Changes in daily defined doses (DDD) of antibiotics after restricted use in medical inpatients
B. Jayakar, N.A Aleykutty, Santhosh M Mathews
Pages: 220-222

Efficacy of international approaches to medicine price regulation and control: A scoping review
Mohammad Bashaar, Mohamed Azmi Hassali, Fahad Saleem, Alian A ALrasheedy, Vijay Thawani, Zaheer-Ud-Din Babar
DOI: 10.7324/JAPS.2017.70434Pages: 227-241

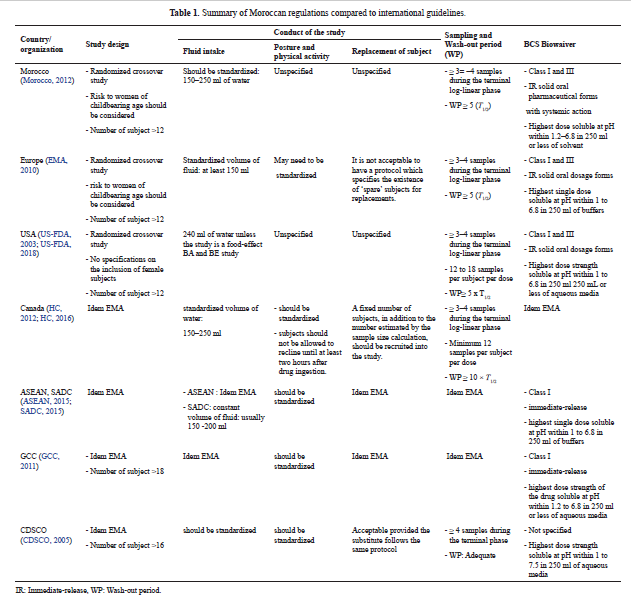
Bioequivalence regulation in emerging countries: Example of Moroccan regulations on immediate release formulations and comparison with international guidelines
Casimir Adade Adade, Amine Cheikh, Yahia Cherrah, Mustapha Bouatia, Jean Michel Cardot
DOI: 10.7324/JAPS.2019.91104Pages: 028-035
Study of breast cancer products’ lifecycle for mapping regulatory challenges
Medha A. Bijapur, Pradeep M. Muragundi, Bhavana Bhat, Virendra S. Ligade
DOI: 10.7324/JAPS.2023.166407Pages: 297-304
.png)
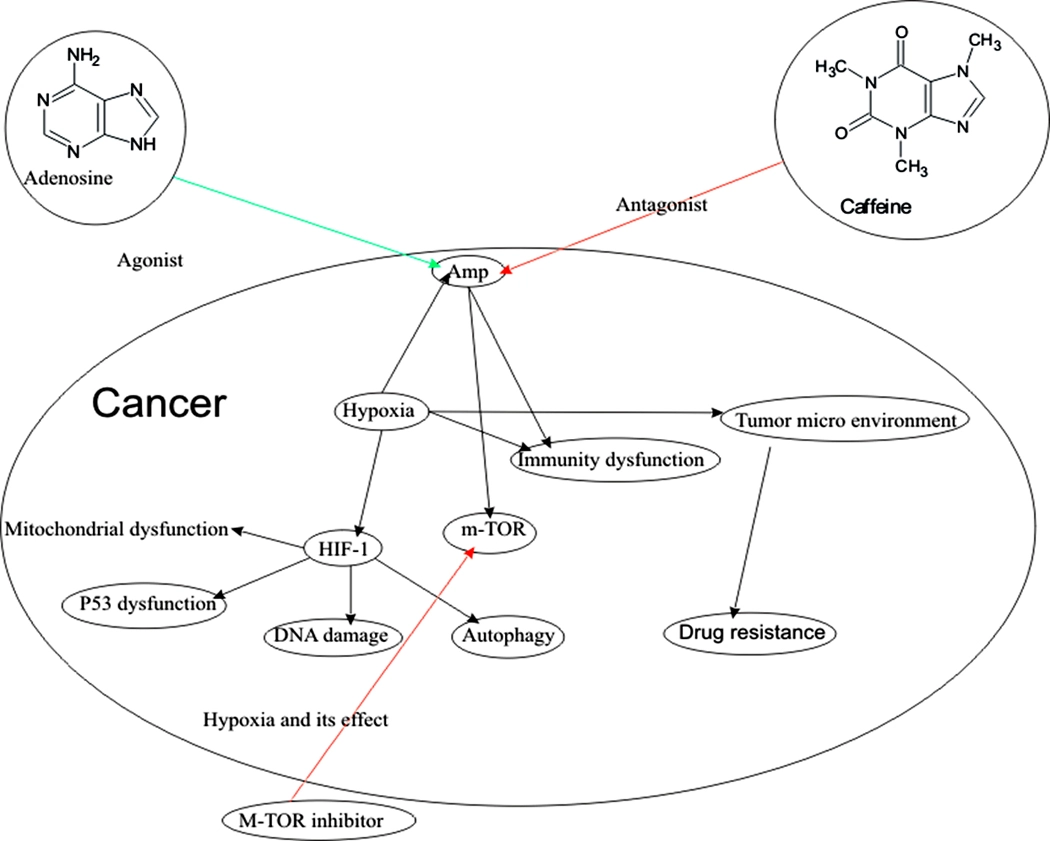
Overcoming multifaceted challenges in cancer treatment: Targeting signal transduction pathways and tumor microenvironment for enhanced therapeutic efficacy
Abdul Majeed Ansari, Naresh Kumar Sharma, Kashinath Tripathi, Gautam Bhardwaj, Ritu Chauhan, Rajiv Kumar Tonk, Moyad Shahwan, Hardeep Singh Tuli, Abhishek Chauhan
DOI: 10.7324/JAPS.2025.204012Pages: 040-063