpinska J. The application of spectroscopic techniques in combination with chemometrics for detection adulteration of some herbs and spices. Microchem J 2020; 153:104278. CrossRef
Kumar R, Sharma V. Chemometrics in forensic science. Trends Anal Chem, 2018; 105:191–201. CrossRef
Laasonen M, Harmia-pulkkinen T, Simard CL, Michiels E. Fast identification of Echinacea purpurea dried roots using near-infrared spectroscopy. Anal Chem, 2002; 74:2493–99. CrossRef
Lee P, Guo H, Huang C, Chan C. Chemical composition of leaf essential oils of Syzygium samarangense (BL.) Merr. et Perry Cv. Pink at three maturity stages. Int J App Res Nat Prod, 2016; 9:9–13.
Li GQ, Zhang YB, Wu P, Chen NH, Wu N, Yang L, Qiu RX, Wang GC, Li YL. New phloroglucinol derivatives from the fruit tree Syzygium jambos and their cytotoxic and antioxidant activities. J Agri Food Chem, 2015; 63(47):10257–62. CrossRef
Li Y, Kong D, Wu H. Comprehensive chemical analysis of the flower buds of five Lonicera species by ATR-FTIR, HPLC-DAD, and chemometric methods. Braz J Pharmacogn, 2018; 28:533–41. CrossRef
Li Y, Shen Y, Yao CL, Guo DA. Quality assessment of herbal medicines based on chemical fingerprints combined with chemometrics approach: a review. J Pharm Biomed Anal, 2020; 185:113215. CrossRef
Lin M, Rasco BA, Cavinato AG, Al-Holy M. Infrared (IR) spectroscopy-near infrared spectroscopy and mid-infrared spectroscopy. In: Sun D-W (ed.). Sun infrared spectroscopy for food quality analysis and control, Elsevier Inc., London, UK, 2009. CrossRef
Louhibi S, Amiri S, Elghadraoui L. Chemical composition and antimicrobial activity of essential oils, Thymus vulgaris L and Mentha L. Pulegium against the major post harvest diseases of citrus in Morocco. Int J Sci Res, 2015; 4(8):2013–16.
Lourenço SC, Moldão-Martins M, Alves VD. Antioxidants of natural plant origins: from sources to food industry applications. Molecules, 2019; 24(22):14–6. CrossRef
Ma Y, He H, Wu J, Wang C, Chao K, Huang Q. Assessment of polysaccharides from mycelia of genus ganoderma by mid-infrared and near-infrared spectroscopy. Sci Rep, 2018a; 8(10):1–10. CrossRef
Ma Y, Zhang Q, Zhang Q, He H, Chen Z, Zhao Y, Wei D, Kong M, Huang Q. Improved production of polysaccharides in Ganoderma Lingzhi mycelia by plasma mutagenesis and rapid screening of mutated strains through infrared spectroscopy. Plos One, 2018b; 13(9):e0204266. CrossRef
Manheim J, Doty KC, Mclaughlin G, Lednev IK. Forensic hair differentiation using attenuated total reflection fourier transform infrared (ATR FT-IR) spectroscopy. App Spectroscop, 2016; 70(7):1109–17. CrossRef
Mazivila SJ, Olivieri AC. Chemometrics coupled to vibrational spectroscopy and spectroscopic imaging for the analysis of solid-phase pharmaceutical products: a brief review on non-destructive analytical methods. Trends Anal Chem, 2018; 108:74–87. CrossRef
Melo CR, Blank AF, Oliveira BMS, Santos ACC, Cristaldo PF, Araújo APA, Bacci L. Formicidal activity of essential oils of Myrcia lundiana chemotypes on Acromyrmex balzani. Crop Protect, 2021; 139:105343. CrossRef
Mesquita PRR, Nunes EC, dos Santos FN, Bastos LP, Costa MAPC, Rodrigues FM, de Andrade JB. Discrimination of Eugenia uniflora L. biotypes based on volatile compounds in leaves using HS-SPME/GC–MS and chemometric analysis. Microchem J, 2017; 130:79–87. CrossRef
Mohamed SB, Amine FM, Kameli A, Walid K, Boukhatem MN, Saidi F. Quality assessment of the essential oil from Eucalyptus globulus labil of Blida (Algeria) origin. Phys Astron, 2015; 36:303–15. CrossRef
Moura GS, Franzener G. Eugenia Uniflora L.: potential uses as a bioactive plant. Arq Inst Biol, 2018; 85:1–9.
Muro CK, Doty KC, Bueno J, Hala L, Lednev IK. Vibrational spectroscopy: recent developments to revolutionize forensic science. Anal Chem, 2015; 87:306–27. CrossRef
Nikzad-langerodi R, Ortmann S, Pferschy-wenzig EM, Bochkov V, Zhao YM. Assessment of antiinflammatory properties of extracts from Honeysuckle (Lonicera sp. L., Caprifoliaceae) by ATR-FTIR spectroscopy. Talanta, 2017; 175:264–72. CrossRef
Oliveri P. Class-modelling in food analytical chemistry: development, sampling, optimisation and validation issues - a tutorial. Anal Chim Acta, 2017; 982:9–19. CrossRef
Osunsanmi FO, Shode FO, Opoku AR. Antiinflammatory activity of betulinic acid and its acetyl derivative from Melaleuca bracteata. South Afr J Bot, 2016; 103:342. CrossRef
Ozgenç Ö, Durmaz S, Boyaci IH, Eksi-Kocak H. Determination of chemical changes in heat-treated wood using ATR-FTIR and FT raman spectrometry. Spectrochim Acta Part A Mol Biomol Spectroscop, 2017; 171:395–400. CrossRef
Padalia RC, Verma RS, Chauhan A, Goswami P, Verma SK, Darokar MP. Chemical composition of Melaleuca linarrifolia Sm. from India: a potential source of 1,8-cineole. Ind Crops Prod, 2015; 63:264–68. CrossRef
Paosen S, Saising J, Septama AW, Voravuthikunchai SP. Green synthesis of silver nanoparticles using plants from Myrtaceae family and characterization of their antibacterial activity. Mater Lett, 2017; 209:201–6. CrossRef
Parhizkar E, Ghazali M, Ahmadi F, Sakhteman A. PLS-LS-SVM based modeling of ATR-IR as a robust method in detection and qualification of alprazolam. Spectrochim Acta Part A Mol Biomol Spectroscop, 2017; 173:87–92. CrossRef
Payn KG, Dvorak WS, Janse BJH, Myburg AA. Microsatellite diversity and genetic structure of the commercially important tropical tree species Eucalyptus urophylla, endemic to seven Islands in Eastern Indonesia. Tree Genet Genom, 2008; 4(3):519–30. CrossRef
Peets P, Leito I, Pelt J, Vahur S. Identification and classification of textile fibres using ATR-FTIR spectroscopy with chemometric methods. Spectrochim Acta Part A Mol Biomol Spectroscop, 2017; 173:175–81. CrossRef
Pereira NLF, Aquino PEA, Júnior JGAS, Cristo JS, Filho MAV, Moura FF, Ferreira NMN, Silva MKN, Nascimento EM, Correia FMA, Cunha FAB, Boligon AA, Coutinho HDM, Ribeiro-Filho J, Matias EFF, Guedes MIF. Antibacterial activity and antibiotic modulating potential of the essential oil obtained from Eugenia jambolana in association with led lights. J Photochem Photobiol B Biol, 2017; 174:144–49. CrossRef
Prieto N, Pawluczyk O, Dugan MER, Aalhus JL. A Review of the principles and applications of near-infrared spectroscopy to characterize meat, fat, and meat products. App Spectroscop, 2017; 17:1403–26. CrossRef
Ranghoo-Sanmukhiya VM, Chellan Y, Soulange JG, Lambrechts IA, Stapelberg J, Crampton B, Lall N. Biochemical and phylogenetic analysis of Eugenia and Syzygium species from Mauritius. J App Res Med Aroma Plants, 2019; 12:21–9. CrossRef
Rocchetti G, Lucini L, Ahmed SR, Saber FR. In vitro cytotoxic activity of six Syzygium leaf extracts as related to their phenolic profiles: an untargeted UHPLC-QTOF-MS approach. Food Res Int, 2019; 126:108715. CrossRef
Rohman A. The employment of fourier transform infrared spectroscopy coupled with chemometrics techniques for traceability and authentication of meat and meat products. J Adv Vet Anim Res, 2019; 6(1):9–17. CrossRef
Saad MH, Rahman SNSA, Navanesan S, Tan CH, Manickam S, Abd Malek SN, Sim KS. Evaluation of antioxidant activity and phytochemical composition of Baeckea frutescens and Leptospermum javanicum essential oils. South Afr J Bot, 2021; 141:474–9. CrossRef
Sankari G, Krishnamoorthy E, Jayakumaran S, Gunasekaran S, Priya VV, Subramaniam S, Subramaniam S, Mohan SK. Analysis of serum immunoglobulins using fourier transform infrared spectral measurements. Biol Med, 2010; 2(3):42–8.
Santos DN, De Souza LL, De Oliveira CAF, Silva ER, De Oliveira AL. Arginase inhibition, antibacterial and antioxidant activities of Pitanga Seed (Eugenia uniflora L.) extracts from sustainable technologies of high pressure extraction. Food Biosci, 2015; 12:93–9. CrossRef
Sardi JCO, Freires IA, Lazarini JG, Infante J, de Alencar SM, Rosalen PL. Unexplored endemic fruit species from Brazil: antibiofilm properties, insights into mode of action, and systemic toxicity of four Eugenia spp. Microb Pathogen, 2017; 105:280–87. CrossRef
Schulz H, Baranska M. Identification and quantification of valuable plant substances by IR and Raman spectroscopy. Vibrat Spectroscop, 2007; 43(1):13–25. CrossRef
Schulz H, Baranska M, Belz HH, Rösch P, Strehle MA, Popp JÜ. Chemotaxonomic characterisation of essential oil plants by vibrational spectroscopy measurements. Vibrat Spectroscop, 2004; 35(1–2):81–6. CrossRef
Schulz H, Özkan G, Baranska M, Krüger H, Özcan M. Characterisation of essential oil plants from Turkey by IR and Raman Spectroscopy. Vibrat Spectroscop, 2005; 39(2):249–56. CrossRef
Sebei K, Sakouhi F, Herchi W, Khouja ML, Boukhchina S. Chemical composition and antibacterial activities of seven Eucalyptus species essential oils leaves. Biol Res, 2015; 48:1–5. CrossRef
Seraglio SKT, Schulz M, Nehring P, Betta FD, Valese AC, Daguer H, Gonzaga LV, Fett R, Costa ACO. Nutritional and bioactive potential of Myrtaceae fruits during ripening. Food Chem, 2018; 239:649–56. CrossRef
Sharifi-Rad J, Salehi B, Varoni EM, Sharopov F, Yousaf Z, Ayatollahi SA, Kobarfard F, Sharifi-Rad M, Afdjei MH, Sharifi-Rad M, Iriti M. Plants of the Melaleuca genus as antimicrobial agents: from farm to pharmacy. Phytother Res, 2017a, 31(10):1475–94. CrossRef
Sharifi-Rad J, Sureda A, Tenore GC, Daglia M, Sharifi-Rad M, Valussi M, Tundis R, Sharifi-Rad M, Loizzo MR, Ademiluyi AO, Sharifi-Rad R, Ayatollahi SA, Iriti M. Biological activities of essential oils: from plant chemoecology to traditional healing systems. Molecules, 2017b; 22:1–55. CrossRef
Shi T, Wu G, Jin Q, Wang X. Camellia oil authentication: a comparative analysis and recent analytical techniques developed for its assessment. A review. Trends Food Sci Technol, 2020; 97:88–99. CrossRef
Siddique S, Parveen Z, Firdaus-e-Bareen, Chaudhary MN, Mazhar S, Nawaz S. The essential oil of Melaleuca armillaris (Sol. Ex Gaertn.) Sm. leaves from Pakistan: a potential source of eugenol methyl ether. Ind Crops Prod. 2017; 109:912–17. CrossRef
Siddique S, Parveen Z, Firdaus-e-Bareen, Mazhar S. Chemical composition, antibacterial and antioxidant activities of essential oils from leaves of three Melaleuca species of Pakistani flora. Arab J Chem, 2020; 13(1):67–74. CrossRef
Siddique S, Perveen Z, Nawaz S, Shahzad K, Ali Z. Chemical composition and antimicrobial activities of essential oils of six species from family Myrtaceae. J Essent Oil Bear Plants, 2015; 18(4):950–56. CrossRef
Sonboli A, Salehi P, Gharehnaghadeh S. Chemical variability in the essential oil composition of Salvia hypoleuca, an endemic species from Iran. J Essent Oil Bear Res, 2016; 28(5):421–27. CrossRef
Stöbener A, Naefken U, Kleber J, Liese A. Determination of trace amounts with ATR FTIR spectroscopy and chemometrics: 5-(hydroxymethyl)furfural in honey. Talanta, 2019; 204:1–5. CrossRef
Syama HP, Sithara T, Krishnan SL, Jayamurthy P. Syzygium cumini seed attenuates LPS induced inflammatory response in murine macrophage cell line RAW264.7 through NF-ΚB translocation. J Funct Foods, 2018; 44:218–26. CrossRef
Tankeu S, Vermaak I, Kamatou G, Viljoen A. Vibrational spectroscopy as a rapid quality control method for Melaleuca alternifolia Cheel (tea tree oil). Phytochem Anal, 2014; 25(1):81–8. CrossRef
Tarhan ?. A robust method for simultaneous quantification of eugenol, eugenyl acetate, and β-caryophyllene in clove essential oil by vibrational spectroscopy. Phytochemistry, 2021; 191:112928. CrossRef
Tavares A, Beiroz W, Fialho A, Frazão F, Macedo R, Louzada J, Audino L. Eucalyptus plantations as hybrid ecosystems: implications for species conservation in the Brazilian atlantic forest. Forest Ecol Manage, 2019; 433:131–39. CrossRef
Thompson TJU, Gauthier M, Islam M. The application of a new method of fourier transform infrared spectroscopy to the analysis of burned bone. J Archaeol Sci, 2009; 36(3):910–14. CrossRef
Thornhill AH, Ho SYW, Külheim C, Crisp MD. Interpreting the modern distribution of myrtaceae using a dated molecular phylogeny. Mol Phylogen Evol, 2015; 93:29–43. CrossRef
Tran DB, Dargusch P, Moss P, Hoang TV. An assessment of potential responses of Melaleuca genus to global climate change. Mitig Adapt Strateg Glob Chang, 2013; 18(6):851–67. CrossRef
Ullah S, Park Y, Ikram M, Lee S, Park C, Kang D, Yang J, Akter J, Yoon S, Chun P, Moon HR. Design, synthesis and anti-melanogenic effect of cinnamamide derivatives. Bioorgan Med Chem, 2018; 26(21):5672–81. CrossRef
van de Voort FR, Ghetler A, García-González DL, Li YD. Perspectives on quantitative Mid-FTIR spectroscopy in relation to edible oil and lubricant analysis: evolution and integration of analytical methodologies. Food Anal Meth, 2008; 1(3):153–63. CrossRef
Wang LH, Sung WC. Rapid evaluation and quantitative analysis of eugenol derivatives in essential oils and cosmetic formulations on human skin using attenuated total reflectance-infrared spectroscopy. Spectroscop, 2011; 26(1):43–52. CrossRef
Wilson PG, O’Brien MM, Gadek PA, Quinn CJ. Myrtaceae revisited: a reassessment of infrafamilial groups. Am J Bot, 2001; 88(11):2013–25. CrossRef
Xiong X, Yu IKM, Tsang DCW, Bolan NS, Ok YS, Igalavithana AD, Kirkham MB, Kim KH, Vikrant K. Value-added chemicals from food supply chain wastes: state-of-the-art review and future prospects. Chem Eng J, 2019; 375:121983. CrossRef
Yang Y, Kong W, Feng H, Dou X, Zhao L, Xiao Q, Yang M. Quantitative and fingerprinting analysis of Pogostemon cablin based on GC-FID combined with chemometrics. J Pharm Biomed Anal, 2016; 121:84–90. CrossRef
Yi L, Dong N, Yun Y, Deng B, Ren D, Liu S, Liang Y. Chemometric methods in data processing of mass spectrometry-based metabolomics: a review. Anal Chim Acta, 2016; 914:17–34. CrossRef
Zeng QH, Zhao JB, Wang JJ, Zhang XW, Jiang JG. Comparative extraction processes, volatile compounds analysis and antioxidant activities of essential oils from Cirsium japonicum Fisch. Ex DC and Cirsium setosum (Willd.) M.Bieb. LWT Food Sci Technol, 2016; 68:595–605. CrossRef
Zhang J, Sun G. Assessment of quality consistency in traditional Chinese medicine using multi-wavelength fusion profiling by integrated quantitative fingerprint method: Niuhuang Jiedu Pill as an example. J Separat Sci, 2019 42(2):509–21. CrossRef
Zhang Y, Wang C, Yang F, Sun G. A strategy for qualitative and quantitative profiling of glycyrrhiza extract and discovery of potential markers by fingerprint-activity relationship modeling. Sci Rep, 2019; 9(1):1–11. CrossRef