INTRODUCTION
The ongoing COVID-19 pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has enveloped the entire globe with severe impositions for almost every phase of human life (Nicola et al., 2020; The World Bank, 2020). Globally, 174,439,909 confirmed cases of COVID-19, including 3,768,987 deaths, as reported by World Health Organization (WHO). As of June 11, 2021, a total of 2,156,550,767 vaccine doses have already been administered (World Health Organization, 2021a). The rapid upsurge in active COVID-19 cases produced a significant health care crisis due to a lack of alertness to confront a sudden pandemic, especially in the developing nations (Blumenthal et al., 2020). At the onset of COVID-19, disease management faced several issues because of no availability of specific effective medication (Aygün et al., 2020; Elengoe, 2020). Hence, a hit and trial strategy of drug repositioning/repurposing with older medication invented for different indications was implanted to find an answer to this dreadful disease (Harrison, 2020; Li, 2019). However, several therapies were being tried and are still under clinical trials to prove their efficacy, yet infection control measures, sanitation, symptomatic, and supportive therapy has been the cornerstone of effective clinical management of the COVID-19 (Blumenthal et al. 2020; Bundgaard et al., 2021; Lio et al., 2021). Later, Food and Drug Administration (FDA) approved Remdesivir for the treatment of COVID-19 on October 22, 2020 (FDA, 2020a).
As there was no definitive therapy in conjunction with a tremendous rise in the number of cases, an effective vaccine vestiges the only answer in building immunity to halt the disease’s further progression (Bundgaard et al., 2021; Chowdhury et al., 2020; Green, 2020; Haque, 2020; Kaur & Gupta, 2020; Tabish & Basch, 2020; World Health Organization, 2021b). Multiple institutes, including the Organization for Economic Co-operation and Development (OECD), WHO reported that vaccine development and vaccination of the majority population most probably the best remedy to achieve the ultimate, long-standing defensive action strategy against COVID-19 miseries (Organization for Economic Co-operation and Development, 2021; World Health Organization, 2020). Additionally, the SARS-CoV-2 (coronavirus), the principal offender of COVID-19 disease, the genetic sequence was publicly available on January 11th, 2020. This discovery instigated several robust research targeting vaccine development against COVID-19 (Thanh et al., 2020). Consequently, connoisseurs, statesmen, politicians, opinion leaders, and many professional groups believe and expect that in the current pandemic situation, the vaccine can only minimize morbidity, mortality, and transmission and offers the most remarkable optimism of coming back to everyday life (Shrotri et al., 2021). Later, WHO developed a new webpage named “COVID-19 vaccine tracker and landscape” reported a total of 287 vaccine candidates under development and of which 102 were in the clinical trial phase, and 185 were under preclinical development till June 11, 2021 (World Health Organization, 2021b).
The COVID-19 vaccines in clinical development are mostly protein subunit vaccines, viral vector (non-replicating), nano-particles, DNA, inactivated virus, and RNA vaccines (Kaur & Gupta, 2020; Kyriakidis et al., 2021; Shin et al., 2020; Yan et al., 2021). Most of the trials regarding COVID-19 vaccine underway and few clearing phase-3 studies, the pharmaceutical industries applied obtained for the emergency use authorization (EUA) of few vaccines (Oliver et al., 2020, 2021; Rizk et al., 2021). There was a total of nine vaccines that have been approved around the world, namely, Comirnaty (BNT162b2), Moderna COVID-19 Vaccine (mRNA-1273), CoronaVac, COVID-19 Vaccine AstraZeneca (AZD1222), a vaccine from Sinopharm, and the Wuhan Institute of Virology, Sputnik V, BBIBP-CorV, EpiVacCorona, and Covaxin, as of January 29, 2021 (Craven, 2021). On December 11, 2020, the FDA gave EUA the Pfizer-BioNTech vaccine for COVID-19 to be distributed in the United States of America for individuals aged 16 years and older (Oliver et al., 2020). Later, by December 18, 2020, FDA also approved the Moderna vaccine for COVID-19 for use in individuals 18 years of age and older (Oliver et al., 2021). However, FDA reported that emphasizing the EUA is only based on limited efficacy safety data, and it is not full approval of these COVID-19 vaccines (Angelis & Darrow; 2021; National Center for Immunization and Respiratory Diseases, 2021). In India, two vaccines, namely, Covishield from Serum Institute of India and Covaxin from Bharat Biotech, received Restricted Emergency Approval for prevention of COVID-19 (Press Information Bureau Government of India, 2021). Although these COVID-19 vaccines have been approved for emergency use, their long-term efficacy is yet to be established (Cyranoski, 2020; Singh and Upshur, 2021).
It is incredibly critical to monitor the vaccine safety and/or serious adverse events (SAEs) using Pharmacovigilance which is defined as “the science and activities related to the detection, assessment, understanding, and prevention of adverse effects or any other drug-related problem” (World Health Organization, 2021c). WHO maintains a global database of adverse events (AEs) through VigiBase®, which maintains the global safety data of various therapeutic interventions as Individual Case Safety Reports (ICSRs) for implementation strict pharmacovigilance (Bergvall et al., 2014; Charan et al., 2021; Dutta, 2018; Kaur et al., 2020; Kuemmerle et al., 2011). The VigiBase, the WHO global ICSR database system, came into existence in 1968 and consists of over 20 million ICSR from over 130 countries (Lindquist, 2008). ICSRs are also known as spontaneous or voluntary reports generated in the post-marketing phase of the drug (Gliklich et al., 2014; Moore et al., 2020; Sharrar & Dieck, 2013). Each ICSR contains information regarding patients’ demographics, drugs, AEs, and administrative information (Kröger et al., 2015; Upsala Monitoring Center, 2021; Wysowski & Swartz, 2005).
This descriptive analysis is an extension to the studies conducted using the same database on drugs used in COVID-19 therapeutics, including Favipiravir, Remdesivir, and Tocilizumad (Charan et al., 2021a, 2021b; Kaur et al., 2020), and primarily focuses on identifying and describing various SAEs reported for the COVID-19 vaccines through the WHO database, thereby facilitating identification of safer vaccines, preventing patients from unnecessary tribulations, and reducing the hospitalization and treatment costs in order to assure rational vaccination regimens and strategies.
METHODOLOGY
Data source
This study was conducted on data obtained from the VigiBase®, maintained by the WHO Uppsala monitoring center, Uppsala, Sweden. All vaccine safety data reported from December 15th, 2020 to January 24th, 2021 were analyzed. Data were cleaned from duplicates and irrelevant entries by the first and second authors (SD, RK), and any discrepancy for removal or retention of individual entries for the analysis was resolved by discussion and consensus in the presence of the first corresponding author (JC). This database has all the data reported in the form of adverse drug events associated with COVID-19 vaccines using ICSRs, the VigiBase– —the unique WHO global database, Uppsala Monitoring Centre, Uppsala, Sweden (https://www.who-umc.org/vigibase/vigibase/). In addition, the detailed information regarding patient’s demographics (age, gender, country, and medical history); drugs (an indication of use, route of administration, start and end date); AEs (date of onset, seriousness, outcome, dechallenge and rechallenge outcomes, and causality) and administrative information (type and source of report) were recorded.
Data interpretation and analysis
Each report in VigiBase® represents an individual AEs, and there could be more than one report for a single individual; thus, the number of reported AEs were more than the number of individuals who had an adverse event. Hence, the data were cleaned manually to remove the duplicates in the same AEs reported for the same individual in different terminologies.
All the adverse drug reactions in the ICSR are automatically coded as per medical dictionary for regulatory activities (MedDRA) (https://www.who-umc.org/vigibase/vigibase/) and WHO-ART terminology (https://www.who-umc.org/vigibase/vigibase/know-more-about-vigibase/). MedDRA is the hierarchical terminology that is composed of five levels: lowest level terms, preferred term (PTs), high-level group terms, high–level terms, and system organ classification (SOCs) (ICH 2021a, 2021b).
In the present study, the SOC and PT categories of AEs were only employed for the analysis. Here, PT refers to a clinical condition in the form of symptom, sign, diagnosis, investigation, medical, social, family history, and characteristic surgical or medical procedures (ICH, 2021b). Each PT is linked to specific SOC, which is grouping by manifestation site (e.g. Cardiovascular disorder), etiology (infections and infestations), and purpose (surgical and medical procedures) (Bousquet et al., 2005). The age, gender, and severity of all the AEs were compared with the SOC and PT. The adverse event's seriousness was decided as per the ICH E2B criteria, which identifies SAEs as those leading to either life-threatening event, hospitalization, disability, congenital abnormality, or death (ICH, 2021c).
Ethical approval
This study had no direct interaction with human participants and was based on the WHO’s database (VigiBase®); hence, ethical approval was not required.
Statistical analysis
Descriptive statistics were reported in the form of frequency and percentages. The cross-tabulation function of Statistical Package for Social Science (SPSS) version 23 (IBM Corp. Released 2015. IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp.) was used for the analysis.
RESULTS
A total of 103,954 AEs were reported till January 24th, 2021 from 32,044 subjects (Average 3.24 AEs per person). Out of the total subjects, 5,731 (17.9%) were males, and 25,652 (80%) were females. Thus, 28,799 (27.7%) AEs from 8,007 individuals were categorized as SAEs (Fig. 1). The majority of SAEs were reported in females and between the age group of 18 and 64 years. Around 83% of the SAEs were reported from European countries (n = 23,987), followed by Americas (n = 4,795) and Asia (n = 17) (Fig. 2). In almost 74% of cases, the BNT162b2 (Pfizer) vaccine was used. Around 1% of SAEs were fatal (Table 1).
The majority of the SAEs were reported from the broad category “general disorders and administration site conditions” (30%), followed by “Nervous system disorders” (19.1%), “Musculoskeletal and connective tissue disorders” (11.2%), and “Gastrointestinal disorders” (10.7%) (Table 2).
Upon analyzing the PTs in the broad categories, 28,799 SAEs were reported. Headache (8.11%) of various types were the most common SAEs, followed by pyrexia (7.09%), fatigue (5.18%), nausea (4.4%), chills (4.2%), and myalgia (3.9%). Pain (1.93%), pain in extremity (1.98%), and vaccination site /injection site/administration site pain (1.88) accounted for another major portion of SAEs (Table 3).
| Figure 1. Schematic Diagram of assessment of Adverse Events associated with COVID-19 vaccines in VigiBase database. |
Table 4 describes the distribution of serious and non-serious AEs between the type of vaccines, gender, and age groups. Comparing serious versus non-serious AEs with various vaccine candidates shows the probability of serious AEs is comparatively low compared to the non-serious AEs. The age group > 65 years had more serious AEs as compared to other age groups. There was an equal distribution of serious and non-serious AEs between males and females.
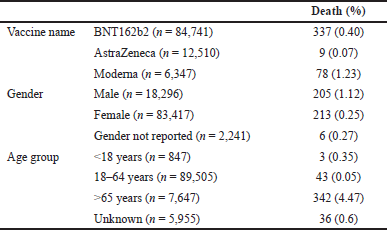
There was a total of 424 deaths. The distribution of these deaths per the vaccines, gender, and age group has been mentioned in Table 5.
DISCUSSION
The present study was conducted to assess the SAEs reported in the global pharmacovigilance database (VigiBase®) associated with various COVID-19 vaccines like BNT162b2 (Pfizer) AZD1222/ChAdOx1 nCoV-19(AstraZeneca), Moderna, etc. that are currently being used across the world for vaccination. Approximately, one-third of the total AEs reported were serious in nature. The majority of the AEs reported were from the female subjects and age group 18–64 years. In addition, a significant chunk of AEs was reported from Europe, and in the majority of the cases, the BNT162b2 (Pfizer) vaccine was used. The reason for More AE reporting for BNT162b2 is that during the study period, maximum vaccination was done with BNT162b2, whereas the number of individuals vaccinated with other vaccines was comparatively less; thus, it is imperative to observe maximum AE with BNT162b2. The AEs reported were commonly classified under general disorders and administration site conditions with headache, fever, and fatigue as the commonest AEs observed.
| Figure 2. Distribution of Serious Adverse Events reported in VigiBase associated with COVID-19 vaccines across continents. |
| Table 1. Characteristics of SAEs (28,799 SAEs reported from 8,007 individuals) reported for COVID-19 vaccines in WHO database (n = 28799). |
In the current analysis, the ratio of serious and non-serious AEs was similar amongst males and females, but more numbers were reported from females. Multiple US studies reported that after the first dose of Pfizer-BioNTech COVID-19 Vaccine, almost 90% of the anaphylactic reactions and non-anaphylactic allergic reactions were observed in females, among which 81% of them with anaphylaxis and 67% with non-anaphylactic allergic reactions had a history of allergic reactions (CDC COVID-19 Response Team; Food and Drug Administration, 2021; Shimabukuro & Nair, 2021a; Shimabukuro, 2021b). However, in the above report, about 62% of the Pfizer-BioNTech COVID-19 vaccines were received by females, which can be a reason for the preponderance of allergic reactions in females (CDC COVID-19 Response Team; Food and Drug Administration, 2021b). Similarly, CDC again reported that Moderna COVID-19 Vaccine females were principal (~61%) recipients of this vaccine. Virtually 100% of the anaphylactic reaction and 91% of non-anaphylactic allergic reactions were observed in females, amongst which 90% of them with anaphylaxis and 60% with non-anaphylactic allergic reactions had a history of allergic reactions (Centers for Disease Control and Prevention, 2021).
The proportion of the female population in the United States was 50.51% (The World Bank, 2019a). In Europe, it was 51.12% in 2019 (The World Bank, 2019b). WHO studied regarding difference among health care professionals (HCPs) in Europe and America in 2019. This study revealed that the percentage of female physicians in the USA and Europe was 46% and 53%, respectively. In contrast, the nursing workforce explicitly percentage dominated by females was 86% and 53% in the USA and Europe, respectively (World Health Organization, 2019). Therefore, the more AEs reported in females could be attributed to many females as health workers. During the early rollout of the vaccine, it was preferably given to the HCPs, and the above data shows a female predominance in the HCPs hence more female HCPs might have received the vaccine as compared to the male ones, which could be a reason for the more number AEs reported by the females. However, the reporting difference is too skewed to be justified only based on this reason.
| Table 2. Distribution of SAEs reported from the COVID-19 vaccines as per the broad system-based classification (n = 28,779). |
The study conducted on assessing the gender-specific differences in AEs reporting with various vaccines in Ontario during 2012–2015 emphasized that most of the AEs reported (66.2%) were associated with females (Harris et al. 2017). However, there was a more even distribution observed while analyzing the SAEs of either gender [57.5% female, and the relative risk reduction (RRR) 1.3] (Huang et al., 2014). Literature reveals that previous study (Huang et al. 2014), 63% on the MF59®-adjuvant H5N1 influenza vaccine. One more study (Halsey et al., 2013) on H1N1 vaccines reported female predominance in the AEs associated with the respective vaccinations. Multiple studies were conducted on the impact of gender and response to vaccines in elderly reported that the AEs with females as compared to males were consistently higher with the response to various vaccines like influenza, pneumococcal, herpes zoster, tetanus, and pertussis (Bayas et al., 2001; Beyer et al., 1996; Cook, 2007; Engler et al., 2008; Fink & Klein, 2015; Gergen, 1995; Hillebrand, 2015). The reactions observed by either gender were similar. However, female vaccine recipients reported more local reactions, like injection site pain, redness, and swelling, as well as some of the systemic reactions like joint pain, myalgia, headache, back pain, abdominal pain, fever, chills, and hypersensitivity reactions (Beyer et al., 1996; Fink & Klein, 2015). Few probable explanations in favor of females with higher AEs can be due to increased humoral and cell-mediated immune reactions to antigens, vaccines, and infections compared to males (Fish, 2008; Klein, 2012).
| Table 3. Common individual Adverse Events reported from the COVID-19 vaccines. |
The SAEs with the Pfizer-BioNTech COVID-19 Vaccine from their clinical trial experience in the 16–55 years of age were reported by 0.4% of the recipients and 0.8% of the participants with more than 56 years of age and older (FDA, 2020b). In the present analysis, the SAEs constituted 25.23% of the total AEs reported in the VigiBase, and deaths were observed in 0.40% of total SAEs associated with the Pfizer-BioNTech vaccine. As per the data reported from clinical trials, death was reported in two (0.01%) vaccine recipients, and both were above 55 years of age (Centers for Disease Control and Prevention, 2021; FDA 2020a). The proportion of non-fatal SAEs was 0.6% with the vaccine, and the most common AEs reported were appendicitis (0.04%), acute myocardial infarction (0.02%), and cerebrovascular accident (0.02%) (FDA, 2020a).
As per the clinical trial experience of Moderna COVID-19 Vaccine, the proportion of vaccine recipients who developed at least one AEs was 1%. In our analysis, the SAEs constituted 26.73% of the total AEs reported in the VigiBase, and death was observed in 1.23% of total SAEs associated with the Moderna vaccine. As per the data reported to FDA, there were six deaths reported after vaccination, and most of them were above 70 years of age and associated co-morbid conditions. In the vaccine recipient group, the commonest SAEs reported were myocardial infarction (0.03%), cholecystitis (0.02%), and nephrolithiasis (0.02%). Three SAEs were also considered likely caused by the vaccines, one case of intractable nausea/vomiting, and two of facial swelling concerning the FDA’s opinion (FDA, 2020a; CDC, 2020). This discrepancy in the proportion of SAEs between our study and the one reported to the FDA could be attributed to the fact that we had calculated the proportion of total AEs reported and not from the total patients vaccinated.
| Table 4. Distribution of serious and non-serious adverse events as per the vaccine, gender, and age groups. |
| Table 5. Distribution of death events per the vaccine, age, and gender (n = 103,954). |
The AEs from the COVID-19 vaccine from AstraZeneca as reported to Medicines & Healthcare products Regulatory Agency (MHRA) were not classified as serious or non-serious but reported general disorders and administration site conditions like injection site reaction/pain, fatigue, headache, and nausea to be commonest SAEs (Medicines & Healthcare products Regulatory Agency, 2021). A recent study published based on an interim analysis of four clinical trials conducted in Brazil, South Africa, and the UK has also reported that 79 (0.7%) of whom received ChAdOx1 nCoV-19 suffered gastrointestinal disorders, injury, poisoning and procedural complications, infections/infestations, and nervous system disorders (Voysey et al., 2021). More serious AEs were reported in older age groups in our analysis than in the younger age groups. This warrants a cautious approach when administrating vaccines to the old age group people in the form of longer follow-up and watchful vigilance.
Limitations of the study
The data analyzed in this study have been adapted from VigiBase, a WHO global database for ICSRs. The data are collected from several sources, and the probability that the suspected adverse effect is drug-related is not the same in all cases. In the absence of proper reporting of other parameters and technical problems in causality assessment, it is not appropriate to attribute all of these events to the vaccine; hence in this paper, we used the term AEs and not the adverse drug reaction, which is more definitely linked to the drug. The data presented in this study does not represent the Uppsala Monitoring Centre or the WHO’s opinion. Also, VigiBase do not give information about the total number of individuals vaccinated, and thus it was not possible to find out the ratio between the total individuals vaccinated and the individuals who had AE with vaccination. Besides this, the duration of the study is small, but the number of cases reported in this duration is sufficient to apply adequate statistical analysis and draw preliminary conclusions.
CONCLUSION
This study observed that the pattern of AEs reported in the database was in sync with the vaccines’ reactogenicity. However, there is an urgent need for systematic analysis regarding different AEs reported in this study to measure causalities through proper review of reports and generating data in primary studies.
The present study is not directed toward accentuating the imperfections related to any specific vaccine and is only intended to spread awareness regarding the commonly reported AE with COVID-19 vaccines so that the recipients’ possible follow-up may be done to avert any serious event. The victory or defeat of the world’s largest vaccination drive to successfully control the pandemic depends mainly upon the information regarding adversities associated with vaccination and awareness about their association with other co-morbidities.
ACKNOWLEDGMENTS
The authors want to acknowledge “mapchart.net,” a free service used to prepare a map diagram for this study. In addition, a portion of this study was earlier published in pre-print server https://www.medrxiv.org/content/10.1101/2021.03.23.21253433v1.
AUTHOR CONTRIBUTIONS
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
CONSENT FOR PUBLICATION
All authors reviewed and approved the final version and have agreed to be accountable for all aspects of the work, including any issues related to accuracy or integrity.
DISCLOSURE
The authors declare that they do not have any financial involvement or affiliations with any organization, association, or entity directly or indirectly with the subject matter or materials presented in this article. This also includes honoraria, expert testimony, employment, ownership of stocks or options, patents or grants received or pending, or royalties.
DATA SHARING
The data that support the findings of this study are available from the Corresponding author (JC), upon reasonable request.
FUNDING
This paper was not funded.
REFERENCES
Angelis A, Darrow J. Safeguarding evidence-based decision making in the FDA for COVID-19 vaccines. Vaccine, 2021; 39(17):2328–30. CrossRef
Aygün Ä°, Kaya M, Alhajj R. Identifying side effects of commonly used drugs in the treatment of Covid 19. Sci Rep, 2020; 10(1):21508. CrossRef
Bayas JM, Vilella A, Bertran MJ, Vidal J, Batalla J, Asenjo MA, Salleras LL. Immunogenicity and reactogenicity of the adult tetanus-diphtheria vaccine. How many doses are necessary? Epidemiol Infect, 2001; 127(3):451–60. CrossRef
Bergvall T, Norén GN, Lindquist M. vigiGrade: a tool to identify well-documented individual case reports and highlight systematic data quality issues. Drug Saf, 2014; 37(1):65–77. CrossRef
Beyer WE, Palache AM, Kerstens R, Masurel N. Gender differences in local and systemic reactions to inactivated influenza vaccine, established by a meta-analysis of fourteen independent studies. Eur J Clin Microbiol Infect Dis, 1996; 15(1):65–70. CrossRef
Blumenthal D, Fowler EJ, Abrams M, Collins SR. COVID-19 – Implications for the Health Care System. N Engl J Med, 2020; 383(15):1483–8. CrossRef
Bousquet C, Lagier G, Louët AL-L, Le Beller C, Venot A, Jaulent M-C. Appraisal of the MedDRA conceptual structure for describing and grouping adverse drug reactions. Drug Saf, 2005; 28(1):19–34. CrossRef
Bundgaard H, Bundgaard JS, Raaschou-Pedersen DET, von Buchwald C, Todsen T, Norsk JB, Pries-Heje MM, Vissing CR, Nielsen PB, Winsløw UC, Fogh K, Hasselbalch R, Kristensen JH, Ringgaard A, Porsborg Andersen M, Goecke NB, Trebbien R, Skovgaard K, Benfield T, Ullum H, Torp-Pedersen C, Iversen K. Effectiveness of adding a mask recommendation to other public health measures to prevent SARS-CoV-2 infection in danish mask wearers: a randomized controlled trial. Ann Intern Med, 2021; 174(3):335–43. CrossRef
CDC COVID-19 Response Team; Food and Drug Administration. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine – the United States, December 14–23, 2020. MMWR Morb Mortal Wkly Rep, 2021a; 70(2):46–51. CrossRef
CDC COVID-19 Response Team; Food and Drug Administration. Allergic reactions including anaphylaxis after receipt of the first dose of moderna COVID-19 vaccine – United States, December 21, 2020-January 10, 2021. MMWR Morb Mortal Wkly Rep, 2021b; 70(4):125–9; doi:10.15585/mmwr.mm7004e1 CrossRef
Centers for Disease Control and Prevention. Local reactions, systemic reactions, adverse events, and serious adverse events: Pfizer-BioNTech COVID-19 vaccine. 2020. Available via https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html (Accessed 8 March 2021).
Charan J, Kaur RJ, Bhardwaj P, Haque M, Sharma P, Misra S, Godman B. Rapid review of suspected adverse drug events due to remdesivir in the WHO database; findings and implications. Expert Rev Clin Pharmacol, 2021a; 14(1):95–103. CrossRef
Charan J, Dutta S, Kaur R, Bhardwaj P, Sharma P, Ambwani S, Jahan I, Abubakar AR, Islam S, Hardcastle TC, Rahman NAA, Lugova H, Haque M. Tocilizumab in COVID-19: a study of adverse drug events reported in the WHO database. Expert Opin Drug Saf, 2021b; 28:1–12; doi: 10.1080/14740338.2021b. CrossRef
Chowdhury MA, Hossain N, Kashem MA, Shahid MA, Alam A. Immune response in COVID-19: a review. J Infect Public Health, 2020; 13(11):1619–29. CrossRef
Cook IF, Pond D, Hartel G. Comparative reactogenicity and immunogenicity of 23 valent pneumococcal vaccines administered by intramuscular or subcutaneous injection in elderly adults. Vaccine, 2007; 25(25):4767–74. CrossRef
Craven J. COVID-19 vaccine tracker. Regulatory affairs professionals society (RAPS). 2021. Available via https://www.raps.org/news-and-articles/news-articles/2020/3/covid-19-vaccine-tracker (Accessed 29 January 2021).
Cyranoski D. Why emergency COVID-vaccine approvals pose a dilemma for scientists. Nature, 2020; 588(7836):18–9. CrossRef
Dutta S. Pharmacovigilance in India: evolution and change in scenario in India. Int J Sci Res, 2018; 7:976–8.
Elengoe A. COVID-19 outbreak in Malaysia. Osong Public Health Res Perspect, 2020; 11(3):93–100. CrossRef
Engler RJ, Nelson MR, Klote MM, VanRaden MJ, Huang CY, Cox NJ, Klimov A, Keitel WA, Nichol KL, Carr WW, Treanor JJ, Walter Reed Health Care System Influenza Vaccine Consortium. Half- vs. full-dose trivalent inactivated influenza vaccine (2004–2005): age, dose, and sex effects on immune responses. Arch Intern Med, 2008; 168(22):2405–14. CrossRef
FDA. Briefing Document. Pfizer-BioNTech COVID-19 Vaccine. Vaccines and Related Biological Products Advisory Committee Meeting, held on December 10, 2020. 2020a. Available via https://www.fda.gov/media/144245/download (Accessed 8 March 2021).
FDA. FDA approves first treatment for COVID-19. FDA news release. 2020b. Available via https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-covid-19 (Accessed 11 June 2021).
Fink AL, Klein SL. Sex and gender impact immune responses to vaccines among the elderly. Physiology, 2015; 30(6):408–16.
Fish EN. The X-files in immunity: sex-based differences predispose immune responses. Nat Rev Immunol, 2008; 8(9):737–44. CrossRef
Gergen PJ, McQuillan GM, Kiely M, Ezzati-Rice TM, Sutter RW, Virella G. A population-based serologic survey of immunity to tetanus in the United States. N Engl J Med, 1995; 332(12):761–6. CrossRef
Gliklich RE, Dreyer NA, Leavy MB (eds). Registries for evaluating patient outcomes: a user's guide [Internet]. 3rd edition, Agency for Healthcare Research and Quality (US), Rockville, MD, 2014, Adverse Event Detection, Processing, and Reporting. Available via https://www.ncbi.nlm.nih.gov/books/NBK208615 (Accessed 12 June 2021)
Green DR. SARS-CoV2 vaccines: slow is fast. Sci Adv, 2020; 6(28):eabc7428. CrossRef
Halsey NA, Griffioen M, Dreskin SC, Dekker CL, Wood R, Sharma D, Jones JF, LaRussa PS, Garner J, Berger M, Proveaux T, Vellozzi C, Hypersensitivity Working Group of the Clinical Immunization Safety Assessment Network, Broder K, Setse R, Pahud B, Hrncir D, Choi H, Sparks R, Williams SE, Engler RJ, Gidudu J, Baxter R, Klein N, Edwards K, Cano M, Kelso JM. Immediate hypersensitivity reactions following monovalent 2009 pandemic influenza A (H1N1) vaccines: reports to VAERS. Vaccine, 2013; 31(51):6107–12. CrossRef
Haque M. Handwashing in averting infectious diseases: relevance to COVID-19. J Popul Ther Clin Pharmacol, 2020; 27(S Pt 1):e37–e52. CrossRef
Harris T, Nair J, Fediurek J, Deeks SL. Assessment of sex-specific differences in adverse events following immunization reporting in Ontario, 2012–15. Vaccine, 2017; 35(19):2600–4; doi:10.1016/j.vaccine.2017.03.035 CrossRef
Harrison C. Coronavirus puts drug repurposing on the fast track. Nat Biotechnol, 2020; 38(4):379–81. CrossRef
Hillebrand K, Bricout H, Schulze-Rath R, Schink T, Garbe E. Incidence of herpes zoster and its complications in Germany, 2005–2009. J Infect, 2015; 70(2):178–86. CrossRef
Huang WT, Chang CH, Peng MC. Telephone monitoring of adverse events during an MF59®-adjuvanted H5N1 influenza vaccination campaign in Taiwan. Hum Vaccin Immunother, 2014; 10(1):100–3. CrossRef
International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Maintenance of the ICH guideline on clinical safety data management: data elements for transmission of individual case safety reports E2B(R2). 2021c. Available via https://admin.ich.org/sites/default/files/inline-files/E2B_R2_Guideline.pdf (Accessed 9 March 2021).
International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). Medical dictionary for regulatory activities (MedDRA). MedDRA Version. 2021a. Available via https://www.meddra.org/how-to-use/support-documentation/english/welcome (Accessed 12 June 2021).
International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). MedDRA hierarchy. 2021b. Available via https://www.meddra.org/how-to-use/basics/hierarchy (Accessed 12 June 2021).
Kaur RJ, Charan J, Dutta S, Sharma P, Bhardwaj P, Sharma P, Lugova H, Krishnapillai A, Islam S, Haque M, Misra S. Favipiravir use in COVID-19: analysis of suspected adverse drug events reported in the WHO database. Infect Drug Resist, 2020; 13:4427–38. CrossRef
Kaur SP, Gupta V. COVID-19 vaccine: a comprehensive status report. Virus Res, 2020; 288:198114.
Klein SL. Sex influences immune responses to viruses and efficacy of prophylaxis and treatments for viral diseases. Bioessays, 2012; 34(12):1050–9. CrossRef
Kröger E, Mouls M, Wilchesky M, Berkers M, Carmichael PH, van Marum R, Souverein P, Egberts T, Laroche ML. Adverse drug reactions reported with cholinesterase inhibitors: an analysis of 16 years of individual case safety reports from VigiBase. Ann Pharmacother, 2015; 49(11):1197–206. CrossRef
Kuemmerle A, Dodoo AN, Olsson S, Van Erps J, Burri C, Lalvani PS. Assessment of global reporting of adverse drug reactions for anti-malarials, including artemisinin-based combination therapy, to the WHO Programme for International Drug Monitoring. Malar J, 2011; 10:57. CrossRef
Kyriakidis NC, López-Cortés A, González EV, Grimaldos AB, Prado EO. SARS-CoV-2 vaccines strategies: a comprehensive review of phase 3 candidates. NPJ Vaccines, 2021; 6(1):28. CrossRef
Li CC, Wang XJ, Wang HR. Repurposing host-based therapeutics to control coronavirus and influenza virus. Drug Discov Today, 2019; 24(3):726–36. CrossRef
Lindquist M. VigiBase, the WHO global ICSR database system: basic facts. Ther Innov Regul Sci, 2008; 42:409–19. CrossRef
Lio CF, Cheong HH, Lei CI, Lo IL, Yao L, Lam C, Leong IH. Effectiveness of personal protective health behavior against COVID-19. BMC Public Health, 2021; 21(1):827. CrossRef
Medicines & Healthcare products Regulatory Agency. Information for UK recipients on COVID 19 Vaccine AstraZeneca. 2021. Available via https://www.gov.uk/government/publications/regulatory-approval-of-covid-19-vaccine-astrazeneca/information-for-uk-recipients-on-covid-19-vaccine-astrazeneca (Accessed 9 March 2021).
Moore TJ, Morrow RL, Dormuth CR, Mintzes B. US Food and Drug Administration Safety Advisories and Reporting to the Adverse Event Reporting System (FAERS). Pharmaceut Med, 2020; 34(2):135-40. CrossRef
National Center for Immunization and Respiratory Diseases. Interim clinical considerations for use of COVID-19 vaccines currently authorized in the United States. 2021. Available via https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html (Accessed 29 January 2021)
Nicola M, Alsafi Z, Sohrabi C, Kerwan A, Al-Jabir A, Iosifidis C, Agha M, Agha R. The socio-economic implications of the coronavirus pandemic (COVID-19): a review. Int J Surg, 2020; 78:185–93. CrossRef
Oliver SE, Gargano JW, Marin M, Wallace M, Curran KG, Chamberland M, McClung N, Campos-Outcalt D, Morgan RL, Mbaeyi S, Romero JR, Talbot HK, Lee GM, Bell BP, Dooling K. The advisory committee on immunization practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine – United States, December 2020. MMWR Morb Mortal Wkly Rep, 2020; 69(50):1922–4. CrossRef
Oliver SE, Gargano JW, Marin M, Wallace M, Curran KG, Chamberland M, McClung N, Campos-Outcalt D, Morgan RL, Mbaeyi S, Romero JR, Talbot HK, Lee GM, Bell BP, Dooling K. The advisory committee on immunization practices’ interim recommendation for use of moderna COVID-19 vaccine – United States, December 2020. MMWR Morb Mortal Wkly Rep, 2021; 69(5152):1653–6. CrossRef
Organization for Economic Co-operation and Development. OECD policy responses to coronavirus (COVID-19). Coronavirus (COVID-19) vaccines for developing countries: an equal shot at recovery. 2021. Available via https://www.oecd.org/coronavirus/policy-responses/coronavirus-covid-19-vaccines-for-developing-countries-an-equal-shot-at-recovery-6b0771e6/ (Accessed 12 June 2021).
Press Information Bureau Government of India. Press statement by the Drugs Controller General of India (DCGI) on restricted emergency approval of COVID-19 virus vaccine. 2021. Available via https://vaccine.icmr.org.in/images/pdf/HFW-DCGI-authorisation-3rdJanuary.pdf (Accessed 29 January 2021).
Rizk JG, Forthal DN, Kalantar-Zadeh K, Mehra MR, Lavie CJ, Rizk Y, Pfeiffer JP, Lewin JC. Expanded access programs, compassionate drug use, and emergency use authorizations during the COVID-19 pandemic. Drug Discov Today, 2021; 26(2):593–603. CrossRef
Shimabukuro T. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine – United States, December 14–23, 2020. Am J Transplant, 2021; 21(3):1332–7. CrossRef
Sharrar RG, Dieck GS. Monitoring product safety in the postmarketing environment. Ther Adv Drug Saf, 2013; 4(5):211–9. doi:10.1177/2042098613490780 CrossRef
Shimabukuro T, Nair N. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine. JAMA, 2021; 325(8):780–1. CrossRef
Shin MD, Shukla S, Chung YH, Beiss V, Chan SK, Ortega-Rivera OA, Wirth DM, Chen A, Sack M, Pokorski JK, Steinmetz NF. COVID-19 vaccine development and a potential nanomaterial path forward. Nat Nanotechnol, 2020; 15(8):646–55. CrossRef
Shrotri M, Swinnen T, Kampmann B, Parker EPK. An interactive website tracking COVID-19 vaccine development. Lancet Glob Health, 2021; 9(5):e590–2. CrossRef
Singh JA, Upshur REG. The granting of emergency use designation to COVID-19 candidate vaccines: implications for COVID-19 vaccine trials. Lancet Infect Dis, 2021; 21(4):e103–9. CrossRef
Tabish HB, Basch CH. Back to the basics: hand washing is public health 101, and it works to slow down the spread of viruses. Infect Dis Health, 2020; 25(4):319–20. CrossRef
Thanh Le T, Andreadakis Z, Kumar A, Gómez Román R, Tollefsen S, Saville M, Mayhew S. The COVID-19 vaccine development landscape. Nat Rev Drug Discov, 2020; 19(5):305–6. CrossRef
The World Bank. Population, female (% of total population) – European Union. 2019b. Available via https://data.worldbank.org/indicator/SP.POP.TOTL.FE.ZS?locations=EU (Accessed 8 March 2021).
The World Bank. Population, female (% of total population) – United States. 2019a. Available via https://data.worldbank.org/indicator/SP.POP.TOTL.FE.ZS?locations=US (Accessed 8 March 2021).
The World Bank. The Global Economic Outlook during the COVID-19 Pandemic: a changed world. 2020. Available via https://www.worldbank.org/en/news/feature/2020/06/08/the-global-economic-outlook-during-the-covid-19-pandemic-a-changed-world (Accessed 28 January 2021).
Upsala Monitoring Center. Guideline for using VigiBase data in studies. 2021. Available via https://www.who-umc.org/media/164772/guidelineusingvigibaseinstudies.pdf (Accessed 12 June 2021)
Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, Bibi S, Briner C, Cicconi P, Collins AM, Colin-Jones R, Cutland CL, Darton TC, Dheda K, Duncan CJA, Emary KRW, Ewer KJ, Fairlie L, Faust SN, Feng S, Ferreira DM, Finn A, Goodman AL, Green CM, Green CA, Heath PT, Hill C, Hill H, Hirsch I, Hodgson SHC, Izu A, Jackson S, Jenkin D, Joe CCD, Kerridge S, Koen A, Kwatra G, Lazarus R, Lawrie AM, Lelliott A, Libri V, Lillie PJ, Mallory R, Mendes AVA, Milan EP, Minassian AM, McGregor A, Morrison H, Mujadidi YF, Nana A, O'Reilly PJ, Padayachee SD, Pittella A, Plested E, Pollock KM, Ramasamy MN, Rhead S, Schwarzbold AV, Singh N, Smith A, Song R, Snape MD, Sprinz E, Sutherland RK, Tarrant R, Thomson EC, Török ME, Toshner M, Turner DPJ, Vekemans J, Villafana TL, Watson MEE, Williams CJ, Douglas AD, Hill AVS, Lambe T, Gilbert SC, Pollard AJ; Oxford COVID Vaccine Trial Group. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomized controlled trials in Brazil, South Africa, and the UK. Lancet, 2021; 397(10269):99–111. CrossRef
World Health Organization. Coronavirus disease (COVID-19): Herd immunity, lockdowns, and COVID-19. 2020. Available via https://www.who.int/news-room/q-a-detail/herd-immunity-lockdowns-and-covid-19 (Accessed 12 June 2021).
World Health Organization. COVID-19 vaccine tracker and landscape. 2021b. Available via https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (Accessed 12 June 2021).
World Health Organization. Gender equity in the health workforce: analysis of 104 countries. Health Workforce Working paper 1. 2019. Available via https://apps.who.int/iris/bitstream/handle/10665/311314/WHO-HIS-HWF-Gender-WP1-2019.1-eng.pdf (Accessed 8 March 2021)
World Health Organization. Regulation and prequalification. What is pharmacovigilance? 2021c. Available via https://www.who.int/teams/regulation-prequalification/pharmacovigilance (Accessed 31 January 2021).
World Health Organization. WHO coronavirus (COVID-19) Dashboard. 2021a. Available via https://covid19.who.int/ (Accessed 11 June 2021).
Wysowski DK, Swartz L. Adverse drug event surveillance and drug withdrawals in the United States, 1969-2002: the importance of reporting suspected reactions. Arch Intern Med, 2005; 165(12):1363–9. CrossRef
Yan ZP, Yang M, Lai CL. COVID-19 vaccines: a review of the safety and efficacy of current clinical trials. Pharmaceuticals (Basel), 2021; 14(5):406. CrossRef