Development and validation of a stability-indicating RP-HPLC method of cholecalciferol in bulk and pharmaceutical formulations: Analytical quality by design approach
Dilipkumar Suryawanshi, Durgesh Kumar Jha, Umesh Shinde, Purnima D. Amin
DOI: 10.7324/JAPS.2019.90604Pages: 021-032

Formulation of somatostatin analog tablets using quality by design approach
Zoya Shprakh
DOI: 10.7324/JAPS.2021.110412Pages: 096-105

Analytical quality by design approach for estimating rosuvastatin calcium in pharmaceutical formulation by green HPLC method: Ecologically evaluated and stability-indicating
Seetharaman Rathinam, Lakshmi Karunanidhi Santhana
DOI: 10.7324/JAPS.2021.1101119Pages: 150-160
The development of biodegradable hemostatic and absorbable sponges containing chlorhexidine digluconate and their in vitro characterization—A QbD approach
Bohdana Pavliuk, Mariana Chubka, Taras Hroshovyi, Mariana Demchuk, Iryna Stechyshyn
DOI: 10.7324/JAPS.2021.120206Pages: 056-065

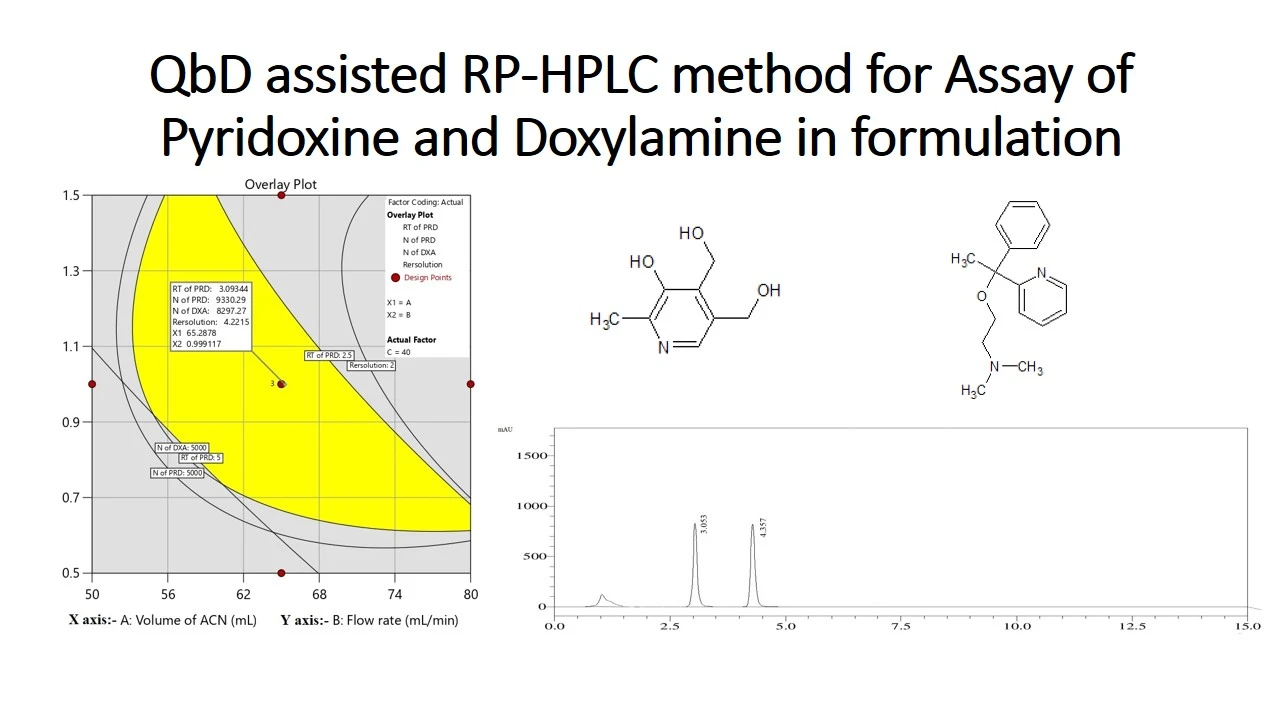
QbD assisted RP-HPLC method for determination of Pyridoxine and Doxylamine in pharmaceutical formulation using central composite design
Gangu Naidu Challa, Daniel Raju Kunda, Sheik Jakir Hussain Mustaq, Nagabharathi Marni, Srilekhya Ketha, Urmila Gorle, Shravitha Jakkula, Bhagavan Rajesh Babu Koppisetty
DOI: 10.7324/JAPS.2025.205442Pages: 072-083
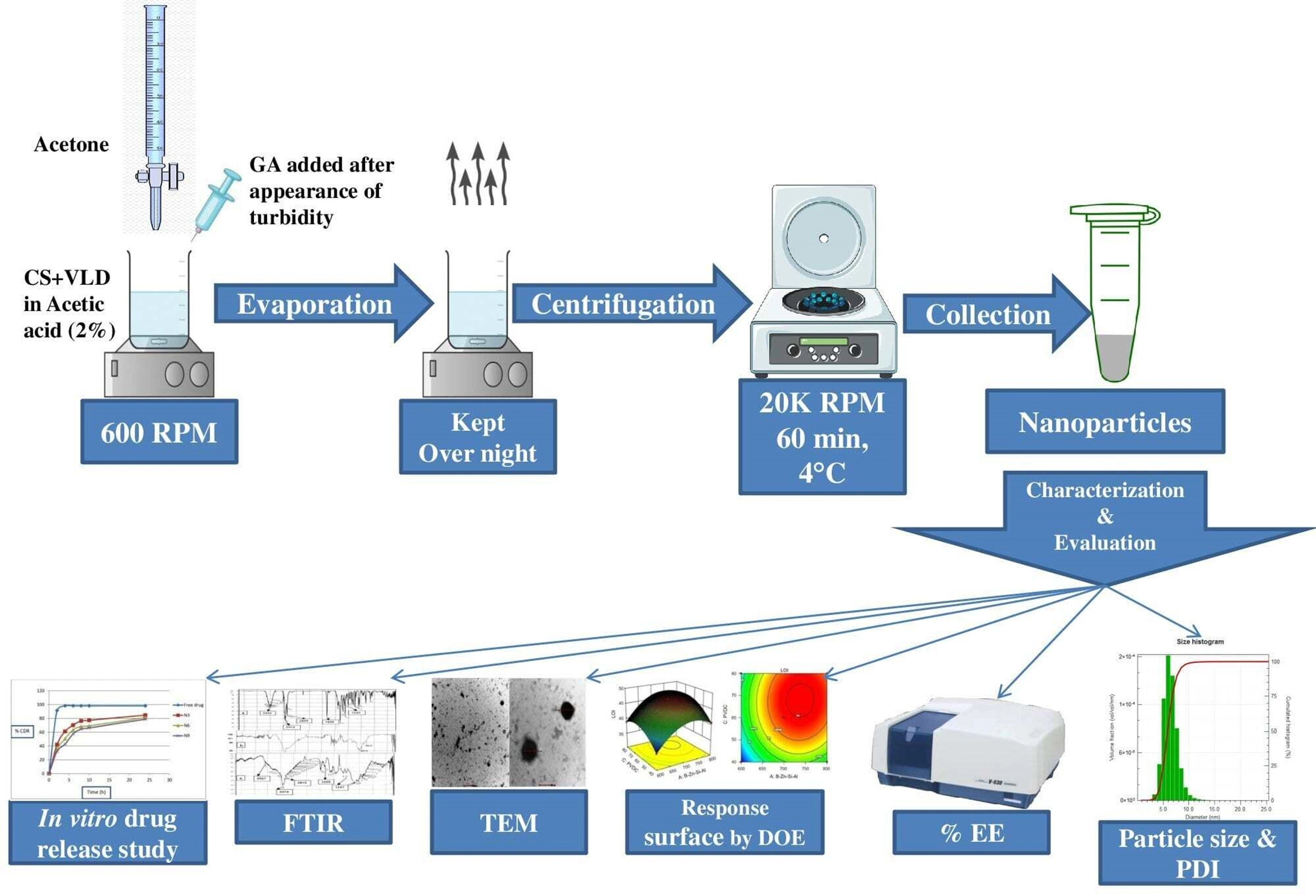
Quality by design approach assisted development and optimization of Chitosan–Vildagliptin nanoparticles using a simple desolvation technique
Anand Shripal Ammanage, Vinayak Shivamurthi Mastiholimath
DOI: 10.7324/JAPS.2025.202888Pages: 174-182
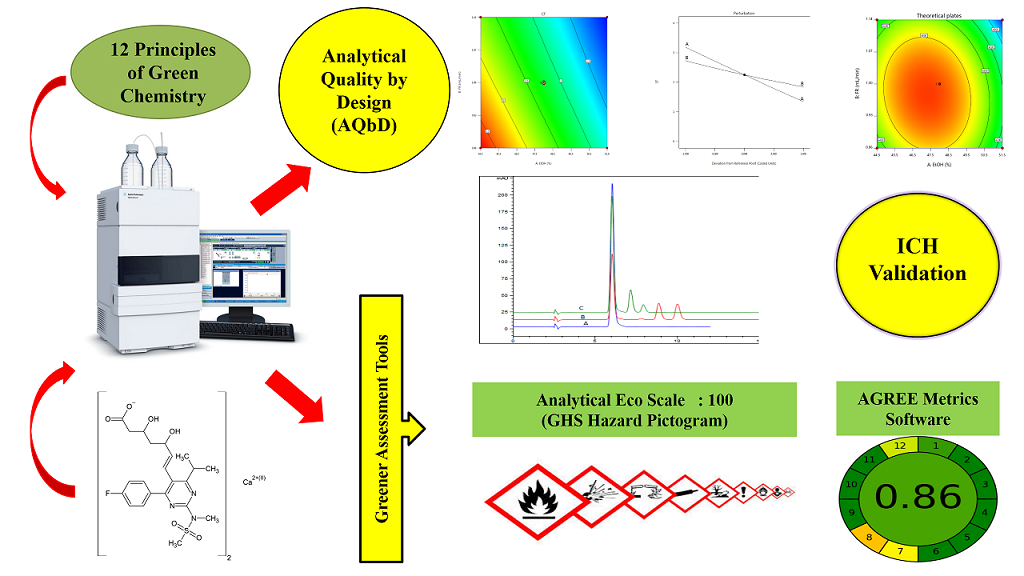
A quality by design approach with comprehensive green analytical chemistry assessment: Development, validation, and application of a high-performance liquid chromatographic method for quantifying meropenem trihydrate in nanosponges and marketed formulations
Ashwini T, Sanjay Garg, Padmaja A. Shenoy, Raghu Chandrashekhar, Yogendra Nayak, Usha Y. Nayak
DOI: 10.7324/JAPS.2025.236517Pages: 070-095
