INTRODUCTION
Cyanoacetamides are extremely reactive compounds possessing both electrophilic and nucleophilic properties. Amide and cyano functional groups of those compounds are appropriately situated to enable reactions with suitable reagents to create various heterocycles. In addition, active methylene of cyanoacetamides will endure a range of condensation and substitution reactions (Fadda et al., 2008). Knoevenagel condensation using piperidine and acetic acid or Lewis acid is an important reaction of active methylene compounds (Sobrinho et al., 2017). N-substituted cyanoacetamides on knoevenagel condensation with aryl aldehydes yield substituted 2-cyano-3-phenylacrylamides. Entacapone, which is chemically known as E-2-cyano-N,N-diethyl-3-(3,4-dihydroxy-5-nitrophenyl)acrylamide, a selective catechol O-methyltransferase inhibitory agent clinically used as an anti-parkinsonian drug possess 2-cyano-3-phenylacrylamide structure (Zhou et al., 2009).
Organic products with branched tert-butyl groups exemplify moderately significant active compounds. Over 200 compounds containing tert-butyl moiety were identified as natural products with exciting bioactivities. Biochemical studies on 5, 5′-thiobis(2-tert-butyl-4-methylphenol) and octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propanoate, compounds found in Paenibacillus odorifer bacterial strain associated with crustose lichen, prompted the recognizable identification of tert-butylphenol derivatives (Nguyen et al., 2018). Various 3,5-di-tert-butyl-4-hydroxyphenyl derivatives are indispensable structures and found in many pharmaceutically important compounds (Inagaki et al., 2000; Kuchana et al., 2019, 2020).
Aminobenzoic acids occur extensively in natural and synthetic compounds. 2-Aminobenzoic acid also called as ortho-aminobenzoic acid or anthranilic acid produced as metabolite in kynurenine pathway (Schwarcz and Stone, 2017). It plays an important role in biosynthesis of tryptophan and its derivatives, as well as in various alkaloids (Wiklund and Bergman, 2006). Anthranilic acid and its esters are used in preparing perfumes, insect repellents and pharmaceuticals. Fenamates, a class of nonsteroidal anti-inflammatory drugs, are derivatives of anthranilic acid (Garg and Sanguinetti, 2012). Tranilast, a synthetic and functional analog of anthranilic acid, used as anti-allergic agent in the treatment of several inflammatory diseases (Dharkhshan and Pour, 2015). 3-Aminobenzoic acid or meta-aminobenzoic acid (MABA) used as an intermediate for dyes, pesticides, and other organic synthesis. MABA chemical structure appears in an anti-inflammatory drug 5-aminosalicylic acid. 4-Aminobenzoic acid also called as para-aminobenzoic acid (PABA) found in folic acid and several food stuffs. It shows a wide variety of therapeutic actions and used as antioxidant, antibacterial, antimutagenic, anticoagulant, fibrinolytic, and immunomodulating agent. PABA has beneficial effect against ultraviolet radiation and used in diagnostic investigations (Crisan et al., 2014). PABA is used as a building block in design and synthesis of several drugs. Examples of drugs containing PABA motif include acedoben, isoprinosine, 4-aminosalicylic acid, aminopterin, methotrexate, benzocaine, tetracaine, procaine, procainamide, padimate-O, and balsalazide (Kratky et al., 2020).
Considering the therapeutic activity of molecular structures 2-cyano-3-phenylacrylamide, 3,5-di-tert-butyl-4-hydroxyphenyl derivatives and aminobenzoic acids, the present research aimed to design and synthesize new ortho, meta and para-(2-cyano-3-(3,5-di-tert-butyl-4-hydroxyphenyl)acrylamido)benzoic acids by assembling all the above structural units. The study also aimed to perform in silico screening of precursors and the title compounds for drug-likeness and bioactivity.
MATERIALS AND METHODS
Reagents and instruments
All chemicals were obtained from Alfa Aeser and SD fine chemicals of AR grade. Melting points determined in open capillaries on the tempo melting point apparatus and are uncorrected. The IR spectra recorded using KBr pellet on Bruker Infrared spectrophotometer. 1H NMR spectra recorded on Bruker Avance 400 (400 MHz) spectrometer using dimethyl sulfoxide (DMSO) as a solvent; chemical shifts (δ ppm) and coupling constants (Hz) are reported in standard manner with reference to internal standard tetramethylsilane. 13C NMR spectra recorded on Bruker Avance 400 (100 MHz) spectrometer using DMSO as a solvent. High-resolution mass spectra recorded on an Agilent 6538 UHD Q-TOF with dual electrospray ionization. The purity of the compounds verified using glass plates coated with Silica gel-G and the spots detected by iodine vapor.
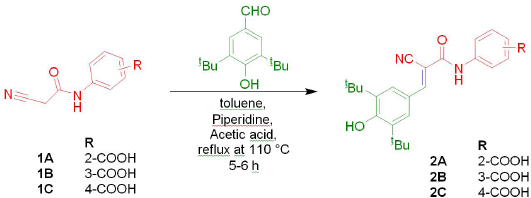
Preparation of ortho, meta and para-(2-cyano-3-(3,5-di-tert-butyl-4-hydroxyphenyl)acrylamido)benzoic acids (2A–2C)
To the oven-dried round bottom flask, added 1.02 g (0.005 mol) of cyanoacetyl aminobenzoic acid (1A or 1B or 1C), 1.17 g (0.005 mol) of 3,5-di-tert-butyl-4-hydroxybenzaldehyde and 10 ml of toluene as a solvent, stirred well. To this, 0.65 ml of glacial acetic acid and 0.175 ml of piperidine were added and refluxed for 5–6 hours at 110°C (Scheme 1). The reaction was monitored by thin layer chromatography and after completion of reaction the solvent was removed by vacuum. Finally, the product obtained was dried and recrystallized with a suitable solvent.
2-(2-cyano-3-(3,5-di-tert-butyl-4-hydroxyphenyl)acrylamido)benzoic acid (2A)
Mol. Formula: C25H28N2O4; Mol.Wt: 420.5090; M.P: 200°C–203°C; Yield:71%.
IR (KBr): 3603 (O-H str), 3406 (N-H str), 2954 (C-H str), 2220 (C≡N str), 1682 (C=O str) cm-1.
1H NMR (400 MHz, DMSO): δ 14.32 (s, 1H, COOH), 8.45 (d, J = 8.0 Hz, 1H, Ar-H), 8.20 (s, 1H, -CH=), 8.01 (d, J = 8.0 Hz, 1H, Ar-H), 7.90 (s, 2H, Ar-H), 7.45 (t, J = 8.0 Hz, 1H, Ar-H), 7.14 (t, J = 8.0 Hz, 1H, Ar-H), 1.40 (s, 18H, -di-C(CH3)3) ppm.
3-(2-cyano-3-(3,5-di-tert-butyl-4-hydroxyphenyl)acrylamido)benzoic acid (2B)
Mol. Formula: C25H28N2O4; Mol.Wt: 420.5090; M.P: 196°C –200°C; Yield:88%.
IR (KBr): 3566 (O-H str), 3336 (N-H str), 3076, 2958 (C-H str), 2221 (C≡N str), 1710 (C=O str) cm-1.
| Scheme 1. Preparation of ortho, meta and para-(2-cyano-3-(3,5-di-tert-butyl-4-hydroxyphenyl)acrylamido)benzoic acids (2A–2C). |
1H NMR (400 MHz, DMSO): δ 13.02 (s, 1H, COOH), 10.38 (s, 1H, NH), 8.29 (t, J = 1.8 Hz, 1H, Ar-H), 8.25 (s, 1H, -CH=), 7.96 (ddd, J = 8.1, 2.1, 1.0 Hz, 1H, Ar-H), 7.92 (s, 2H, Ar-H), 7.70 (dt, J = 8.0, 1.3 Hz, 1H, Ar-H), 7.50 (t, J = 8.0 Hz, 1H, Ar-H), 1.43 [s, 18H, -di-C(CH3)3] ppm.
13C NMR (100 MHz, DMSO): δ 167.55, 161.99, 159.46, 152.49, 139.45, 139.25, 131.76, 129.48, 128.79, 125.30, 125.02, 123.47, 121.76, 117.68, 102.43, 35.27, 30.44 ppm.
Mass: [M+H]+ 421.2124.
4-(2-cyano-3-(3,5-di-tert-butyl-4-hydroxyphenyl)acrylamido)benzoic acid (2C)
Mol. Formula: C25H28N2O4; Mol.Wt: 420.5090; M.P: 249°C–252°C; Yield:76%.
IR (KBr): 3538 (O-H str), 3402 (N-H str), 2955, 2918 (C-H str), 2210 (C≡N str), 1712 (C=O str) cm−1.
1H NMR (400 MHz, DMSO): δ 10.41 (s, 1H, NH), 8.21 (s, 1H, -CH=), 7.95 (d, J = 8.0 Hz, 2H, Ar-H), 7.91 (s, 2H, Ar-H), 7.82 (d, J = 8.0 Hz, 2H, Ar-H), 1.43 (s, 18H, -di-C(CH3)3) ppm.
13C NMR (100 MHz, DMSO): δ 167.39, 162.34, 160.63, 152.55, 143.17, 139.47, 130.73, 128.92, 126.30, 122.84, 120.12, 117.92, 101.19, 35.27, 30.42 ppm.
Mass: [M+H]+ 421.2135.
Prediction of drug-likeness properties and bioactivity score of ortho, meta and para-(2-cyano-3-(3,5-di-tert-butyl-4-hydroxyphenyl)acrylamido)benzoic acids (2A-2C) and cyanoacetyl aminobenzoic acids (1A-1C) by Molinspiration Cheminformatics software
Molinspiration Cheminformatics is online software used for the prediction of physicochemical properties and bioactivity score. Various physicochemical properties like logP, topological polar surface area (TPSA), hydrogen bond donor (HBD), hydrogen bond acceptor (HBA), molecular weight, molecular volume, and the number of rotatable bonds can be predicted by using this software. It was used to evaluate the drug-likeness property of title compounds and the corresponding cyanoacetyl aminobenzoic acids based on Lipinski’s rule of five. The software was used to find ligands modulating Nuclear receptors, G-protein coupled receptors (GPCR), Ion channels and also to identify the ligands as Kinase inhibitors, Protease inhibitors and Enzyme inhibitors.
RESULTS AND DISCUSSION
Chemistry
In the present study, new ortho, meta, and para-(2-cyano-3-(3,5-di-tert-butyl-4-hydroxyphenyl)acrylamido)benzoic acids (2A-2C) were prepared by knoevenagel condensation of active methylene group of respective cyanoacetyl aminobenzoic acids (1A-1C) with 3,5-di-tert-butyl-4-hydroxybenzaldehyde. All the reactants of this knoevenagel condensation reaction were prepared by adopting the procedures available in the literature (Cahoy, 1972; Madhavi and Sudeepthi, 2013). The synthesized title compounds were purified by recrystallization with alcohol and characterized by physical and spectral analytical methods. The percentage yield of title compounds ranges from 71 to 88 and found good when compared with previously reported 2-cyano-3-(3,5-di-tert-butyl-4-hydroxyphenyl)acrylamide derivatives of aminophenols (Kuchana and Kummari, 2018; Madhavi et al., 2019).
The IR spectrum of compound 2A revealed the presence of absorption band at 3603 cm−1 for free O−H bond stretching and at 3405 cm−1 for N-H stretching. The absorption band representing C−H stretching appeared at 2954 cm−1 and the C≡N stretching appeared at 2220 cm−1. The C=O stretching vibration appeared at 1682 cm−1. The 1H NMR spectrum of compound 2A revealed the presence of a singlet peak at δ 14.32 ppm for proton of COOH group. One singlet appeared at δ 8.20 ppm for the –CH = proton. Two ring protons of benzoic acid moiety appeared as two doublets at δ 8.45 and δ 8.01 with coupling constant J = 8.0 ppm. Another two ring protons of benzoic acid moiety appeared as two triplets at δ 7.45 ppm and δ 7.14 ppm with coupling constant J = 8.0 ppm. The two protons of 3,5-di-tert-butyl-4-hydroxyphenyl moiety appeared as singlet signal at δ 7.90 ppm. A more intense singlet peak appeared at δ 1.40 ppm for the 18 protons of di-tert-butyl group.
The IR spectrum of compound 2B showed absorption bands at 3566 and 3336 cm−1 because of free O−H stretching and N-H stretching. The absorption bands due to C−H stretching vibrations appeared at 3076 and 2958 cm−1. The IR peaks at 2221 and 1710 cm-1 indicative of C≡N and C=O stretching vibrations. The 1H NMR spectrum of the compound 2B revealed the presence of a singlet peak for proton of carboxylic acid at δ 13.02 ppm and another singlet peak for the proton of amide at δ 10.38 ppm. A singlet signal appeared at δ 8.25 ppm for the -CH= proton. The two aromatic protons of benzoic acid moiety appeared as two triplets at δ 8.29 ppm and δ 7.50 ppm with the coupling constants J = 1.8 Hz and J = 8.0 Hz respectively. Another two aromatic protons of benzoic acid appeared as doublet of doublet of doublet at δ 7.96 ppm with the coupling constants J = 8.1, 2.1, 1.0 Hz and as doublet of triplet at δ 7.70 ppm with the coupling constants J = 8.0, 1.3 Hz. The two ring protons of 3,5-di-tert-butyl-4-hydroxyphenyl moiety appeared as singlet signal at δ 7.92 ppm. A more intense singlet peak appeared at 1.43 ppm for the 18 protons for di-tert-butyl group. Further, the compound was characterized by 13C NMR spectrum which showed assignable peaks at δ 167.55 (COOH), 161.99 (CONH), 159.46, 152.49, 139.45(2C), 139.25, 131.76, 129.48, 128.79(2C), 125.30, 125.02, 123.47, 121.76, 117.68, 102.48 (C≡N), 35.27(2C), 30.44(6C) ppm. The mass spectrum of compound 2B showed a characteristic peak at 421.2124 for [M+H]+ confirming its structure.
The IR spectrum of compound 2C revealed the presence of an absorption band at 3538 cm−1 because of free O−H bond stretching vibration. The absorption band at 3402 cm−1 represents N-H stretching vibration. IR peaks at 2955 and 2918 cm−1 appeared due to C−H stretching vibrations. The absorption bands at 2210 and 1712cm−1 represents C≡N and C=O stretching vibrations. The 1H NMR spectrum of compound 2C showed a singlet at δ 10.41 ppm indicative of N-H proton of amide. One singlet appeared at δ 8. 21 ppm for the –CH = proton. The ring protons of benzoic acid moiety appeared as two doublets at δ 7.95 ppm and 7.82 ppm with the coupling constants J = 8.0 Hz. The two ring protons of 3,5-di-tert-butyl-4-hydroxyphenyl moiety appeared as singlet signal at δ 7.91 ppm. A more intense singlet peak appeared at δ 1.43 ppm for the 18 protons of di-tert-butyl group. Furthermore, the compound was characterized by 13C NMR spectrum which showed assignable peaks at δ 167.39 (COOH), 162.34 (CONH), 160.63, 152.55, 143.17, 139.47(2C), 130.73(2C), 128.92(2C), 126.30, 122.84, 120.12 (2C), 117.92, 101.19(C≡N), 35.27(2C), 30.42(6C) ppm. The mass spectrum of compound 2C showed a characteristic peak at 421.2135 for [M + H]+ confirming its structure.
Drug-likeness properties and bioactivity score of ortho, meta and para-(2-cyano-3-(3,5-di-tert-butyl-4-hydroxyphenyl)acrylamido)benzoic acids (2A-2C) and cyanoacetyl aminobenzoic acids (1A-1C) by Molinspiration Cheminformatics software
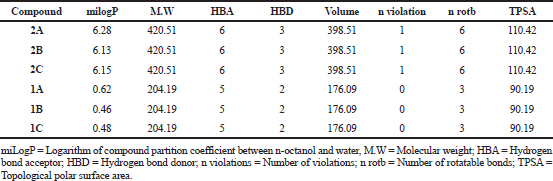
Drug likeness properties of title compounds (2A–2C) and cyanoacetyl aminobenzoic acids (1A–1C) were calculated through Molinspiration Cheminformatics software and the data presented in Table 1. The drug-likeness of a molecule depends on the molecular properties. These properties were assessed using theoretical calculations following Lipinski’s rule of five, this rule says that the orally active drug must possess a molecular weight 500, LogP value ≤ 5, number of HBDs ≤ 5, number of HBAs ≤ 10. The logP value indicates the lipophilicity of a molecule, which is a major determining factor in its absorption, distribution in the body, penetration across vital membranes and biological barriers, metabolism and excretion. The results revealed that the logP values of title compounds are greater than five indicating high lipophilicity. The molecular weights of all the investigated compounds are within the limit ≤ 500, indicates good absorption. The number of HBDs and acceptors are within the limit. The number of rotatable bonds indicates the flexibility of the molecule. The literature states that an orally active drug generally has no more than one violation of calculated molecular properties (Kuchana et al., 2019). Hence, it has been considered that all the evaluated compounds obeyed Lipiniski’s rule of five and having drug-likeness properties.
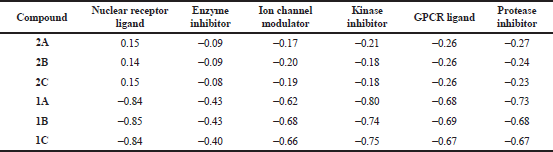
The bioactivity of title compounds (2A–2C) and cyanoacetyl aminobenzoic aicds (1A–1C) was estimated by calculating the activity score as GPCR ligand, Ion channel modulator, Nuclear receptor ligand, Kinase inhibitor, Protease inhibitor and Enzyme inhibitor using Molinspiration Cheminformatics software. The results obtained are listed in Table 2. Higher the bioactivity score, more probability of the investigated compound to be active. The compounds having bioactivity score greater than 0.00 are very active, while values between 0.00 and −0.50 are moderately active, if less than −0.50 are inactive. The results indicated that the title compounds were more active as Nuclear receptor ligands with bioactivity score more than 0.00. The highest bioactivity score (0.15) observed for the compounds 2A and 2C closely followed by 2B (0.14). This may be assigned due to the high lipophilic character of the title compounds. The study also revealed that the investigated title compounds were moderately active as Enzyme inhibitors, Ion channel modulators, Kinase inhibitors, GPCR ligands and Protease inhibitors. All the cyanoacetylated aminobenzoic acids (1A–1C) were found moderately active as Enzyme inhibitors and inactive as Nuclear receptor ligands, Ion channel modulators, Kinase inhibitors, GPCR ligands and Protease inhibitors. From the above observations it can be emphasized that introduction of 3,5-di-tert-butyl-4-hydroxyphenyl moiety on to the active methylene group of cyanoacetyl aminobenzoic acids (1A–1C) through a carbon–carbon double bond generated new ortho, meta, and para-(2-cyano-3-(3,5-di-tert-butyl-4-hydroxyphenyl)acrylamido)benzoic acids possessing drug-likeness properties and good bioactivity score as Nuclear receptor ligands.
| Table 1. Drug likeness properties of ortho, meta and para-(2-cyano-3-(3,5-di-tert-butyl-4-hydroxyphenyl)acrylamido)benzoic acids (2A–2C) and cyanoacetyl aminobenzoic acids (1A–1C). |
| Table 2. Bioactivity score of ortho, meta and para-(2-cyano-3-(3,5-di-tert-butyl-4-hydroxyphenyl)acrylamido)benzoic acids (2A–2C) and cyanoacetyl aminobenzoic acids (1A–1C). |
CONCLUSION
To function as a drug molecule, a compound requires some important structural features to interact with receptor binding sites and should possess a set of physicochemical properties. Several computational methods are available for calculating the molecular physicochemical properties and bioactivity. The screening is based on identification of structural fragments and their orientation typical for the active molecules. Therefore, in silico screening experiments were used as a guide to design new compounds with better drug-likeness properties and bioactivity. In the present study, new ortho, meta, and para-(2-cyano-3-(3,5-di-tert-butyl-4-hydroxyphenyl)acrylamido)benzoic acids were designed by considering the important pharmacophoric groups 2-cyano-3-phenylacrylamide, 3,5-di-tert-butyl-4-hydroxyphenyl moiety and aminobenzoic acids. The knoevenagel condensation reaction was utilized to synthesize the title compounds using cyanoacetyl aminobenzoic acids and 3,5-di-tert-butyl-4-hydroxybenzaldehyde. The in silico screening results indicated that all the investigated compounds obeyed Lipinski’s rule and therefore possess drug-likeness properties. The title compounds were identified as bioactive ligands modulating Nuclear receptors which indicate the crucial role and impact of 2-cyano-3-phenylacrylamide structure with 3,5-di-tert-butyl-4-hydroxy group on phenyl ring and benzoic acid moiety on nitrogen atom.
ACKNOWLEDGMENTS
The authors express thanks to the DST-CURIE Center, Sri Padmavati Mahila Visvavidyalayam (Women’s University) for giving IR spectra and Indian Institute of Chemical Technology, Hyderabad for giving 1H NMR, 13C NMR and Mass spectra.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Cahoy RP. Inventor. Chevron Research and Technology Co, Gulf Research and Development Co, Assignee. Combating weed with O-acyl-3, 5-dialkyl-4-hydroxybenzaldehydoxime. US patent 3644524A. 1972.
Crisan ME, Bourosh P, Maffei ME, Forni A, Pieraccini S, Sironi M, Chumakov YM. Synthesis, crystal structure and biological activity of 2-hydroxyethylammonium salt of p-aminobenzoic acid. PLoS One, 2014; 9(7):e101892. CrossRef
Dharkhshan S, Pour AB. Tranilast: a review of its therapeutic applications. Pharmacol Res, 2015; 91:15–28.
Fadda AA, Bondak S, Rabie R, Etman HA. Cyanoacetamide derivatives as synthons in heterocyclic synthesis. Turk J Chem, 2008; 32(3):259–86.
Garg P, Sanguinetti MC. Structure-activity relationship of fenamates as Slo2.1channel activators. Mol Pharmacol, 2012; 82(5):795–802. CrossRef
Inagaki M, Tsuri T, Jyoyama H, Ono T, Yamada K, Kobayashi M, Hori Y, Arimura A, Yasui K, Ohno K, Kakudo S, Koizumi K, Suzuki R, Kato M, Kawai S, Matsumito S. Novel antiarthritic agents with 1,2-isothiazolidine-1,1-dioxide (gamma-sultam) skeleton: cytokine suppressive dual inhibitors of cyclooxygenase-2 and 5-lipoxygenase. J Med Chem, 2000; 43(10):2040–8. CrossRef
Kratky M, Konecna K, Janousek J, Brablikova M, Jandourek O, Trejtnar F, Stolarikova J, Vinsova J. 4-Aminobenzoic acid derivatives: converting folate precursor to antimicrobial and cytotoxic agent. Biomolecules, 2020; 10(1):9. CrossRef
Kuchana M, Bethapudi DR, Ediga RK, Sisapuram Y. Synthesis, in-vitro antioxidant activity and in-silico prediction of drug-likeness properties of a novel compound: 4-(3,5-Di-tert-butyl-4-hydroxybenylidene)-3-methylisoxazol-5(4H)-one. J Appl Pharm Sci, 2019; 9(9):105–10. CrossRef
Kuchana M, Kummari R. Synthesis and evaluation of novel α-cyano-N-(2-hydroxyphenyl)cinnamamides for antioxidant, antibacterial and anti-inflammatory activities: in silico prediction of drug likeness properties. Int J Pharm Res, 2018; 10(3):300–10. CrossRef
Kuchana M, Pulavarthi M, Potthuri S, Manduri V, Jaggarapu VD. In silico study of molecular properties, bioactivity and toxicity of 2-(substituted benzylidene)succinic acids and some selected anti-inflammatory drugs. Int J Pharm Sci Drug Res, 2020; 12(4):353–9. CrossRef
Madhavi K, Sudeepthi P. Synthesis of cyanoacetylated derivatives of some heteroaryl amines as analgesic and antioxidant agents. Int J Pharm Sci Nanotechnol, 2013; 5(4):1879–84. CrossRef
Madhavi K, Swathi K, Anitha B, Reddy Usha Sree G, Sravanthi G, Aswini G. Synthesis and evaluation of novel α-cyano-N-(4-hydroxyphenyl)cinnamamides for antioxidant and anti-inflammatory activities: in silico prediction of drug likeness properties. Int J Pharm Sci Res, 2019; 10(1): 203-213.
Nguyen TBL, Delalande O, Rouaud I, Ferron S, Chaillot L, Pedeux R, Tomasi S. tert-Butylphenolic derivatives from Paenibacillus odorifer–a case of bioconversion. Molecules, 2018; 23(8):1951. CrossRef
Schwarcz R, Stone TW. The kynurenine pathway and the brain: challenges, controversies and promises. Neuropharmacology, 2017; 112(Pt B):237–47. CrossRef
Sobrinho RCMA, de Oliverira PM, Montes D’Oca CR, Russowsky D, Montes D’Oca MG. Solvent-free knoevenagel reaction catalysed by reusable pyrrolidinium base protic ionic liquids (PyrrlLs): synthesis of long chain alkylidenes. RSC Adv, 2017; 7(6):3214–21. CrossRef
Wiklund P, Bergman J. The chemistry of anthranilic acid. Curr Org Synth, 2006; 3(3):379–402. CrossRef
Zhou W, Li HB, Xia CN, Zheng XM, Hu WX. The synthesis and biological evaluation of some caffeic acid amide derivatives: E-2-cyano-(3-substituted phenyl) acrylamides. Bioorg Med Chem Lett, 2009; 19(7):1861–5. CrossRef