Development and Validation of a Stability-Indicating High Performance Thin Layer Chromatography (HPTLC) Method for estimation of Canagliflozin in bulk and Pharmaceutical Dosage Form
Ishpreet Kaur, Sharad Wakode, Harsharan Pal Singh
DOI: 10.7324/JAPS.2016.60508Pages: 051-057

A Study of Method Development, Validation and Forced Degradation for Quantification of Buprenorphine Hydrochloride in a Microemulsion Formulation
Dhanashree Arun Mundhey, Vishal V. Rajkondawar, Anwar S. Daud, Nidhi P. Sapkal
DOI: 10.7324/JAPS.2016.601022Pages: 159-169

A new rapid Stability indicating RP-PDA-UPLC method for the estimation of Assay of Pemetrexed disodium-An anti-Lung cancer drug from lyophilized parenteral formulation
Vamsi Krishna Galla, V. Archana, Rajeswari Jinka
DOI: 10.7324/JAPS.2017.71019Pages: 131-137

Development and validation of a stability-indicating RP-HPLC method of cholecalciferol in bulk and pharmaceutical formulations: Analytical quality by design approach
Dilipkumar Suryawanshi, Durgesh Kumar Jha, Umesh Shinde, Purnima D. Amin
DOI: 10.7324/JAPS.2019.90604Pages: 021-032

Quantification of rasagiline mesylate by stability indicating RP-HPLC method: Development and validation
Rohith Ganapathi Bhatta, Sathesha Babu Birur Kotappa, Sadashivaiah Rudragangaiah
DOI: 10.7324/JAPS.2019.90908Pages: 059-065

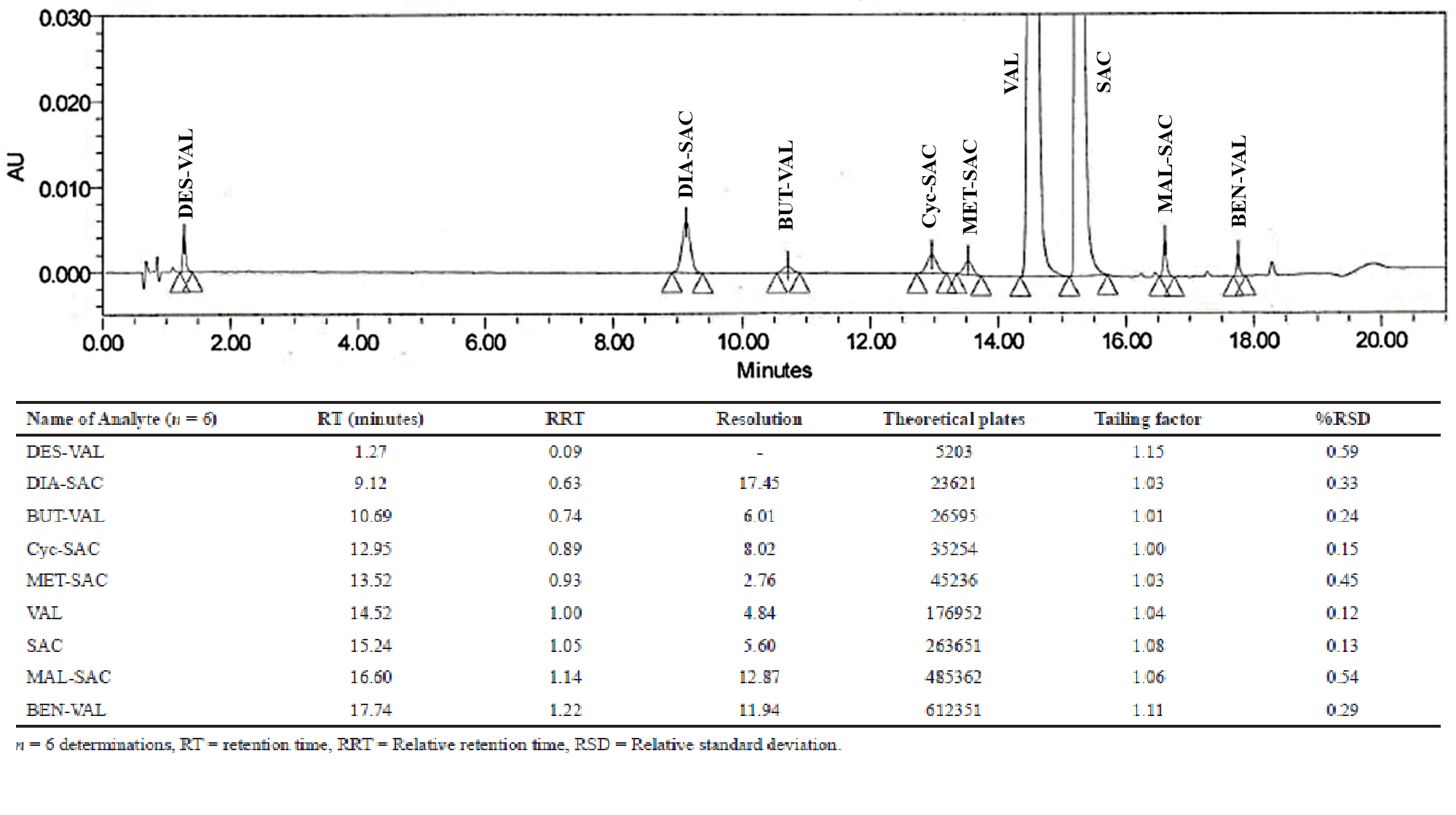
Development and validation of a stability indicating UHPLC method for Sacubitril/Valsartan complex in the presence of impurities and degradation products
Pintu Prajapati, Dhara Bhayani, Priti Mehta
DOI: 10.7324/JAPS.2020.102015Pages: 097-107
Forced degradation study of efonidipine HCl ethanolate, characterization of degradation products by LC-Q-TOF-MS and NMR
Charu P. Pandya, Sadhana J. Rajput
DOI: 10.7324/JAPS.2020.104012Pages: 075-099

A new approach for evolution and quantification of Triamcinolone acetonide in medication shots by using RP-HPLC
Manikantha Naveen Vuddagiri, Veeraswami Boddu
DOI: 10.7324/JAPS.2021.1101216Pages: 169–174

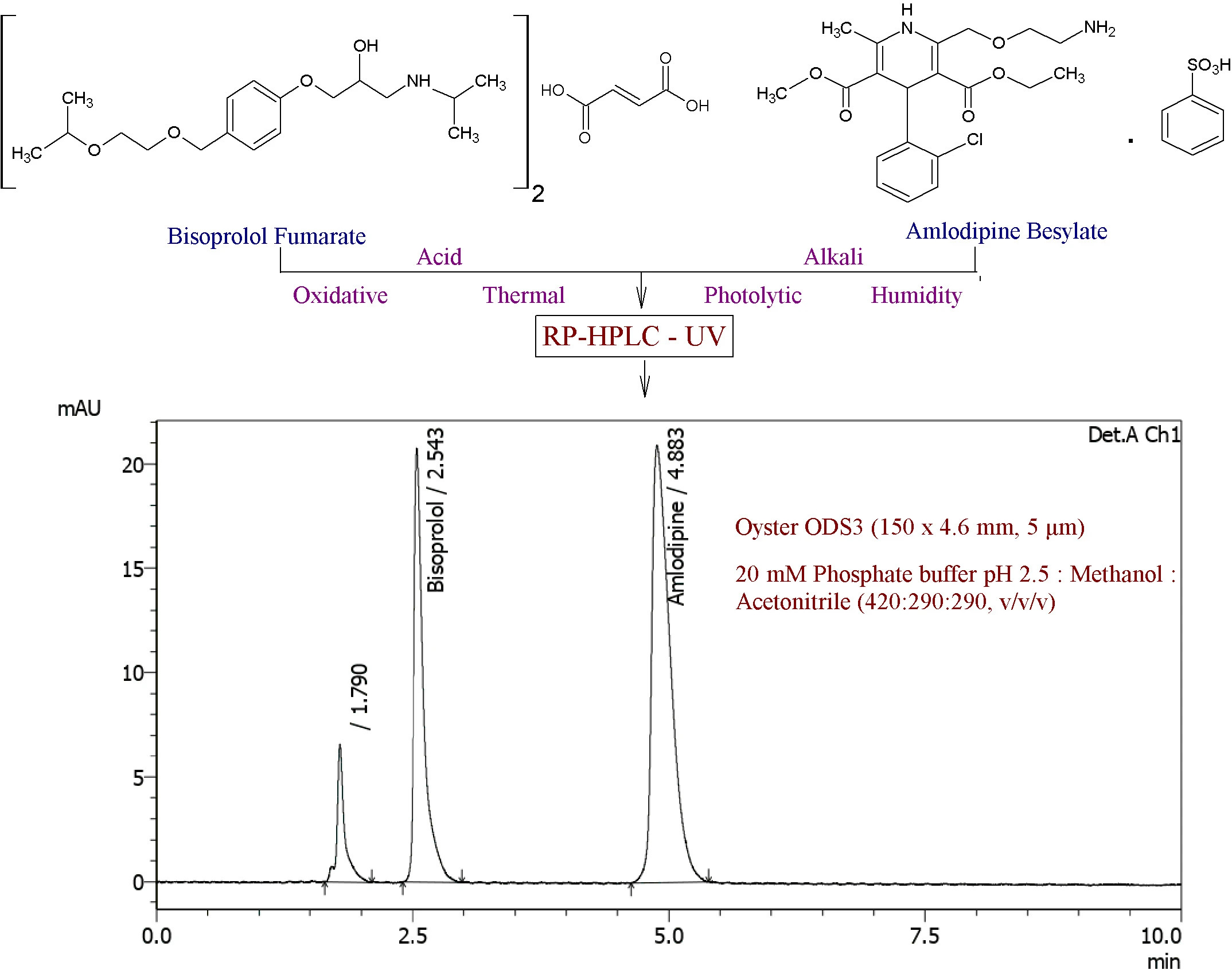
Stability-indicating RP-HPLC method development and validation for simultaneous estimation of bisoprolol fumarate and amlodipine besylate in bulk and in tablet dosage form
Rameshwar Bhausaheb Gholve, Sanjay Sudhakar Pekamwar, Tukaram Mohanrao Kalyankar
DOI: 10.7324/JAPS.2021.1101211Pages: 121–134
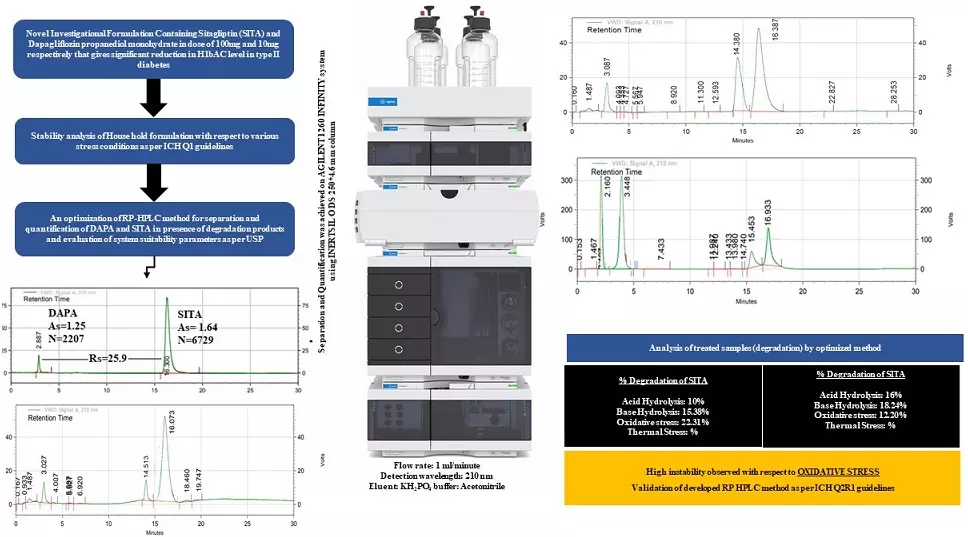
Quantitative computation and stability evaluation of phase III composition comprising sitagliptin and dapagliflozin propanediol monohydrate by RP-HPLC
Yesha Darshak Patel, Pinak Rameshbhai Patel, Jigna Bhatt, Binny Mehta, Krunal Detholia
DOI: 10.7324/JAPS.2022.120614Pages: 148-155