INTRODUCTION
p style="text-indent:0pt; text-align:justify; margin-top:0.5em; margin-bottom:0.5em;">Coronavirus disease 2019 (COVID-19) caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has turned into a global health, social, and economic crisis. It was first reported and identified in Wuhan, China, in December 2019 as a cluster of pneumonia cases of unknown etiology (Lu et al., 2020). Initially referred to as 2019 novel coronavirus (2019-nCoV), the disease spread rapidly, causing millions of deaths across the world. In January 2020, the World Health Organization (WHO) declared the outbreak as a public health emergency of international concern (Sohrabi et al., 2020). Subsequently, after due research and analysis, this nCoV was named SARS-CoV-2 and the associated disease was referred to as COVID-19 (WHO, 2021a). With the global spread and increasing severity of the disease, the WHO characterized COVID-19 as a pandemic on March 11, 2020, and called it COVID-19/coronavirus pandemic (Cucinotta and Vanelli, 2020). As of June 10, 2021, more than 174 million confirmed cases and 3.7 million deaths have been reported worldwide. To this day, the USA is the worst hit country with more than 33 million confirmed cases and 0.59 million deaths, followed by India with 29.1 million confirmed cases and 0.36 million deaths (WHO, 2021b, COVID-19 dashboard).India started the COVID-19 vaccination program on January 16, 2021 and currently three vaccines have been approved for emergency public use, including COVAXIN, Covishield, and recently approved Sputnik-V. The emergency restricted use approval of COVAXIN was questioned due to lack of proper safety and efficacy data leading to lack of confidence and distrust among common people for indigenously developed vaccine. Moreover, COVAXIN trials also came under heavy scrutiny with unethical trial allegations. The approval was highly criticized by media and the public. People were hesitant in receiving COVAXIN and some Indian states delayed the vaccination drive owing to safety and lack of efficient data. In this article, we have made an effort to review the safety, immunogenicity, and efficacy of COVAXIN.
COVID-19 VACCINES
Vaccines represent a key weapon in fighting against COVID-19 for saving lives and ending this pandemic. Vaccines help human bodies to develop immunity against SARS-CoV-2 through T and B lymphocytes. Being an nCoV, vaccine development presented with considerable challenges. However, researchers all over the globe have carried out a commendable job in developing novel, safe, and effective COVID-19 vaccines in a limited timeline with extraordinary international collaborations.
Currently, a broad range of COVID-19 vaccines are in the clinical development process for evaluation of quality, safety, and efficacy. As of June 10, a total of 287 vaccines were currently in the developmental process, of which 102 vaccine candidates are in clinical development and 185 vaccine candidates are in the pre-clinical development phase (WHO, 2021c. Draft landscape of COVID-19 vaccines). Fifteen COVID-19 vaccine candidates have been approved for emergency public use. COVID-19 vaccines are classified into different platforms including mRNA, replicating viral vector, non-replicating viral vector, DNA, inactivated virus, live attenuated virus, and other protein subunit vaccines. The 16 COVID-19 vaccines authorized for the public use includes 7 inactivated vaccines (COVAXIN, BBIBP-CorV, WIBP-CorV, CoronaVac, CoviVac, QazVac, and 1 unnamed by Minhai Biotechnology Co.), 2 RNA-based vaccines (BNT162b2 and mRNA-1,273), 4 viral vector vaccines (AZD1222-Covishield, Ad26.COV2.S, Sputnik-V, and Ad5-nCoV), and 2 protein subunit vaccines (EpiVacCorona and RBD-Dimer) (WHO, 2021c. Draft landscape of COVID-19 vaccines).
COVAXIN
Inactivated vaccines are traditional vaccines in which the pathogens are killed/modified rendering the pathogen unable to replicate but retaining its immunogenicity so that the immune system can detect it and produce an immune response against such pathogens. The advantages of inactivated vaccines include better safety in populations with the compromised immune system, ease and low cost of production (Iversen and Bavari, 2021). Bharat Biotech in collaboration with the Indian Council of Medical Research and National Institute of Virology (NIV) developed India’s first indigenous COVID-19 vaccine called COVAXIN (BBV152). COVAXIN is a whole-virion β-propiolactone inactivated SARS-CoV-2 vaccine formulated with an imidazoquinoline molecule (Algel-IMDG), which is a toll-like receptor (TLR 7/8) agonist (Ella et al., 2021a).
In public interest, COVAXIN was granted restricted emergency approval by the Central Drugs Standard Control Organization on January 3, 2021, based on phase-I/II safety and immunogenicity clinical trials only (Mohapatra and Mishra, 2021). India started one of the world’s largest vaccination programs for COVID-19 on January 16, 2021, despite controversy and safety concerns (Bagcchi 2021; Bhuyan 2021). COVAXIN is a two-dose regimen administered 28 days apart and is supplied as ready to use liquid formulation in multi-dose vials stable at 2°C–8°C. Table 1 provides the list of preclinical and clinical research studies of COVAXIN published in various peer-reviewed journals.
The clinical trials were initiated after establishing the safety and protective efficacy in preclinical trials conducted in hamsters and non-human primates. The results of preclinical studies were published in nature communications and iScience–CellPress journals. The vaccine candidates successfully induced significant titers of SARS-CoV-2-specific IgG and neutralizing antibodies (Mohandas et al., 2020; Yadav et al., 2021a). Ganneru et al. (2021) also reported Th1 skewed antibody responses with an elevated IgG2a/IgG1 ratio and increased levels of SARS-CoV-2-specific IFN-γ+ CD4+ T-lymphocyte response induced due to inactivated vaccine formulation (Ganneru et al., 2021). The results of pre-clinical studies confirmed the safety and immunogenic potential of the vaccine candidates and supported human clinical trials.
Phase-I clinical trial
The results of the double-blind, randomized phase-I trial for safety and immunogenicity of inactivated SARS-CoV-2 vaccine, COVAXIN, were published in The Lancet on January 21, 2021 (Ella et al., 2021b). A total of 375 participants aged 18–55 years were randomized into four groups, three groups (n = 100 each) to be administered one of the three test vaccine formulations: 3 μg with Algel-IMDG, 6 μg with Algel-IMDG, 6 μg with Algel, and an Algel only control arm (n = 75). Two intramuscular (deltoid muscle) doses (0.5 ml/dose) of COVAXIN were administered on day 0 and day 14 to each participant. The primary outcome was an assessment of local and systemic reactogenicity events and secondary outcome was seroconversion rates. The study reported a good safety profile with pain at injection site, headache, fatigue, and fever as most common adverse events and no serious adverse events were observed. Overall, the incidence of adverse events reported was 14%–25%. The observed adverse events were mild (69%) and moderate (31%) in nature. Based on the micro-neutralization test (MNT50), seroconversion rates of 87.9%, 91.9%, and 82.8% were reported in 3 μg with Algel-IMDG, 6 μg with Algel-IMDG, and 6 μg with Algel groups, respectively. Based on the plaque-reduction neutralization test (PRNT50), seroconversion rates of 93.4%, 86.4% and 86.6% were reported in 3 μg with Algel-IMDG, 6 μg with Algel-IMDG, 6 μg with Algel groups respectively. Algel-IMDG based 3 and 6 μg formulations of COVAXIN enhanced the humoral and cellular immune response in study participants and both were selected for phase-II clinical trial (Ella et al., 2021b).
Phase-II clinical trial
The results of the double-blind, randomized phase-II trial evaluating safety and immunogenicity of COVAXIN were published on March 8, 2021, in The Lancet along with 3-month follow-up of phase-I trial (Ella et al., 2021a). In this study, a total of 380 participants aged 12–65 years were randomly assigned into two groups of 3 μg with Algel-IMDG (n = 190) and 6 μg with Algel-IMDG (n = 190). Two intramuscular (deltoid muscle) doses (0.5 ml/dose) of COVAXIN were administered to each participant on day 0 and day 28. Both vaccine formulations were well tolerated and showed similar safety profiles with no serious adverse events. The primary outcome of the phase-II immunogenicity study was Anti-IgG responses against spike protein (S1) glycoprotein, receptor-binding domain, and nucleocapsid protein of SARS-CoV-2 expressed as geometric mean titers (GMTs). MNT50 and PRNT50 were used to assess neutralizing antibody titers in serum samples (Ella et al., 2021a).
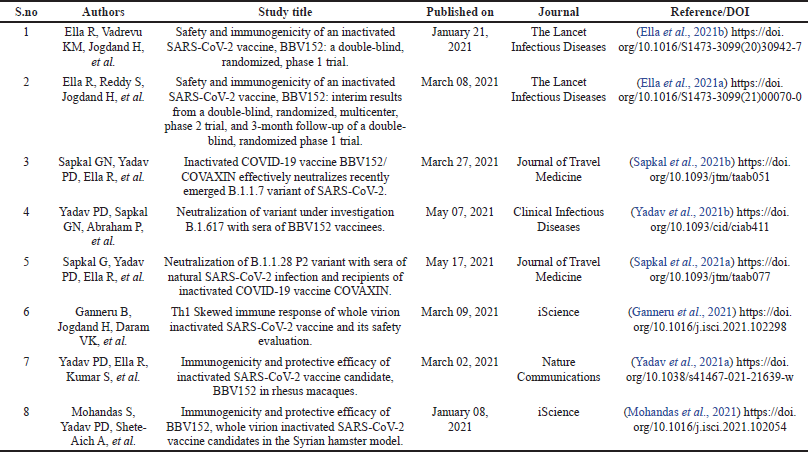 | Table 1. Published clinical and pre-clinical research studies of COVAXIN. [Click here to view] |
The researchers reported a seroconversion rate of 92.9% in 3 μg with Algel-IMDG group and 98.3% in 6 μg with Algel-IMDG group based on PRNT50. Based on MNT50, a seroconversion rate of 88.0% and 96.6% was reported in 3 μg with Algel-IMDG group and 98.3% in 6 μg with Algel-IMDG group, respectively. GMTs (PRNT50 and MNT50) were significantly higher in 6 μg with Algel-IMDG group than 3 μg with Algel-IMDG group. Both the study groups elicited T-cell response biased to Th1 phenotype. Three-month post-second dose follow-up results reported by Ella et al. (2021) showed that GMTs (MNT50) were 39.9, 69.5, and 53.3 in 3 μg with Algel-IMDG, 6 μg with Algel-IMDG, and 6 μg with Algel groups, respectively. Based on the interim results of phase-II clinical trial, researchers selected COVAXIN 6 μg with Algel-IMDG formulation for phase-III efficacy trial (Ella et al., 2021a).
Both phase-I and phase-II clinical trials of inactivated SARS-CoV-2 vaccine demonstrate that COVAXIN is safe and well tolerated with no serious adverse effects and significant neutralizing antibody response. The phase-I/II trials reported no association between the dose of vaccine and observed adverse events (Ella et al., 2021a, 2021b). However, evaluation of long-term safety outcomes needs phase-III trials. The phase-II trial reported better reactogenicity and more enhanced humoral and cell-mediated immune responses as compared to phase-I trial. The 3-month post-second dose follow-up showed that neutralizing antibody responses persisted and T-cell memory responses were more pronounced in 6 μg with Algel-IMDG group (Ella et al., 2021a). Phase-I trial also established that COVAXIN induced T-cell memory responses (antigen recall memory) as demonstrated by increased antigen-specific CD4+ T cells supporting the results of phase-I trial.
The phase-I/II safety and immunogenicity clinical trials of COVAXIN vaccine were based on NIV-2020-770 homologous and two heterologous strains. However, Sapkal et. al. (2021b) demonstrated a comparable neutralization activity of COVAXIN vaccinated human serum against SARS-CoV-2 variant B.1.1.7 (UK-variant) and other heterologous strains (Bharat Biotech, 2021).
Phase-III clinical trial
COVAXIN is currently undergoing randomized, double-blind, and placebo controlled phase-III clinical trial. Bharat Biotech announced the first interim analysis of phase-III results. According to the report posted on Bharat Biotech’s official website, the phase-III trial involved 25,800 participants aged 18–98 years (including 2,433 above the age of 60 and 4,500 with comorbidities). The report states that COVAXIN has demonstrated an efficacy of 77.8% against symptomatic COVID-19 disease and 93.4% effective against severe COVID-19 disease (Bharat Biotech, 2021).
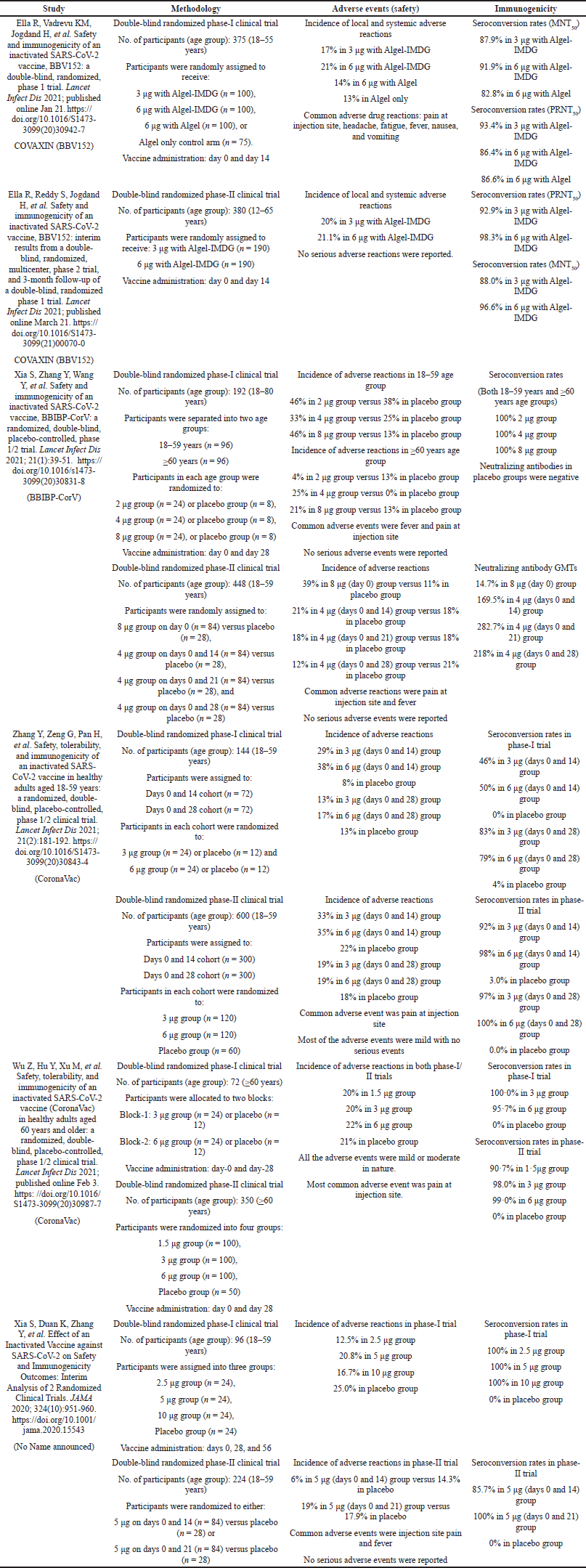 | Table 2. Description of the results from published phase-I/II safety and immunogenicity studies of inactivated COVID-19 vaccines. [Click here to view] |
DISCUSSION
COVAXIN, a whole virion inactivated SARS-CoV-2 vaccine was granted restricted emergency approval based on safety and immunogenicity studies only without phase-III clinical trial and evidence on efficacy of the vaccine. The incomplete evidence-based regulatory approval was criticized, leading to controversy regarding safety concerns for the indigenously developed vaccine. The WHO has recommended that a minimum criterion for any acceptable COVID-19 vaccine should be a clear demonstration of efficacy (on a population basis) ideally with ~50% point estimate and the efficacy can be assessed against the “disease, severe disease, shedding or transmission” endpoints (WHO, 2021d. Target product profiles for COVID-19 vaccines).
The safety profile and incidence of adverse events due to COVAXIN were similar to other inactivated SARS-CoV-2 vaccines like BBIBP-CorV and CoronaVac (Xia et al., 2021; Zhang et al., 2021). However, the local and systemic adverse events due to COVAXIN are lower when compared to other vaccine platforms like mRNA and viral-vector-based vaccines. COVAXIN has also demonstrated a similar immunogenicity profile and enhanced immune response as reported by other inactivated vaccines (Wu et al., 2021; Xia et al., 2020; Xia et al., 2021; Zhang et al., 2021). The detailed description of safety and immunogenicity of inactivated COVID-19 vaccine candidates reported in published studies from phase-I/II clinical trials is presented in Table 2.
One of the significant results reported from the phase-I/II trials was demonstration of enhanced humoral as well as cell-mediated immune response among COVAXIN recipients (Ella et al., 2021a; Ella et al., 2021b). Although CD4+ and CD8+ T-cell responses were reported in a subset of participants only in phase-I trial but phase-II trial reported much enhanced cell-mediated immune response (Ella et al., 2021b). COVAXIN enhanced the T-cell memory response as indicated by increased CD4+, CD45RO+, and CD27+ T-cell population confirming the antigen recall memory response (Ella et al., 2021b). Again the 3-month follow-up results of phase-I trial reported that the COVAXIN-induced neutralizing antibody response persisted in all study participants after the 3-month follow-up period (Ella et al., 2021a). Other inactivated COVID-19 vaccines have not reported any cell-mediated immunity development in their phase-I/II clinical trials. Moreover COVAXIN vaccinated human serum has shown comparable antibody neutralization activity against SARS-CoV-2 variant B.1.1.7, B.1.617, and other heterologous strain (Sapkal et al., 2021a; Sapkal et al., 2021b; Yadav et al., 2021b).
While traditional inactivated vaccines are formulated with alum as an adjuvant, COVAXIN is formulated with a toll-like- receptor (TLR 7/8) agonist adjuvant molecule (Ella et al., 2021b). The alum-based inactivated vaccines typically develop Th2 biased responses leading to safety concerns. Th2 cell-mediated response is implicated in the development of eosinophilic lung immunopathology (Bessa and Bachmann, 2010). However, the TLR 7/8 agonist adjuvant in COVAXIN primarily produces Th1 biased response with minimal Th2 response providing protection against vaccine-induced lung pathology (Ella et al., 2021a; Ella et al., 2021b). Moreover, TLR 7/8 agonist also induces IgA production, thereby contributing to the immunogenicity of the vaccine (Meiler et al., 2008; Bessa and Bachmann, 2010).
Bharat Biotech’s report states that COVAXIN has demonstrated high clinical efficacy of 78% in preventing COVID-19 among individuals without prior infection after two dose regimen (Bharat Biotech, 2021). The Sinopharm inactivated vaccine (BBIBP-CorV) has reportedly shown an efficacy of 86% in efficacy trials conducted in UAE and Bahrain, but the efficacy data have not been published yet (Cyranoski, 2020). The stated efficacy of CoronaVac developed by Sinovac has reported varying efficacy results. Researchers in Brazil reported that CoronaVac showed an efficacy of 78% which was later revised to 50.4% efficacy in preventing severe and mild COVID-19 in trials conducted in Brazil, which is significantly lower than the reported efficacy of other inactivated vaccines. However, the CoronaVac Turkish trials have reported an efficacy of 91.25% which was again revised to 83.5% in final analysis (Mallapaty, 2020). The efficacy data on all inactivated vaccines including COVAXIN has not been made public yet and the data needs to be peer-reviewed.
Based on the preliminary results of phase-III trial results, COVAXIN has a lower efficacy (78%) than other types of COVID-19 vaccines mRNA-1,273 (Baden et al., 2021) which is mRNA-based COVID-19 vaccine developed by Moderna showing 95% efficacy and Comirnaty (BNT162b2) (Polack et al., 2020) which is also an mRNA-based vaccine developed by Pfizer and BioNTech showing 95% vaccine efficacy in two dose regimen in protection against COVID-19 in phase-III safety efficacy trials. However, the stated efficacy of COVAXIN is higher than Covishield (AZD1222) an adenovirus vaccine developed by AstraZeneca and Oxford vaccine group reporting an overall vaccine efficacy of 70.4% after two doses and 64.1% after a single dose (Voysey et al., 2021). The safety and efficacy data from Moderna (mRNA-1,273), Comirnaty (BNT162b2), and Covishield (AZD1222) have been published in peer-reviewed journals (Baden et al., 2021; Polack et al., 2020; Voysey et al., 2021).
CONCLUSION
Evidence suggests that COVAXIN is a safe, well-tolerated and immunogenic vaccine. COVAXIN has also demonstrated comparable effectiveness against mutant SARS-CoV-2 strains as well. The safety and immunogenicity of COVAXIN has been demonstrated in adolescents, adults, and elderly people. The preliminary efficacy significantly exceeds the WHO and FDA recommended minimum acceptable criteria for COVID-19 vaccine approval. However, the COVAXIN phase-III clinical trial data needs to be made public and peer reviewed for better transparency and building confidence in indigenously developed vaccine and shedding COVAXIN hesitancy among common masses.
CONFLICTS OF INTERESTS
The authors report no financial or any other conflicts of interest in this work.
FUNDING
There is no funding to report.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
AUTHORS’ CONTRIBUTIONS
Mukhtar Ahmad Dar and Sameer Dhingra designed the review. Mukhtar Ahmad Dar and Priyadarshani Chauhan contributed to the literature review, drafting the manuscript, and editing throughout the process. Pawan Kumar contributed to literature search and manuscript writing. Sameer Dhingra contributed as supervisor, manuscript editing, and revising process. Richa Chauhan, Krishna Murti, Jaykaran Charan, and Velayutham Ravichandiran contributed in manuscript editing, revisions, and proofreading. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines. The final draft of the manuscript was read and approved by all the authors for submission.
REFERENCES
Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med, 2021; 384(5):403–16. CrossRef
Bagcchi S. The world’s largest COVID-19 vaccination campaign. Lancet Infect Dis, 2021; 21(3):323. CrossRef
Bessa J, Bachmann MF. T Cell-dependent and -independent IgA responses: role of TLR signalling. Immunol Invest, 2010; 39(4-5):407–28. CrossRef
Bharat Biotech. Bharat Biotech announces phase 3 results of COVAXIN. Bharat Biotech, 2021. Available via https://www.bharatbiotech.com/images/press/covaxin-phase3-efficacy-results.pdf (Accessed 10 June 2021)
Bhuyan A. India begins COVID-19 vaccination amid trial allegations. Lancet, 2021; 397(10271):264. CrossRef
Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed, 2020; 91(1):157–60.
Cyranoski D. Arab nations first to approve chinese COVID vaccine. Nature, 2020; 588:2. CrossRef
Ella R, Reddy S, Jogdand H, Sarangi V, Ganneru B, Prasad S, Das D, Raju D, Praturi U, Sapkal G, Yadav P, Reddy P, Verma S, Singh C, Redkar SV, Gillurkar CS, Kushwaha JS, Mohapatra S, Bhate A, Rai S, Panda S, Abraham P, Gupta N, Ella K, Bhargava B, Vadrevu KM. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: interim results from a double-blind, randomised, multicentre, phase 2 trial, and 3-month follow-up of a double-blind, randomised phase 1 trial. Lancet Infect Dis, 2021a; 21(7):950–61. CrossRef
Ella R, Vadrevu KM, Jogdand H, Prasad S, Reddy S, Sarangi V, Ganneru B, Sapkal G, Yadav P, Abraham P, Panda S, Gupta N, Reddy P, Verma S, Kumar Rai S, Singh C, Redkar SV, Gillurkar CS, Kushwaha JS, Mohapatra S, Rao V, Guleria R, Ella K, Bhargava B. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: a double-blind, randomised, phase 1 trial. Lancet Infect Dis, 2021b; 21(5):637–46. CrossRef
Ganneru B, Jogdand H, Daram VK, Das D, Molugu NR, Prasad SD, Kannappa SV, Ella KM, Ravikrishnan R, Awasthi A, Jose J. Th1 skewed immune response of whole virion inactivated SARS CoV 2 vaccine and its safety evaluation. iScience, 2021; 24(4):102298. CrossRef
Iversen PL, Bavari S. Inactivated COVID-19 vaccines to make a global impact. Lancet Infect Dis, 2021; 21(6):746–8. CrossRef
Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle. J Med Virol, 2020; 92(4):401–2. CrossRef
Mallapaty S. China COVID vaccine reports mixed results — what does that mean for the pandemic? Nature, 2020; 1–6. CrossRef
Meiler F, Klunker S, Zimmermann M, Akdis CA, Akdis M. Distinct regulation of IgE, IgG4 and IgA by T regulatory cells and toll-like receptors. Allergy Eur J Allergy Clin Immunol, 2008; 63(11):1455–63. CrossRef
Mohandas S, Yadav PD, Shete-Aich A, Abraham P, Vadrevu KM, Sapkal G, Mote C, Nyayanit D, Gupta N, Srinivas VK, Kadam M, Kumar A, Majumdar T, Jain R, Deshpande G, Patil S, Sarkale P, Patil D, Ella R, Prasad SD, Sharma S, Ella KM, Panda S, Bhargava B. Immunogenicity and protective efficacy of BBV152, whole virion inactivated SARS-CoV-2 vaccine candidates in the Syrian hamster model. iScience, 2020; 24(2): 1–11. CrossRef
Mohapatra PR, Mishra B. Regulatory approval of COVID-19 vaccine for restricted use in clinical trial mode. Lancet Infect Dis, 2021; 21(5):599–600. CrossRef
Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck Jr RW, Hammitt LL, Türeci O, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, ?ahin U, Jansen KU, Gruber WC, C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med, 2020; 383(27):2603–15. CrossRef
Sapkal G, Yadav PD, Ella R, Abraham P, Patil DY, Gupta N, Panda S, Mohan K, Bhargava B. Neutralization of VUI B.1.1.28 P2 variant with sera of COVID-19 recovered cases and recipients of Covaxin an inactivated COVID-19 vaccine. J Travel Med, 2021a; 1–3. CrossRef
Sapkal GN, Yadav PD, Ella R, Deshpande GR, Sahay RR, Gupta N, Vadrevu KM, Abraham P, Panda S, Bhargava B. Inactivated COVID-19 vaccine BBV152/COVAXIN effectively neutralizes recently emerged B.1.1.7 variant of SARS-CoV-2. J Travel Med. 2021b; 28(4):1–3. CrossRef
Sohrabi C, Alsafi Z, O’Neill N, Khan M, Kerwan A, Al-Jabir A, Iosifidis C, Agha R. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19). Int J Surg, 2020; 76(2):71–6. CrossRef
Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, Bibi S, Briner C, Cicconi P, Collins AM, Colin-Jones R, Cutland CL, Darton TC, Dheda K, Duncan CJA, Emary KRW, Ewer KJ, Fairlie L, Faust SN, Feng S, Ferreira DM, Finn A, Goodman AL, Green CM, Green CA, Heath PT, Hill C, Hill H, Hirsch I, Hodgson SHC, Izu A, Jackson S, Jenkin D, Joe CCD, Kerridge S, Koen A, Kwatra G, Lazarus R, Lawrie AM, Lelliott A, Libri V, Lillie PJ, Mallory R, Mendes AVA, Milan EP, Minassian AM, McGregor A, Morrison H, Mujadidi YF, Nana A, O'Reilly PH, Padayachee SD, Pittella A, Plested E, Pollock KM, Ramasamy MN, Rhead S, Schwarzbold AV, Singh N, Smith A, Song R, Snape MD, Sprinz E, Sutherland RK, Tarrant R, Thomson EC, Török ME, Toshner M, Turner DPJ, Vekemans J, Villafana TL, Watson MEE, Williams CJ, Douglas AD, Hill AVS, Lambe T, Gilbert SC, Pollard AJ,Oxford COVID Vaccine Trial Group. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet, 2021; 397(10269):99–111. CrossRef
World Health Organization. Naming the coronavirus disease (COVID-19) and the virus that causes it. World Health Organization, Geneva, Switzerland, 2021a. Available via https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it (Accessed 08 June 2021)
World Health Organization. WHO coronavirus disease (COVID-19) dashboard. World Health Organization, Geneva, Switzerland, 2021b. Available via https://covid19.who.int/ (Accessed 10 June 2021)
World Health Organization. Draft landscape and tracker of COVID-19 candidate vaccines. World Health Organization, Geneva, Switzerland, 2021c. Available via https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (Accessed 10 June 2021)
World Health Organization (WHO). 2021d. WHO target product profiles for COVID-19 vaccines. World Health Organization, Geneva, Switzerland, 2021d. Available via https://www.who.int/publications/m/item/who-target-product-profiles-for-covid-19-vaccines (Accessed 10 June 2021)
Wu Z, Hu Y, Xu M, Chen Z, Yang W, Jiang Z, Li M, Jin H, Cui G, Chen P, Wang L, Zhao G, Ding Y, Zhao Y, Yin W. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis, 2021; 21(6):803–12. CrossRef
Xia S, Duan K, Zhang Y, Zhao D, Zhang H, Xie Z, Li X, Peng C, Zhang Y, Zhang W, Yang Y, Chen W, Gao S, You W, Wang X, Wang Z, Shi Z, Wang Y, Yang X, Zhang L, Huang L, Wang Q, Lu J, Yang Y, Guo J, Zhou W, Wan X, Wu C, Wang W, Huang S, Du J, Meng Z, Pan A, Yuan Z, Shen S, Guo W, Yang X. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA - J Am Med Assoc, 2020; 324(10):951–60. CrossRef
Xia S, Zhang Y, Wang Y, Wang H, Yang Y, Gao GF, Tan W, Wu G, Xu M, Lou Z, Weijin Huang W, Xu W, Huang B, Wang H, Wang W, Zhang W, Li N, Xie Z, Ding L, You W, Zhao Y, Yang X, Liu Y, Wang Q, Huang L, Yang Y, Xu G, Luo L, Wang W, Liu P, Guo W, Yang X. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis, 2021; 21(1):39–51. CrossRef
Yadav PD, Ella R, Kumar S, Patil DR, Mohandas S, Shete AM, Vadrevu KM, Bhati G, Sapkal G, Kaushal H, Patil S. Immunogenicity and protective efficacy of inactivated SARS-CoV-2 vaccine candidate, BBV152 in rhesus macaques. Nat Commun, 2021a; 12(1):1–1. CrossRef
Yadav PD, Sapkal GN, Abraham P, Ella R, Deshpande G, Patil DY, Nyayanit DA, Gupta N, Sahay RR, Shete AM, Panda S, Bhargava B, Mohan VK. Neutralization of variant under investigation B.1.617 with sera of BBV152 vaccinees. Clin Infect Dis, 2021b. CrossRef
Zhang Y, Zeng G, Pan H, Li C, Hu Y, Chu K, Han W, Chen Z, Tang R, Yin W, Chen X, Hu Y, Liu X, Jiang C, Li J, Yang M, Song Y, Wang X, Gao Q, Zhu F. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis, 2021; 21(2):181–92. CrossRef