INTRODUCTION
Immunomodulatory agents derived from natural materials have emerged as promising candidates for treating COVID-19, which is caused by a highly pathogenic novel virus known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Tallei et al., 2021a). SARS-CoV-2 is rapidly spreading and has become the deadliest pandemic, posing significant global health challenges (Gates, 2020; Rakib et al., 2020). Because of a lack of therapeutic options, mortality rates in intensive care units reached as high as 20% in certain population subsets (Goyal et al., 2020). Globally, as of August 23, 2021, there have been 211,730,035 confirmed cases of COVID-19, including 4,430,697 deaths, reported by the WHO. This figure is growing rapidly in South East Asia, with a reported 47% increase. India, Indonesia, and Sri Lanka had the highest rates of new cases, while Indonesia, India, and Nepal had the highest rates of new deaths. This is exacerbated by the emergence of diverse variants as a result of SARS-CoV-2 mutations that affect infectivity, disease severity, and interactions with host immunity (Harvey et al., 2021).
The symptoms of a deadly viral infection can be treated by suppressing the cytokine storm (Ye et al., 2020). Viral infections created an overactivation of adaptive and innate immune responses such as mitogen-activated protein kinase and nuclear factor kappa B (NF-κB), causing excessive cytokine, a proinflammatory substance, being released along with chemokines that provoke severe lung alteration (Hariharan et al., 2021; Li et al., 2020; Tang et al., 2020). The overproduction of tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and interleukin-1beta (IL-1β), as proinflammatory cytokines, created the cytokine storm, prompting a serious problem of multiorgan failure and vascular hyperpermeability (Hu et al., 2021; Jafarzadeh et al., 2020; Sun et al., 2020). There is a crucial need for immunomodulatory agents to overcome this issue by targeting these COVID-19 immunological human host proteins (Henderson et al., 2020; Mehta et al., 2020).
 | Table 1. Receptors’ grid box dimensions and center. [Click here to view] |
The use of natural components to save lives is required due to the time and cost constraints for which new drug approvals are required during a pandemic. Plants are used indefinitely as a source of medicine due to the presence of specific chemicals/active ingredients that have been empirically demonstrated by indigenous people (Aziz et al., 2018; Pan et al., 2014; Tallei et al., 2019). Plants also contain active compounds that have been scientifically and clinically proven to be used in the treatment of a variety of diseases, both infectious and metabolic (Eddouks et al., 2021; Graf et al., 2010; Kwofie et al., 2021; Silva and Júnior, 2010; Wahab et al., 2021). They are sources of novel drug compounds for antiviral (Khairan et al., 2021; Rakib et al., 2020; Singh et al., 2020; Tallei et al., 2020), as well as immunomodulatory agents (Chakraborty et al., 2021; Jantan et al., 2015; Wen et al., 2012).
To identify potential immunomodulatory agents, preliminary screening of various pharmacological agents derived from medicinal plants is required. Because of its efficiency, the in silico approach is a promising method for discovering novel compounds as COVID-19 drug candidates (Noor et al., 2021; Pinzi and Rastelli, 2019). In this research, we analyzed medicinal compounds of betel (Piper betle L.) leaves using a molecular docking method to obtain promising immunomodulatory agents that could be further utilized for COVID-19 management.
MATERIALS AND METHODS
Ligands’ preparations
Our previous study used gas chromatography-mass spectrometry to identify bioactive compounds in betel leaves (Fatimawali et al., 2021). These compounds are used in present studies as ligands. PubChem (http://pubchem.ncbi.nlm.nih.gov) was used to find the ligand structures. The three-dimensional (3D) structures of the ligands were saved in .sdf format. The 3D conformations were generated using Avogadro 1.2.0 (Hanwell et al., 2012) and further assembled using AutoDock tools (Morris et al., 2009). Ligands’ energy was minimized, and the structures were geometrically optimized. The optimized ligand structures were saved in .pdbqt format and then used for molecular docking.
RECEPTOR PREPARATIONS
The 3D structures of the targeted proteins TNF-α (PDB ID: 2AZ5), IL-1β ((PDB ID: 2NVH), IL-6 (PDB ID: 1ALU), and NF-κB p65 (PDB ID: 1OY3) (Fig. 1) determined by X-ray crystallography were retrieved from Protein Data Bank (http://www.rcsb.org/pdb) and used as receptors in this study. The protein structures were optimized with Biovia Discovery Studio Visualizer 2020. Native ligand and water molecules attached to the proteins were removed and then saved in .pdb format. The proteins were modified by adding polar hydrogen using AutoDock tools. The optimized proteins were saved in .pdbqt format and then further used as receptors for molecular docking.
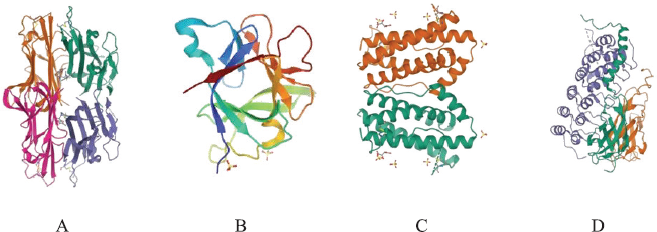 | Figure 1. (A) TNF-α (PDB ID: 2AZ5); (B) IL-1β ((PDB ID: 2NVH); (C) IL-6 (PDB ID: 1ALU); and (D) NF-κB p65 (PDB ID: 1OY3) protein structures. [Click here to view] |
RECEPTOR–LIGAND DOCKING
The docking procedure was based on our previous research (Sailah et al., 2021; Tumilaar et al., 2021). PyRx 0.8 (Dallakyan and Olson, 2015) was used to carry out molecular docking. Previously optimized ligands and receptors were selected and docked with the Vina wizard feature by using the Run Vina option. Table 1 shows the grid box dimensions and center used in the docking protocol.
ANALYSIS AND VISUALIZATION
Ligand–receptor interactions were visualized in both 2D and 3D structures obtained from the molecular docking output file. The output file was split using the command prompt with command: vina_split–input (output file name) .pdbqt. Analysis and visualization of the ligand and receptor interaction were carried out using Biovia Discovery Studio Visualizer 2020. Interactions of ligand and receptor with the highest binding affinity score were visualized and saved as image files.
DOCKING PROTOCOL VALIDATION
The validation was conducted according to Gupta et al. (2021). The control immunomodulatory agents were redocked using AutoDock Vina. The resulting poses were obtained and further analyzed using Pymol. The root mean square error (RMSD) was determined.
ADMET PROPERTIES AND LIPINSKI’S RULE OF FIVE
Ligands with greater binding energy compared to the control were further calculated for their pharmacokinetic characteristic. AdmetSAR 2.0 (Yang et al., 2019) was used to predict the absorption, distribution, metabolism, excretion, and toxicity properties of the compounds, and the number of violations of Lipinski’s rule of five was observed using SwissADME (Daina et al., 2017).
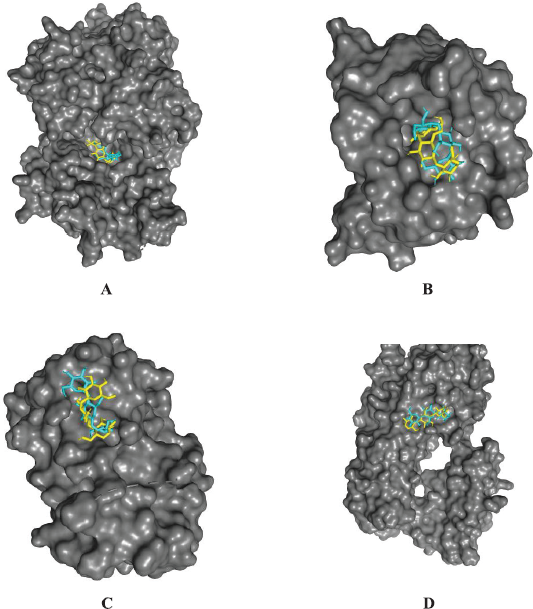 | Figure 2. β-Glucan docking validation poses of (A) TNF-α, (B) IL-1β, (C) IL-6, and (D) NF-κB p65. The light blue color is the docked result with PyRx, and the yellow color is the redocked result pose using AutoDock Vina. [Click here to view] |
RESULTS AND DISCUSSIONS
COVID-19 continues to spread throughout the world, despite unprecedented social isolation and restrictions. Additionally, various variants of SARS-CoV-2 have begun to emerge, prompting researchers to become even more aggressive in their search for an antidote to this virus. According to Feuillet et al. (2021), effective treatments for COVID-19 patients should include a combination of antivirals and immunomodulators. Traditional medicinal plants have been identified as a potentially beneficial source of immunomodulators against COVID-19 (Dutta et al., 2021; Nugraha et al., 2020).
Using a molecular docking approach, we searched for immunomodulatory candidates from betel leaves that have the potential of being developed for COVID-19 management. PyRx was used to carry out the docking. By redocking the control immunomodulatory agents with AutoDock Vina, the docking protocol was validated. The RMSD value of poses was measured to calculate the similarity of poses and coordinates between two molecules. Docking validation poses of β-glucan of TNF-α, IL-1β, IL-6, and NF-κB p65 are shown in Figure 2. Docking poses resulting from PyRx (light blue color) were superimposed by the docking poses resulting from Vina (yellow color), and then the RMSD value was calculated. These results showed the similarity of docking pose results from both applications. RMSD value calculates the similarity of the poses quantitatively. The closer the value to zero, the more similar the poses. RMSD value of less than 2Å is acceptable and suggests that the docking protocol used in this study has been carried out successfully (Xiao et al., 2018). The RMSD values of the poses were 1.040, 1.032, 1.310, and 1.930 Å in interactions with receptor TNF-α (PDB ID: 2AZ5), IL-1β ((PDB ID: 2NVH), IL-6 (PDB ID: 1ALU), and NF-κB p65 (PDB ID: 1OY3), respectively. This indicated that RMSD values were in the acceptable range. Therefore, the present research’s docking protocol is valid, and the result is reliable.
Betel leaf bioactive compounds together with β-glucan and thiopurine as control immunomodulatory agents were docked to TNF-α, IL-1β, IL-6, and NF-κB. The binding energy results are presented in Table 2. Molecular docking has been widely used to design novel components or repurposing drugs (Pinzi and Rastelli, 2019). Molecular docking analyzes binding energy and poses of interactions between ligands and a specific receptor (Ahmed et al., 2019). There are 17 compounds that show lower binding free energy compared to both β-glucan and thiopurine. It suggests that the ligands bind to the proteins with better and stable interactions and are potential as immunomodulatory agents. Androstan-17-one,3-ethyl-3-hydroxy-,(5.alpha.) has the binding free energy of −8.7, −7.1, −7, and −6.8 kcal/mol for TNF-α, IL-1β, IL-6, and NF-κB, respectively. A lower binding free energy indicates that the ligand binds strongly to the receptor and may be capable of inhibiting the protein target’s activities.
When compared to the control immunomodulatory agents, 17 compounds had higher binding free energy, particularly with TNF-α. It suggests that most betel leaf bioactive compounds have better immunomodulatory activity than β-glucan and thiopurine due to lower binding free energy and thus can inhibit the targeted proteins more steadily and strongly. TNF-α, which was initially identified as an endotoxin-induced glycoprotein, is required for cell proliferation, migration, differentiation, and death (Ganeshpurkar and Saluja, 2018; Mercogliano et al., 2020). The inflammatory disease may be treated by the use of TNF-α-blocking drugs (Silva et al., 2010).
Various interactions with inflammatory and immune cells are arbitrated by a class of proteins termed interleukins. Interleukins are proteins that help cells grow, differentiate, and activate their functions. IL-1β (human leukocyte pyrogen/lymphocyte mitogen) and IL-6 are two important interleukins. They are produced by macrophages, T-cells, and bone marrow stromal cells. IL-1β is an important mediator to evoke an immune response. IL-1β contributes towards the progression of pain, inflammation, and cell apoptosis (Marchand et al., 2005).
The NF-κB signaling pathway is regarded as the most commonly involved proinflammation pathway (Liu et al., 2017). Several studies have found that NF-κB regulates a wide range of genes that produce proinflammatory cytokines, adhesion molecules, chemokines, growth factors, and inducible enzymes such as cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) (Shih et al., 2015).
Amino acid residues that are involved in interactions between proteins and androstan-17-one,3-ethyl-3-hydroxy-,(5.alpha.) are shown in 2D images. The ligands’ binding poses are shown in 3D images (Fig. 3). The interactions are shown to form van der Waals, conventional hydrogen, pi-sigma, alkyl, and pi-alkyl bonds. Binding interactions of the control immunomodulatory agents are shown in 3D and 2D interaction images in Figure 4.
The formation of ligand–receptor complexes is mediated by molecular interactions such as hydrogen bonds, electrostatic interactions, and hydrophobic interactions (Tallei et al., 2021b). Figure 3 shows that androstan-17-one,3-ethyl-3-hydroxy-,(5.alpha.) forms 10 van der Waals interactions and 2 H-bonds on TNF-α; 6 van der Waals interactions, 1 pi-sigma bond, 2 pi-alkyl bonds, and 1 H-bond on IL-1β; 3 van der Waals interactions, 1 pi-sigma bond, and 3 H-bonds on IL-6; and 6 van der Waals interactions and 1 pi-alkyl bond on NF-κB p65.
Androstan-17-one,3-ethyl-3-hydroxy-,(5.alpha.) forms H-bonds with Tyr-151 and Gly-121 on the active site of TNF-α, Leu-80 on polar site of IL-1β, and Gln-75, Ser-76, and Ser-176 of IL-6. In biological systems, hydrogen bonds have significant roles, particularly in the stabilization of protein structures (Glowacki et al., 2013). Hydrophobic interactions, such as pi-sigma and pi-alkyl bonds, exist in addition to hydrogen bonds (Dai et al., 2019). Pi-sigma bonds are formed with amino acids Phe-133 of IL-1β and Phe-74 of IL-6, while pi-alkyl bonds are formed with Pro-131 of IL-1β and Cys-240 of NF-κB p65. Van der Waals interactions can form in greater numbers on all target proteins than other bonds. Van der Waals interaction, in combination with hydrogen bonds, contributes to the stability of the formed ligand–protein complexes (He et al., 2005).
TNF-α is a powerful proinflammatory molecule that controls a variety of macrophage functions (Parameswaran and Patial, 2010). Trauma and infection cause a continuous release of TNF-α, resulting in its highest bioavailability in the early stages of tissue inflammation. One of the most important functions is to regulate the production of the proinflammatory cytokine cascade (Ye et al., 2020). TNF-α regulates the formation of a proinflammatory cytokine cascade through molecular interactions (Liu et al., 2016). Tyr-59, Tyr-151, and Tyr-119 are active amino acids that play a crucial role in TNF-α function. By binding to this amino acid, the inhibitor can significantly impair its function (Parves et al., 2021). In addition to the active residue of tyrosine, the TNF-α binding site is composed of Leu-57, Ser-60, Gln-61, Leu-120, Gly-121, and Gly-122, so that binding to these amino acids can cause inhibition of their biological activity (He et al., 2005). The binding free energy of androstan-17-one,3-ethyl-3-hydroxy-,(5.alpha.) is low, implying the formation of a stable complex with TNF-α. This compound has a van der Waals interaction with the amino acids Tyr-59, Tyr-151, and Tyr-119, which are active amino acids of TNF-α, so that this binding will cause the potential for TNF-α inactivation. Interactions also occur with the amino acids Leu-57, Ser-60, Gln-61, Leu-120, Gly-121, Gly-122, and Ile-155, which indicates that this compound binds to the binding site or around the active side of TNF-α. Wang et al. (2020) used the Molecular Operating Environment (MOE)-site Finder in determining the TNF-α binding site (PDB ID: 2AZ5) and found that the binding site was located around the amino acids Tyr-119, Leu-57, Tyr-59, Ser-60, Leu-120, Gly-121, Gly-122, and Tyr151. These findings corroborate the findings of this investigation and may attest to the validity of the binding sites chosen for molecular docking. Apart from functioning as proinflammatory cytokines, TNF-α also enhances the signaling of mediators of lipid transduction, such as prostaglandins and platelet-activating factors (Vassalli, 1992). Based on its roles, TNF-α plays a central role in the activation of inflammatory cells (Yang et al., 2018).
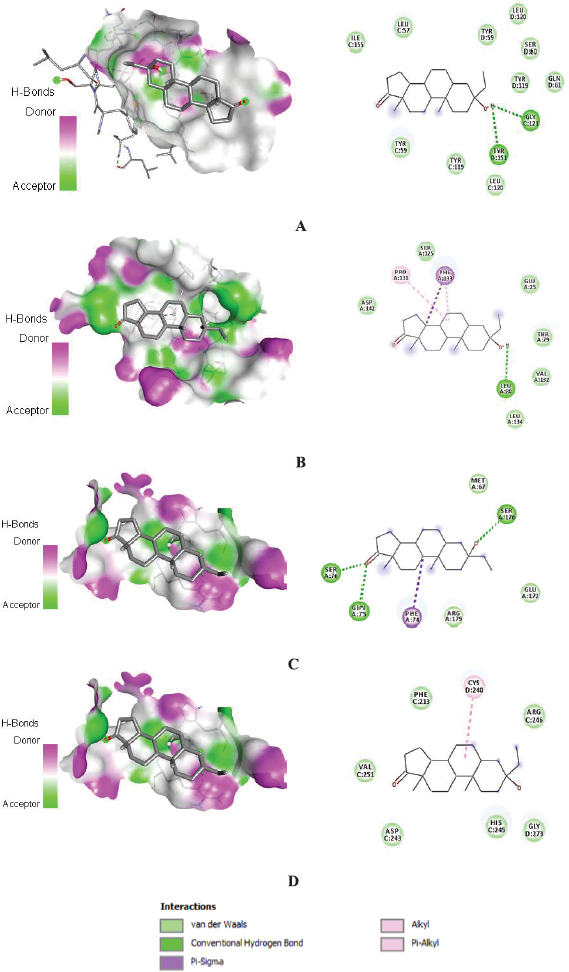 | Figure 3. 3D and 2D configuration of interaction between androstan-17-one,3-ethyl-3-hydroxy-,(5.alpha.) and (A) TNF-α, (B) IL-1β, (C) IL-6, and (D) NF-κB p65. [Click here to view] |
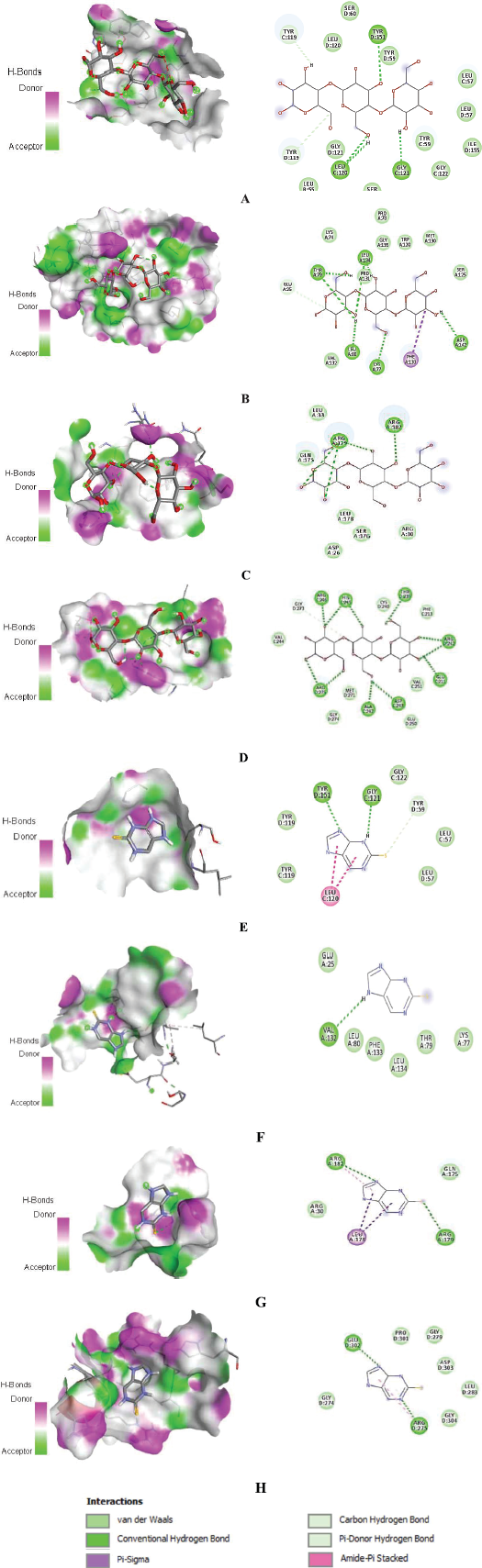 | Figure 4. 3D and 2D interactions of β-glucan binding to (A) TNF-α, (B) IL-1β, (C) IL-6, and (D) NF-κB p65. 3D and 2D interactions of thiopurine binding to (E) TNF-α, (F) IL-1β, (G) IL-6, and (H) NF-κB p65. [Click here to view] |
IL-1 is a proinflammatory cytokine that is released when the innate pathogen-sensing pathway is activated during microbial infection (Browne, 2015). Excessive release of proinflammatory cytokines, on the other hand, causes a cytokine storm, which causes widespread tissue damage and multiorgan failure, which is fatal, resulting in death. In COVID-19 patients, targeting cytokines can improve survival rates and reduce mortality (Ragab et al., 2020). The poses of interaction between betel fruit bioactive compounds and IL-1β were analyzed. The interaction occurs with the amino acids Glu-25, Thr-79, Leu-80, Ser-125, Pro-131, Val-132, Phe-133, Leu-134, and Asp-142 of IL-1β. Quillin et al. (2006) found cavity 4 and cavity 5 of IL-1β, with amino acids in cavity 4 being Lys-16, Ala-127, Met-130, Ser-125, Thr-124, Pro-131, and Ala-28, and in cavity 5 being Leu-10, Val-132, Leu-18, Leu-26, Phe-42, Leu-69, and Ile-122. Cavity 4 is the more polar side and has a small volume (16 Å3), while the cavity is the central apolar side and has a volume of 39 Å3. These amino acids also play a role in the kaurenoic acid–IL-1β and ibuprofen–IL-1β complexes (Karmakar et al., 2019). IL-1β is an inflammatory mediator that causes various biological responses ranging from the central nervous system, hematologically and metaboliccally (Dinarello, 2011). An excessive inflammatory response, on the other hand, can result in tissue damage and disease (Wallach et al., 2014).
IL-6 is the most important immune response and inflammatory mediator induced by infection or injury. More than half of the COVID-19 patients have elevated IL-6 levels (Zhang et al., 2020). In COVID-19 patients, IL-6 levels are linked to an inflammatory response, respiratory failure, and mortality (Herold et al., 2020; Zhang et al., 2020). According to Grifoni et al. (2020), anti-IL-6 drugs can be used to manage cytokine storms caused by SARS-CoV-2, which could be a treatment option for COVID-19. In its interaction with IL-6, androstan-17-one,3-ethyl-3-hydroxy-,(5.alpha.) molecularly binds to Met-67 which is a hydrophobic side chain, with Phe-74, which is part of the disulfide (Cys73-Cys83), with Gln-75 and Ser-76m, which are part of type II β turn (Gln75–Phe78), and with Glu-172, Ser-176, and Arg-179, which are part of the D helix (Gln156–Arg182) of IL-6. Previous studies have shown that changes to Arg-179, Gln-175, Phe-74, and Gly-35 could significantly reduce IL-6 activity. On IL-6, the compound interacts with amino acids on sites 1 and 2, which play a role in mediating IL-6 binding to IL-6r and gp130, respectively. Androstan-17-one,3-ethyl-3-hydroxy-,(5.alpha.), which binds to IL-6 in this study, is predicted to decrease or stop its activity by blocking IL-6 binding to IL-6r and gp130 in signal transduction.
NF-κB proteins are critical regulators of innate and adaptive immune responses, including proliferation, apoptosis inhibition, cell migration and invasion, and angiogenesis and metastasis (Park and Hong, 2016). NF-κB is activated continuously or transiently in response to viral and bacterial infections, necrotic cell products, DNA damage, oxidative stress, and proinflammatory cytokines (Karin and Greten, 2005; Taniguchi and Karin, 2018). NF-κB p65 (along with p50 in the heterodimer form) is the most commonly occurring form of NF-κB activated through the recognized pathway concerning pathological stimuli. The principal point for the discovery and development of new drugs was the NF-κB p6a signaling path (Grancieri et al., 2019). On the basis of a docking study of NF-κB p65, Grancieri et al. (2019) found that the active site was around the amino acid Lys-221, His-245, Arg-246, Gly-273, Gly-274, Arg-275, Leu-282, Leu-283, Pro-285, and Gly-304. The present study also found that androstan-17-one, 3-ethyl-3-hydroxy-, (5.alpha.) interacts with NF-κB p65 in the same binding region, namely the amino acids Phe-213, Cys-240, Asp-243, His-245, Arg-246, Val-251, and Gly-273. This binding site contains amino acids that are involved in the pharmacological activity; thus, binding to or inhibiting this site can result in a decrease in NF-κB p65 activity. NF-κB is a transcription factor that regulates a variety of functions of the innate and adaptive immune systems and plays a critical role in inflammatory responses. NF-κB promotes the expression of a wide range of proinflammatory genes, among them, are those encoding cytokines and chemokines, both of which are involved in the regulation of inflammation. Inhibition of this protein is predicted to reduce cytokine production and alleviate cytokine storms (Liu et al., 2017).
The present study demonstrates that bioactive compounds from betel leaves have a significantly higher binding free energy to all of the targeted proteins compared to controls. A study conducted by Johnson et al. (2020) docked a total of 43 compounds from Phyllanthus nivosus leaves on TNF-α using PyRx. The binding free energies were found to range from the highest −2 kcal/mol to the lowest 6.3 kcal/mol. This is a significantly lower value than the one obtained in the current study. Kaempferol and chlorogenic acid were studied for their binding free energy against IL-1β and IL-6 using PyRx by Karmakar et al. (2019). The binding free energies of −7.0 and −6.8 kcal/mol were found in chlorogenic acid and kaempferol, respectively, to IL-1β, while the binding with IL-6 showed −7.1 and −7.7 kcal/mol.
A total of 17 compounds were subjected to additional pharmacokinetic analysis. Lipinski’s Ro5 criteria of β-glucan, thiopurine, and several of the betel leaf bioactive compounds are presented in Table 3. β-Glucan was found to violate five out of six criteria, suggesting that there will be a major drug system delivery development for the drug. Sixteen of the 17 best betel leaf compounds have been shown to conform to Lipinski’s Ro5 (Lipinski et al., 2001). This indicates that the possible immunomodulatory compounds in betel leaf extract are orally active.
ADMET profiles of β-glucan, thiopurine, and some of the betel leaf bioactive compounds are presented in Table 4. The potential immunomodulatory compounds in betel leaf extract were predicted to have good in vitro activities due to their high binding affinity with the target proteins. The binding free energy value, when combined with the ADMET profile, can be used to predict the safety and efficacy of betel leaf bioactive compounds in in vivo and clinical settings.
All 17 compounds were found to have a high rate of intestinal absorption in humans, indicating that they are well absorbed and can enter the body orally. The blood–brain barrier’s permeability and subcellular localization were used to characterize the compounds’ distribution. A high blood–brain barrier’s permeability demonstrated that the ligands could penetrate and distribute throughout the brain. The subcellular localization of the compounds predicted their ability to distribute and penetrate at the subcellular level (Daneman and Prat, 2015; Soni et al., 2016). The results of the present study showed that betel leaf bioactive compounds can be distributed and penetrate in mitochondria, lysosomes, and plasma membrane. All of the compounds were not CYP2D6 substrates, indicating that they are poorly metabolized in the body. However, metabolism has the potential to change a drug’s effectiveness in the body (Lee et al., 2019).
Hepatotoxicity and acute oral toxicity were chosen as the toxicity parameters for this study. Hepatotoxicity is a proxy for organ toxicity, whereas acute oral toxicity is a proxy for the maximum dose of the studied compounds that the body can tolerate. The findings that the selected compounds are hypothetically nontoxic to the liver imply that the bioactive compounds in betel leaf are unlikely to cause liver damage. The compounds were classified as toxicity class III (500 < LD50 ≤ 5,000 mg/kg), implying that the body can tolerate a dose of up to 500 mg/kg. As a result, it is recommended that these compounds be developed as drugs within that range (Cheng et al., 2011; Gadaleta et al., 2019; Li et al., 2014; Yang et al., 2017).
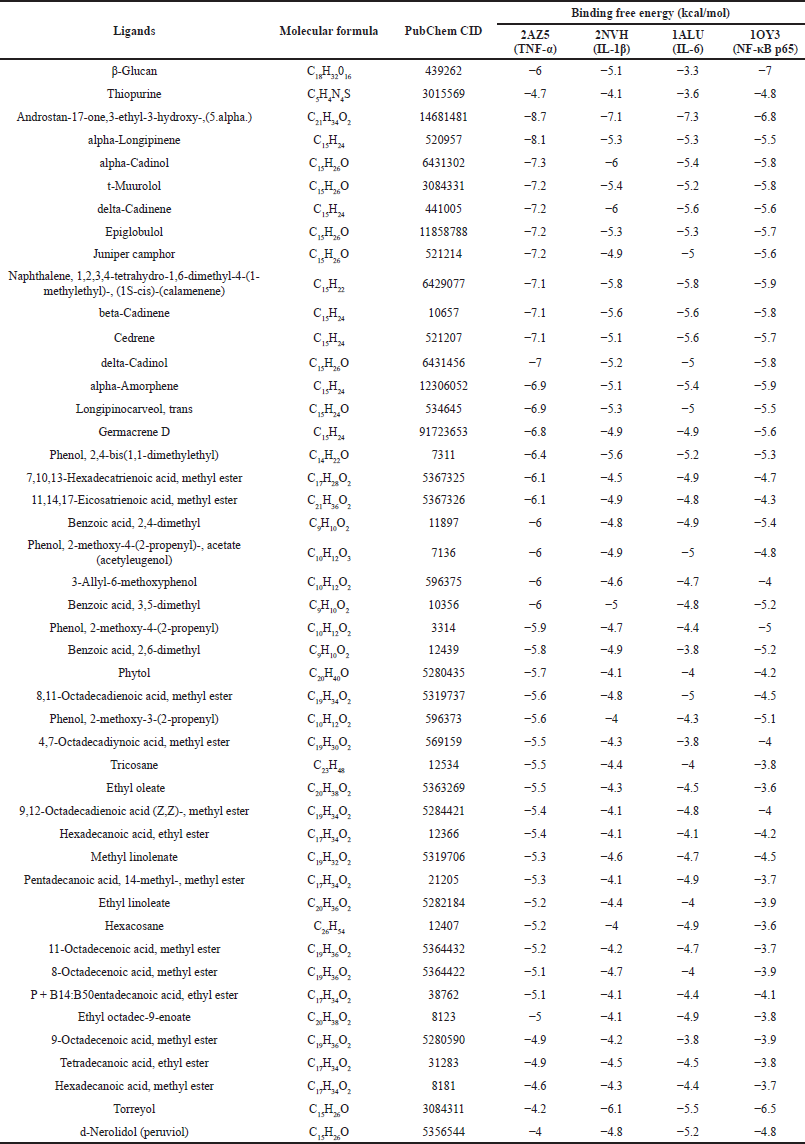 | Table 2. β-Glucan, thiopurine, and betel leaf bioactive compounds binding free energy. [Click here to view] |
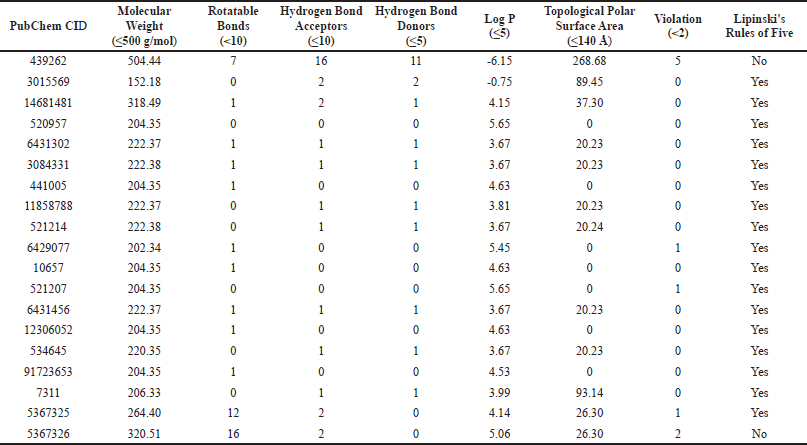 | Table 3. Analysis of Lipinski’s Ro5 of β-glucan, thiopurine, and some of the betel leaf bioactive compounds [Click here to view] |
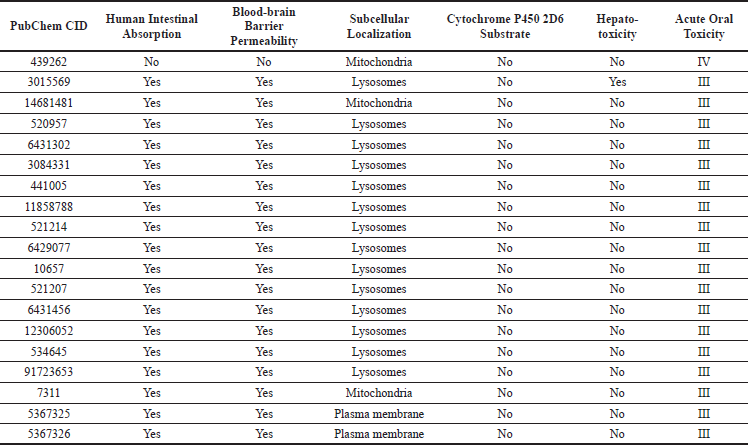 | Table 4. ADMET prediction of β-glucan, thiopurine, and selected betel leaf bioactive compounds [Click here to view] |
CONCLUSION
The betel leaf bioactive compounds have the potential to inhibit the COVID-19 host immunological proteins due to their high affinity with all the targeted proteins. The compounds’ binding free energies ranged from −4 to −8.7 kcal/mol, −4 to −7.1 kcal/mol, −3.8 to −7.3 kcal/mol, and −3.6 to −6.8 kcal/mol for TNF-α, IL-1β, IL-6, and NF-κB, respectively. Seventeen ligands showed higher binding energy than the control immunological agents. Androstan-17-one,3-ethyl-3-hydroxy-,(5.alpha.) has the binding free energy of −8.7, −7.1, −7, and −6.8 kcal/mol for TNF-α, IL-1β, IL-6, and NF-κB p65, respectively. When compared to previous studies, this study discovered that the betel leaf bioactive compounds have a significantly higher binding free energy to all of the targeted proteins. Lipinski’s Ro5 analysis demonstrates that majority of the compounds comply with the criteria and are predicted to be active when administered orally as drugs. The ADMET prediction of the compounds indicates that the ligands have favorable pharmacokinetic properties, implying that they have the potential to be developed as drugs. Hence, betel leaf bioactive compounds have the potential to treat COVID-19 because of having immunomodulatory effects and suppressing the cytokine storm, and they have the potential to be developed as drugs because of their good and safe ADMET profile.
ACKNOWLEDGMENTS
This research was funded by the Directorate of Research and Community Service, Ministry of Research and Technology/National Research and Innovation Agency under the scheme of Excellent Applied Research in Higher Education (Master Contract No. 297/E4.1/AK.04.PT/2021; Derivateve Contract No. 2014/UN12.13/LT/2021; Letter of Assignment No. 1945/UN12.13/LT/2021).
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Ahmed S, Rakib A, Islam MA, Khanam BH, Faiz FB, Paul A, Chy MN, Bhuiya NM, Uddin MM, Ullah SA, Rahman MA. In vivo and in vitro pharmacological activities of Tacca integrifolia rhizome and investigation of possible lead compounds against breast cancer through in silico approaches. Clin Phytosci, 2019; 5:1–13. CrossRef
Aziz MA, Adnan M, Khan AH, Shahat AA, Al-Said MS, Ullah R. Traditional uses of medicinal plants practiced by the indigenous communities at Mohmand Agency, FATA, Pakistan. J Ethnobiol Ethnomed, 2018; 14(1):2; https://doi.org/10.1186/s13002-017-0204-5
Browne EP. An interleukin-1 beta-encoding retrovirus exhibits enhanced replication in vivo. J Virol, 2015; 89(1):155–64; https://doi.org/10.1128/JVI.02314-14
Chakraborty AJ, Mitra S, Tallei TE, Tareq AM, Nainu F, Cicia D, Dhama K, Emran TB, Simal-Gandara J, Capasso R. Bromelain a potential bioactive compound: a comprehensive overview from a pharmacological perspective. Life, 2021; 11(4):317; https://doi.org/10.3390/life11040317
Cheng F, Yu Y, Zhou Y, Shen Z, Xiao W, Liu G, Li W, Lee PW, Tang Y. Insights into molecular basis of cytochrome p450 inhibitory promiscuity of compounds. J Chem Inf Model, 2011; 51(10):2482–95; https://doi.org/10.1021/ci200317s
Dai T, Chen J, McClements DJ, Li T, Liu C. Investigation the interaction between procyanidin dimer and α-glucosidase: spectroscopic analyses and molecular docking simulation. Int J Biol Macromol, 2019; 130:315–22; https://doi.org/10.1016/j.ijbiomac.2019.02.105
Daina A, Michielin O, Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep, 2017; 7(1):42717; https://doi.org/10.1038/srep42717
Dallakyan S, Olson AJ. Small-molecule library screening by docking with PyRx. Methods Mol Biol, 2015; 1263:243–50; https://doi.org/10.1007/978-1-4939-2269-7_19
Daneman R, Prat A. The blood–brain barrier. Cold Spring Harb Perspect Biol, 2015; 7(1):a020412; https://doi.org/10.1101/cshperspect.a020412
Dinarello CA. A clinical perspective of IL-1β as the gatekeeper of inflammation. Eur J Immunol, 2011; 41(5):1203–17; https://doi.org/10.1002/eji.201141550
Dutta M, Nezam M, Chowdhury S, Rakib A, Paul A, Sami SA, Uddin M, Rana M, Hossain S, Effendi Y, Idroes R. Appraisals of the Bangladeshi medicinal plant Calotropis gigantea used by folk medicine practitioners in the management of COVID-19: a biochemical and computational approach. Front Mol Biosci, 2021; 8:1–16; https://doi.org/10.3389/fmolb.2021.625391
Eddouks M, Andrade-Cetto A, Heinrich M, De Feo V, Cho WC. Editorial: mechanisms of traditional medicinal plants used to control type 2 diabetes or metabolic syndrome. Front Pharmacol, 2021; 11:2101; https://doi.org/10.3389/fphar.2020.617018
Fatimawali, Maulana RR, Windah ALL, Wahongan IF, Tumilaar SG, Adam AA, Kepel BJ, Bodhi W, Tallei TE. Data on the docking of phytoconstituents of betel plant and matcha green tea on SARS-CoV-2. Data Brief, 2021; 36:107049; https://doi.org/10.1016/j.dib.2021.107049
Feuillet V, Canard B, Trautmann A. Combining antivirals and immunomodulators to fight COVID-19. Trends Immunol, 2021; 42(1):31–44; https://doi.org/10.1016/j.it.2020.11.003
Gadaleta D, Vukovi? K, Toma C, Lavado GJ, Karmaus AL, Mansouri K, Kleinstreuer NC, Benfenati E, Roncaglioni A. SAR and QSAR modeling of a large collection of LD(50) rat acute oral toxicity data. J Cheminform, 2019; 11(1):58; https://doi.org/10.1186/s13321-019-0383-2
Ganeshpurkar A, Saluja A. In silico interaction of catechin with some immunomodulatory targets: a docking analysis. Indian J Biotechnol, 2018; 17:626–31.
Gates B. Responding to covid-19—a once-in-a-century pandemic? N Engl J Med, 2020; 382(18):1677–9; https://doi.org/10.1056/NEJMp2003762
Glowacki E, Irimia-Vladu M, Bauer S, Sariciftci NS. Hydrogen-bonds in molecular solids—from biological systems to organic electronics. J Mater Chem B, 2013; 1(31):3742–53. CrossRef
Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, Satlin MJ, Campion TR Jr, Nahid M, Ringel JB, Hoffman KL, Alshak MN, Li HA, Wehmeyer GT, Rajan M, Reshetnyak E, Hupert N, Horn EM, Martinez FJ, Gulick RM, Safford MM. Clinical characteristics of covid-19 in New York City. N Engl J Med, 2020; 382(24):2372–4; https://doi.org/10.1056/NEJMc2010419
Graf BL, Raskin I, Cefalu WT, Ribnicky DM. Plant-derived therapeutics for the treatment of metabolic syndrome. Curr Opin Investig Drugs, 2010; 11(10):1107–15. Available via https://pubmed.ncbi.nlm.nih.gov/20872313
Grancieri M, Martino HSD, Gonzalez de Mejia E. Digested total protein and protein fractions from chia seed (Salvia hispanica L.) had high scavenging capacity and inhibited 5-LOX, COX-1-2, and iNOS enzymes. Food Chem, 2019; 289:204–14; https://doi.org/10.1016/j.foodchem.2019.03.036
Grifoni E, Valoriani A, Cei F, Cei F, Lamanna R, Gelli AM, Ciambotti B, Vannucchi V, Moroni F, Pelagatti L, Tarquini R, Landini G. Interleukin-6 as prognosticator in patients with COVID-19. J Infect, 2020; 81(3):452–82; https://doi.org/10.1016/j.jinf.2020.06.008
Gupta A, Ahmad R, Siddiqui S, Yadav K, Srivastava A, Trivedi A, Ahmad B, Khan MA, Shrivastava AK, Singh GK. Flavonol morin targets host ACE2, IMP-α, PARP-1 and viral proteins of SARS-CoV-2, SARS-CoV and MERS-CoV critical for infection and survival: a computational analysis. J Biomol Struct Dyn, 2021:1–32; https://doi.org/10.1080/07391102.2021.1871863
Hanwell M, Curtis D, Lonie DC, Vandermeersch T, Zurek E, Hutchison G. Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J Cheminform, 2012; 4:17. CrossRef
Hariharan A, Hakeem AR, Radhakrishnan S, Reddy MS, Rela M. The role and therapeutic potential of NF-kappa-B pathway in severe COVID-19 patients. Inflammopharmacology, 2021; 29(1):91–100; https://doi.org/10.1007/s10787-020-00773-9
Harvey WT, Carabelli AM, Jackson B, Gupta RK, Thomson EC, Harrison EM, Ludden C, Reeve R, Rambaut A, Peacock SJ, Robertson DL. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol, 2021; 19(7):409–24; https://doi.org/10.1038/s41579-021-00573-0
He MM, Smith AS, Oslob JD, Flanagan WM, Braisted AC, Whitty A, Cancilla MT, Wang J, Lugovskoy AA, Yoburn JC, Fung AD, Farrington G, Eldredge JK, Day ES, Cruz LA, Cachero TG, Miller SK, Friedman JE, Choong IC, Cunningham BC. Small-molecule inhibition of TNF-alpha. Science, 2005; 310(5750):1022–5; https://doi.org/10.1126/science.1116304
Henderson LA, Canna SW, Schulert GS, Volpi S, Lee PY, Kernan KF, Caricchio R, Mahmud S, Hazen MM, Halyabar O, Hoyt KJ, Han J, Grom AA, Gattorno M, Ravelli A, De Benedetti F, Behrens EM, Cron RQ, Nigrovic PA. On the alert for cytokine storm: immunopathology in COVID-19. Arthritis Rheumatol, 2020; 72(7):105–63; https://doi.org/10.1002/art.41285
Herold T, Jurinovic V, Arnreich C, Lipworth BJ, Hellmuth JC, von Bergwelt-Baildon M, Klein M, Weinberger T. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol, 2020; 146(1):128–36.e4; https://doi.org/10.1016/j.jaci.2020.05.008
Hu B, Huang S, Yin L. The cytokine storm and COVID-19. J Med Virol, 2021; 93(1):250–6; https://doi.org/10.1002/jmv.26232
Jafarzadeh A, Chauhan P, Saha B, Jafarzadeh S, Nemati M. Contribution of monocytes and macrophages to the local tissue inflammation and cytokine storm in COVID-19: lessons from SARS and MERS, and potential therapeutic interventions. Life Sci, 2020; 257:118102; https://doi.org/10.1016/j.lfs.2020.118102
Jantan I, Ahmad W, Bukhari SNA. Plant-derived immunomodulators: an insight on their preclinical evaluation and clinical trials. Front Plant Sci, 2015; 6:655; https://doi.org/10.3389/fpls.2015.00655
Johnson T, Odoh KD, Nwonuma C, Akinsanmi A, Adegboyega AE. Biochemical evaluation and molecular docking assessment of the anti-inflammatory potential of Phyllanthus nivosus leaf against ulcerative colitis. Heliyon, 2020; 6:e03893. CrossRef
Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol, 2005; 5(10):749–59; https://doi.org/10.1038/nri1703
Karmakar B, Ghosh I, Talukdar P, Talapatra S. Leaf phytoligands of Annona reticulata Linn.: molecular docking approach against proinflammatory receptors to detect antiinflammatory small molecules. Pharma Innov J, 2019; 8:32–9.
Khairan K, Idroes R, Tallei TE, Jacob MJN and C. Bioactive compounds from medicinal plants and their possible effect as therapeutic agents against COVID-19: a review. Curr Nutr Food Sci, 2021; 17(6):621–33; https://doi.org/10.2174/1573401317999210112201439
Kwofie SK, Broni E, Asiedu SO, Kwarko GB, Dankwa B, Enninful KS, Tiburu EK, Wilson MD. Cheminformatics-based identification of potential novel anti-SARS-CoV-2 natural compounds of African origin. Molecules, 2021; 26(2); https://doi.org/10.3390/molecules26020406
Lee S-B, Wheeler MM, Patterson K, McGee S, Dalton R, Woodahl EL, Gaedigk A, Thummel KE, Nickerson DA. Stargazer: a software tool for calling star alleles from next-generation sequencing data using CYP2D6 as a model. Genet Med, 2019; 21(2):361–72; https://doi.org/10.1038/s41436-018-0054-0
Li G, Fan Y, Lai Y, Han T, Li Z, Zhou P, Pan P, Wang W, Hu D, Liu X, Zhang Q. Coronavirus infections and immune responses. J Med Virol; 2020; 92(4):424–32; https://doi.org/10.1002/jmv.25685
Li X, Chen L, Cheng F, Wu Z, Bian H, Xu C, Li W, Liu G, Shen X, Tang Y. In silico prediction of chemical acute oral toxicity using multi-classification methods. J Chem Inf Model, 2014; 54(4):1061–9; https://doi.org/10.1021/ci5000467
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev, 2001; 46(1–3):3–26; https://doi.org/10.1016/S0169-409X(00)00129-0
Liu Q, Zhou Y, Yang Z. The cytokine storm of severe influenza and development of immunomodulatory therapy. Cell Mol Immunol, 2016; 13(1):3–10; https://doi.org/10.1038/cmi.2015.74
Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther, 2017; 2(1):17023; https://doi.org/10.1038/sigtrans.2017.23
Marchand F, Perretti M, McMahon SB. Role of the immune system in chronic pain. Nat Rev Neurosci, 2005; 6(7):521–32; https://doi.org/10.1038/nrn1700
Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet, 2020; 395(10229):1033–4; https://doi.org/10.1016/S0140-6736(20)30628-0
Mercogliano MF, Bruni S, Elizalde P V, Schillaci R. Tumor necrosis factor α blockade: an opportunity to tackle breast cancer. Front Oncol, 2020; 10:584; https://doi.org/10.3389/fonc.2020.00584
Morris G, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem, 2009; 30(16):2785–91; https://doi.org/10.1002/jcc
Noor H, Ikram A, Rathinavel T, Kumarasamy S, Nasir Iqbal M, Bashir Z. Immunomodulatory and anti-cytokine therapeutic potential of curcumin and its derivatives for treating COVID-19—a computational modeling. J Biomol Struct Dyn, 2021:1–16; https://doi.org/10.1080/07391102.2021.1873190
Nugraha RV, Ridwansyah H, Ghozali M, Khairani AF, Atik N. Traditional herbal medicine candidates as complementary treatments for covid-19: a review of their mechanisms, pros and cons. Evid Based Complement Altern Med, 2020; 2020:2560645; https://doi.org/10.1155/2020/2560645
Pan SY, Litscher G, Gao SH, Zhou SF, Yu ZL, Chen HQ, Zhang SF, Tang MK, Sun JN, Ko KM. Historical perspective of traditional indigenous medical practices: the current renaissance and conservation of herbal resources. Evid Based Complement Alternat Med, 2014; 2014:525340; https://doi.org/10.1155/2014/525340
Parameswaran N, Patial S. Tumor necrosis factor-α signaling in macrophages. Crit Rev Eukaryot Gene Expr, 2010; 20(2):87–103; https://doi.org/10.1615/critreveukargeneexpr.v20.i2.10
Park MH, Hong JT. Roles of NF-κB in cancer and inflammatory diseases and their therapeutic approaches. Cells, 2016; 5(2):15; https://doi.org/10.3390/cells5020015
Parves MR, Mahmud S, Riza YM, Sujon KM, Uddin MA, Chowdhury M, Alam I, Islam M, Tithi FA, Alam M, Jui NR. Inhibition of TNF-alpha using plant-derived small molecules for treatment of inflammation-mediated diseases. Proceedings, 2021; 79(1); https://doi.org/10.3390/IECBM2020-08586
Pinzi L, Rastelli G. Molecular docking: shifting paradigms in drug discovery. Int J Mol Sci, 2019; 20(18):4331; https://doi.org/10.3390/ijms20184331
Quillin ML, Wingfield PT, Matthews BW. Determination of solvent content in cavities in IL-1β using experimentally phased electron density. Proc Natl Acad Sci, 2006; 103(52):19749–53; https://doi.org/10.1073/pnas.0609442104
Ragab D, Salah Eldin H, Taeimah M, Khattab R, Salem R. The COVID-19 cytokine storm; what we know so far. Front Immunol, 2020; 11:1446; https://doi.org/10.3389/fimmu.2020.01446
Rakib A, Paul A, Nazim Uddin Chy M, Uddin N, Sami SA, Baral SK, Majumder M, Tareq AM, Amin MN, Shahriar A, Uddin M. Biochemical and computational approach of selected phytocompounds from Tinospora crispa in the management of COVID-19. Molecules, 2020; 25(17); https://doi.org/10.3390/molecules25173936
Sailah I, Tumilaar SG, Lombogia LT, Celik I, Tallei TE. Molecular docking and dynamics simulations study of selected phytoconstituents of “Pangi” (Pangium edule Reinw ) leaf as anti-SARS-CoV-2. Philipp J Sci, 2021; 150:925–37.
Shih RH, Wang CY, Yang CM. NF-kappaB signaling pathways in neurological inflammation: a mini review. Front Mol Neurosci, 2015; 8:77; https://doi.org/10.3389/fnmol.2015.00077
Silva LCR, Ortigosa LCM, Benard G. Anti-TNF-α agents in the treatment of immune-mediated inflammatory diseases: mechanisms of action and pitfalls. Immunotherapy, 2010; 2(6):817–33; https://doi.org/10.2217/imt.10.67
Silva N, Júnior AF. Biological properties of medicinal plants: a review of their antimicrobial activity. J Venom Anim Toxins Incl Trop Dis, 2010; 16:402–13. CrossRef
Singh S, Sk MF, Sonawane A, Kar P, Sadhukhan S. Plant-derived natural polyphenols as potential antiviral drugs against SARS-CoV-2 via RNA-dependent RNA polymerase (RdRp) inhibition: an in silico analysis. J Biomol Struct Dyn, 2020:1–16; https://doi.org/10.1080/07391102.2020.1796810
Soni S, Ruhela RK, Medhi B. Nanomedicine in central nervous system (CNS) disorders: a present and future prospective. Adv Pharm Bull, 2016; 6(3):319–35; https://doi.org/10.15171/apb.2016.044
Sun X, Wang T, Cai D, Hu Z, Liao H, Zhi L, Wei H, Zhang Z, Qiu Y, Wang J, Wang A. Cytokine storm intervention in the early stages of COVID-19 pneumonia. Cytokine Growth Factor Rev, 2020; 53:38–42; https://doi.org/10.1016/j.cytogfr.2020.04.002
Tallei TE, Fatimawali AY, Idroes R, Kusumawaty D, Emran TB, Yesiloglu TZ, Sippl W, Mahmud S, Alqahtani T, Alqahtani AM, Asiri S. An analysis based on molecular docking and molecular dynamics simulation study of bromelain as anti-SARS-CoV-2 variants. Front Pharmacol, 2021a; 12:2192; https://doi.org/10.3389/fphar.2021.717757
Tallei TE, Pelealu JJ, Pollo HN, Pollo GA, Adam AA, Effendi Y, Karuniawan A, Rahimah S, Idroes R. Ethnobotanical dataset on local edible fruits in North Sulawesi, Indonesia. Data Brief, 2019; 27; https://doi.org/10.1016/j.dib.2019.104681
Tallei TE, Tumilaar SG, Lombogia LT, Adam AA, Sakib SA, Emran TB, Idroes R. Potential of betacyanin as inhibitor of SARS-CoV-2 revealed by molecular docking study. IOP Conf Ser Earth Environ Sci, 2021b; 711(1); https://doi.org/10.1088/1755-1315/711/1/012028
Tallei TE, Tumilaar SG, Niode NJ, Fatimawali, Kepel BJ, Idroes R, Effendi Y, Sakib SA, Emran TB. Potential of plant bioactive compounds as SARS-CoV-2 main protease (Mpro) and spike (s) glycoprotein inhibitors: a molecular docking study. Scientifica (Cairo), 2020; 2020:6307457; https://doi.org/10.1155/2020/6307457
Tang Y, Liu J, Zhang D, Xu Z, Ji J, Wen C. Cytokine storm in COVID-19: the current evidence and treatment strategies. Front Immunol, 2020; 11:1708; https://doi.org/10.3389/fimmu.2020.01708
Taniguchi K, Karin M. NF-κB, inflammation, immunity and cancer: coming of age. Nat Rev Immunol, 2018; 18(5):309–24; https://doi.org/10.1038/nri.2017.142
Tumilaar SG, Fatimawali F, Niode NJ, Effendi Y, Idroes R, Adam AA, Rakib A, Emran TB, Tallei TE. The potential of leaf extract of Pangium edule Reinw as HIV-1 protease inhibitor: a computational biology approach. J Appl Pharm Sci, 2021; https://doi.org/10.7324/JAPS.2021.110112
Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol, 1992; 10:411–52; https://doi.org/10.1146/annurev.iy.10.040192.002211
Wahab S, Ahmad I, Irfan S, Baig MH, Farouk AE, Dong JJ. Use of natural compounds as a potential therapeutic agent against COVID-19. Curr Pharm Des, 2021; 27(9):1144–52; https://doi.org/10.2174/1381612826666210101154118
Wallach D, Kang TB, Kovalenko A. Concepts of tissue injury and cell death in inflammation: a historical perspective. Nat Rev Immunol, 2014; 14(1):51–9; https://doi.org/10.1038/nri3561
Wang Z, Wang Y, Vilekar P, Yang SP, Gupta M, Oh MI, Meek A, Doyle L, Villar L, Brennecke A, Liyanage I. Small molecule therapeutics for COVID-19: repurposing of inhaled furosemide. PeerJ, 2020; 8:e9533; https://doi.org/10.7717/peerj.9533
Wen CC, Chen HM, Yang NS. Developing phytocompounds from medicinal plants as immunomodulators. Adv Bot Res, 2012; 62:197–272; https://doi.org/10.1016/B978-0-12-394591-4.00004-0
Xiao W, Wang D, Shen Z, Li S, Li H. Multi-body interactions in molecular docking program devised with key water molecules in protein binding sites. Molecules, 2018; 23(9); https://doi.org/10.3390/molecules23092321
Yang H, Li X, Cai Y, Wang Q, Li W, Liu G, Tang Y. In silico prediction of chemical subcellular localization via multi-classification methods. Med Chem Commun, 2017; 8(6):1225–34; https://doi.org/10.1039/C7MD00074J
Yang H, Lou C, Sun L, Li J, Cai Y, Wang Z, Li W, Liu G, Tang Y. admetSAR 2.0: web-service for prediction and optimization of chemical ADMET properties. Bioinformatics, 2019; 35(6):1067–9; https://doi.org/10.1093/bioinformatics/bty707
Yang S, Wang J, Brand DD, Zheng SG. Role of TNF–TNF receptor 2 signal in regulatory T cells and its therapeutic implications. Front Immunol, 2018; 9:784; https://doi.org/10.3389/fimmu.2018.00784
Ye Q, Wang B, Mao J. The pathogenesis and treatment of the “Cytokine Storm” in COVID-19. J Infect, 2020; 80(6):607–13; https://doi.org/10.1016/j.jinf.2020.03.037
Zhang ZL, Hou YL, Li DT, Li FZ. Laboratory findings of COVID-19: a systematic review and meta-analysis. Scand J Clin Lab Invest, 2020; 80(6):441–7; https://doi.org/10.1080/00365513.2020.1768587