Formulation and evaluation of Diphenhydramine hydrochloride and Ibuprofen soft gelatin capsules
Shyamala Bhaskaran, Pradeep GC, Lakshmi PK
Pages: 188-190

Development of Oral Colon Specific PH Dependent Microcapsules of NSAID Drug Naproxen
J. K. Saboji, R. B. Gadve, S. M. Patil
DOI: 10.7324/JAPS.2012.2535Pages: 202-211

Model-Based Bioequivalence assessment of a commercial Azithromycin Capsule against Pfizer Zithromax® Tablet marketed in Jamaica
Amusa S. Adebayo and Noel McFarlane
DOI: 10.7324/JAPS.2014.401012Pages: 062-068

Development of oral capsules from Enterica herbal decoction-a traditional remedy for typhoid fever in Ghana
Doris Kumadoh, Joseph Adotey, Kwabena Ofori-Kwakye, Samuel Lugrie Kipo, Thomford Prah, Sowah Patterson
DOI: 10.7324/JAPS.2015.50414Pages: 083-088

Physicochemical quality evaluation of amoxicillin capsules produced in compounding pharmacies at Diadema, Sβo Paulo, Brazil
Fúlvio Gabriel Corazza, Blanca Elena Ortega Markman, Paulo César Pires Rosa
DOI: 10.7324/JAPS.2015.501205Pages: 029-034

Quinine-Loaded Polymeric Nanoparticles: Validation of a simple HPLC-PDA Method to Determine Drug Entrapment and Evaluation of its Photostability
Luana Roberta Michels, Lisiane Bajerski, Tamara Ramos Maciel, Letícia Marques Colomé, Sandra Elisa Haas
DOI: 10.7324/JAPS.2016.60202Pages: 009-015

Development and Validation of a Stability Indicating HPLC Method for the Simultaneous Analysis of Esomeprazole and Itopride in Bulk and In Capsules
M. Nageswara Rao, K. B. M. Krishna, B. Hari Babu
DOI: 10.7324/JAPS.2016.60210Pages: 072-080

Validation of analytical method by HPLC for determination of dapsone in polymeric nanocapsules based on crude rice brain oil
Letícia Marques Colomé, Gaya Mengue Freitas, Janaina de Medeiros Bastiani, Thais Carla Brussulo Pereira, Lisiane Bajerski, Eduardo André Bender, Sandra Elisa Haas
DOI: 10.7324/JAPS.2017.70734Pages: 230-233

A Review on Various Formulation Methods in preparing Colon targeted mini-tablets for Chronotherapy
Mohd Abdul Hadi, N. G. Raghavendra Rao, A. Srinivasa Rao, Tayyaba Mahtab, Sayeeda Tabassum
DOI: 10.7324/JAPS.2018.8321Pages: 158-164

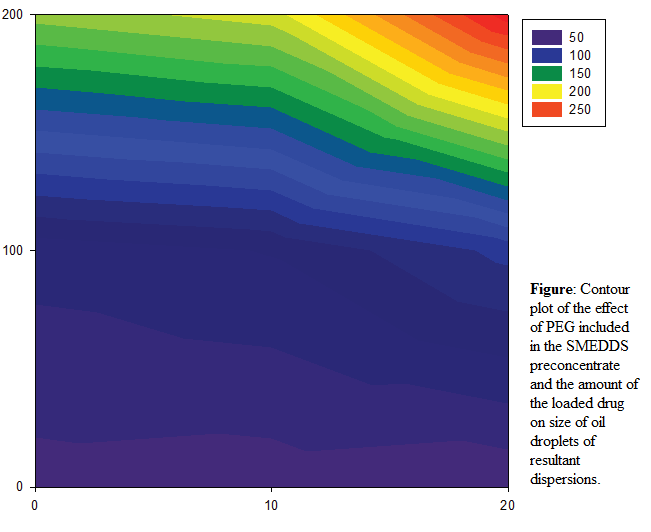
Impact of a formulation design on resultant dispersions of self-microemulsifying lipid systems
Naser M. Y. Hasan
DOI: 10.7324/JAPS.2021.110311Pages: 100-106
Review of fecal microbiota transplantation in autistic children and feasible techniques for fecal microbiota transplant delivery
Darina Malygina, Alexander Volkov, Olga Zhukova, Olga Vorobyova, Dmitry Panteleev, Daria Zykova, Anna Blagonravova
DOI: 10.7324/JAPS.2021.120201Pages: 001-009

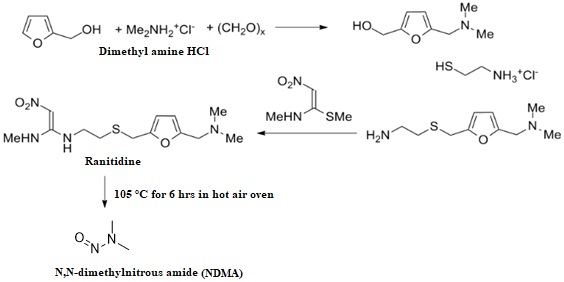
Novel stability indicating LC-MS method for N-Nitroso dimethyl amine genotoxic impurity quantification in ranitidine drug substance and drug product
Ganpisetti Srinivasa Rao, Dharamasoth Ramadevi, B. M. Rao, Nagaraju Rajana, K. Basavaiah
DOI: 10.7324/JAPS.2022.120711Pages: 106-114
Simultaneous determination of chloroquine and colchicine co-nanoencapsulated by HPLC-DAD
Tamara Ramos Maciel, Camila de Oliveira Pacheco, Pietra Fonseca Ramos, Ana Claudia Funguetto Ribeiro, Renata Bem dos Santos, Sandra Elisa Haas
DOI: 10.7324/JAPS.2023.130212Pages: 106-112

Traditional Thai herbal medicine for treating COVID-19
Phayong Thepaksorn, Katanchalee Thabsri, Piyachat Evelyn Denbaes, Suwannee Sroisong, Pussadee Srathong, Suriyan Sukati
DOI: 10.7324/JAPS.2023.126979Pages: 032-039

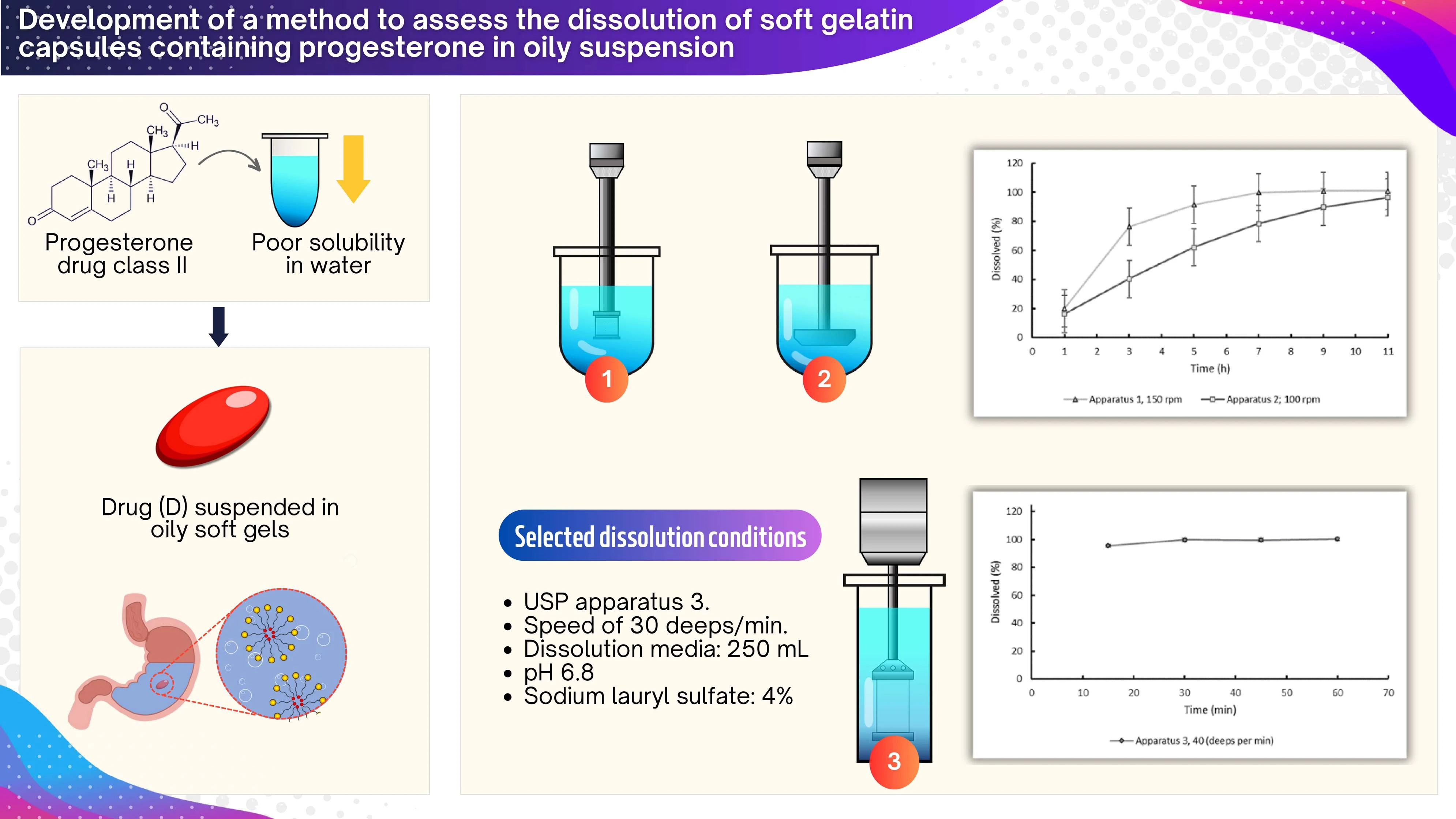
Development of a method to assess the dissolution of soft gelatin capsules containing progesterone in oily suspension
Diego R. Monterroza, Claudia M. Baena-Aristizábal, Luisa Fernanda Ponce D´león, Yolima Baena
DOI: 10.7324/JAPS.2024.158285Pages: 163-170