INTRODUCTION
The skin serves as a protective barrier against bacterial infection. A wound occurs when its normal anatomical structure is disrupted due to surgical procedures or chemical, physical, mechanical, or thermal factors, resulting in impaired skin functions [1]. Polymicrobial organisms, including bacteria, viruses, and fungi, can easily penetrate wounds through subcutaneous tissues, where they can thrive and multiply in a supportive environment [2]. When microorganisms proliferate to the extent that they cause local and or systemic infection, the condition is referred to as a wound infection. Microorganisms in the wound lead to tissue damage locally and prevent the wound from healing [3]. The warm, moist, and nutrient-rich environment of wounds promotes microbial colonization and proliferation, increasing their contagiousness [4]. Wound infections resulting from microbial invasion are among the most common public health concerns. The most frequent pathogenic bacteria that cause wound infection includes Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus spp., Escherichia coli, Pseudomonas aeruginosa, Enterococcus faecalis, Acinetobacter baumannii, Klebsiella pneumoniae, and Proteus species [5,6]. These pathogens can cause significant tissue damage and non-healing wounds by impairing the immune system with its virulence factors [7]. Wounds are classified into two types: acute and chronic. Acute wounds, such as cuts, burns, abrasions, and surgical wounds, result from external factors and typically heal through the natural stages of wound repair [6]. An infected wound can delay the healing rate and negatively impact the quality of life [8]. Conversely, chronic wounds, such as leg or arterial ulcers, take a longer time to heal and are primarily caused by internal factors that can be associated with diseases such as diabetes, over weight, immune deficiencies, and microbial infections, which can further exacerbate the wound [7]. Wound infections constitute one third of nosocomial infections among surgical patients and contribute to 70%–80% of mortality [5,9,10]. The rise of antibiotic-resistant strains, coupled with the scarcity of new-generation antibiotics, and their high-cost increased wound-related morbidity and mortality [11].
Discovering a novel treatment approach to combat wound infections is crucial since these infections negatively impact the patient’s mental health and result in exorbitant medical expenses. More than 80% of people worldwide still rely on traditional medicines to treat a variety of illnesses, according to a survey conducted by World Health Organization [12]. Many plants and natural products possess antibacterial, antifungal, and antiprotozoal properties, making them suitable for both systemic and local applications. The medicinal use of plants is widely preferred due to their strong pharmacological effects, minimal toxicity, and cost-effectiveness compared to synthetic drugs. Medicinal plants are rich in diverse bioactive secondary metabolites, including tannins, terpenoids, alkaloids, saponins, flavonoids, and phenolic compounds, and can have distinct physiological effects on the human body [13].
Medicinal plants are effective in treating infectious diseases and various types of external wounds, including chronic, deep suppurative, open, lacerated, incised, and ulcerated wounds, and have been utilized for these purposes in both humans and various animal species. Their use offers the advantage of minimizing many side effects commonly associated with synthetic antimicrobials [14, 15].
Crassocephalum crepidioides, a wild plant from the Asteraceae family that grows widely in tropical and sub-tropical regions. It is known as Thickhead, Redflower rag leaf, Fireweed, and locally referred to as Terapaibi in Manipur, India. It has been traditionally used for treating a variety of ailments. In Manipur, a paste made from its leaves is commonly applied to minor wounds by the local population [16, 17]. However, there has been less scientific evidence to prove the wound healing potential of C. crepidioides. There are several reports on antibacterial, antioxidant properties [17], anti-inflammatory [18], and antidiabetic properties [19]. However, the antibacterial activity of C. crepidioides leaf extract against the bacterial strains isolated from the infected wound has not yet been reported. Hence, this study aims to analyze the bacterial profile of infected wounds, access their antibiotic susceptibility patterns, and evaluate the in vitro antibacterial activity of C. crepidioides leaf extract against clinical wound isolates.
MATERIALS AND METHODS
Plant collection and extraction
Crassocephalum crepidioides leaves were collected from various regions of Kakching District, Manipur, India, and was authenticated by Dr. P. Palani, Centre for Advanced Studies in Botany, University of Madras, Chennai, with the voucher specimen number MUBL116. The leaves were extracted following the method described in the previous study by Devi et al. [17]. Briefly, the plant material was washed with distilled water, shade dried, and finally ground using an electric grinder. 25 g of powdered plant material was mixed with 250 ml of sterile distilled water and heated in a hot plate at 60° for 2 hours with intermittent shaking. The mixture was then filtered first through muslin clot and subsequently through Whatman No.1 filter paper. The yield percentage was calculated using the formula, weight of the dried extract/weight of the initial dried sample × 100 [20].
Study area and time frame
This cross-sectional study was conducted over a span of 7 months, from November 2023 to June 2024. Wound samples showing signs of infection received at Laboratory of Sathyabama General Hospital, Chennai, were included in the study. Wounds from cuts, burns, abrasions, and surgical wounds with signs of infection before administration of antibiotics were considered in this study. However, samples from wounds of different aetiologies such as leg ulcers, diabetic foot ulcers, and pressure ulcers were excluded. Ethical approval for this study was granted by the Institutional Human Ethical Committee of the Sathyabama Institute of Science and Technology, India, under certificate number 330/IRB-IBSEC/SIST Dated 18th October 2023.
Collection and identification of bacterial wound isolates
A total of 69 wound swab samples were collected from the laboratory of Sathyabama General Hospital and transported within an hour to the Microbiology Laboratory of Sathyabama Dental College and Hospital, Tamil Nadu, India. Subsequently, each specimen was cultured on various agar media, including nutrient agar plates, blood agar, MacConkey agar, mannitol salt agar, and eosin methylene blue. Preliminary bacterial identification was carried out based on colony morphology. Further characterization was performed through Gram staining and Biochemical tests, followed by identification using the automated VITEK MS (Biomerieux).
Inoculum preparation
A single colony of each organism from 24 hours old culture plate was picked and inoculated separately into sterile nutrient broth. The cultures were then incubated at 37°C for 3 hours, after which the turbidity of the bacterial suspension was adjusted to a density of 1.5 × 108 CFU ml−1, equivalent to the 0.5 McFarland standard [21].
Antibiotic susceptibility test
The antibacterial susceptibility of wound isolates was assessed using the Kirby–Bauer disc diffusion method [21]. A lawn culture of the isolates was prepared on sterile Muller–Hinton agar (MHA) plates. Antibiotics discs for Gram-Positive bacteria included Amikacin (AK 10 µg), Amoxicillin (AMX 10 µg), Bacitracin (B 10 units), Cephalothin (CEP 30 µg), Erythromycin (E 15 µg), Novobiocin (NV 30 µg), Oxytetracycline (O 30 µg), and Vancomycin (VA 30 µg). For Gram-negative bacteria, the antibiotics tested were Amikacin (AK 10 µg), Carbericillin (CB 100 µg), Ciprofloxacin (CIP 10 µg), Co-Trimazine (CM 25 µg), Kanamycin (K 30 µg), Nitrofurantoin (NIT 300 µg), Streptomycin (S 10 µg), and Tetracycline (TE 30 µg). Antibiotics used in the study were purchased from HiMedia, Maharashtra, India. After incubating the plates at 37°C for 24 hours, zone diameters were measured in mm and the results were interpreted as sensitive, intermediate, or resistant according to the Clinical Laboratory Standard Institute guidelines [22]. Organisms exhibiting resistance to three or more antibiotics were classified as multidrug-resistant (MDR) [23]. Extensively drug-resistant (XDR) organisms were defined as those resistant to all antimicrobial agents except for two or fewer antimicrobial categories, and pan drug-resistant organisms were the organisms that were resistant to all antimicrobial agents [24, 25].
Antibacterial activity assay
The culture was evenly spread onto MHA using a sterile cotton swab. On each plate, equidistant wells, 8 mm in diameter, were created using a gel puncher, positioned 2 mm from the plate edge. Aseptically, 100 µl of the plant extract (500 mg/ml) was introduced into the respective wells. Ciprofloxacin (5 µg) served as the standard, while distilled water was used as the negative control. The plates were left undisturbed for 40 minutes to allow pre-diffusion, then, incubated at 37°C for 24 hours [26]. The assay was conducted in triplicates.
Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC)
The agar well diffusion method was carried out to determine the MIC of C. crepidioides leaf extract against bacteria isolated from wounds. Lawn culture of the bacterial isolates was made onto the Mueller–Hinton agar plates using sterile cotton swabs. Different concentrations (6.25, 12.5, 25, 50, 100, 200, and 400 mg/ml) of the extract were prepared using double-fold serial dilutions. Wells were then created into the agar plates, and 100 µl from various concentrations was transferred into the respective wells. The plates were left undisturbed for 30 minutes at room temperature to allow proper diffusion, followed by incubation at 37°C for 24 hours. Distilled water was used as the negative control, while ciprofloxacin (5 µg) was used as the standard antibiotic. After incubation, zones of inhibition were measured, and the lowest concentration of the extract that inhibited the growth of microorganisms was recorded as the MIC [27, 28].
MBC was performed by touching the inhibition zone of MIC plates of the four lowest concentrations of the plant extract that showed no visible bacterial growth and subculture onto the Nutrient agar plates. The sub cultured plates were then incubated at 37°C for 24 hours, after which bacterial growth on these plates was assessed. The concentration of the plant extract that did not produce any bacterial growth on freshly inoculated plates was recorded as the MBC [28].
Statistical analysis
Data were expressed as mean ± SD, analyzed using One-way ANOVA, and the statistical difference was evaluated by Dunnett t tests using SPSS software version 25 (p-value ≤ 0.05 is considered statistically significant).
RESULTS
Yield of plant extract and properties
The percentage yield of the hot aqueous extract of C. crepidioides was found to be 16.13%. The obtained extract was brown in color, and amorphous powder in nature.
Identification of bacterial wound isolates
A total of 20 pure bacterial species were isolated from 69 wound samples. The distribution of these isolates was as follows: 5 (25%) GPC and 15 (75%), GNB as shown in Figure 1. The identified organisms included 5 Staphylococcus species (25%), 5 E. coli (25%), 3 Klebsiella spp. (15%), 2 Proteus spp. (10%), 1 P. aeruginosa (5%), 1 A. baumannii (5%), 1 E. hormaechei (5%), and 2 Providencia species (10%) (Fig. 2).
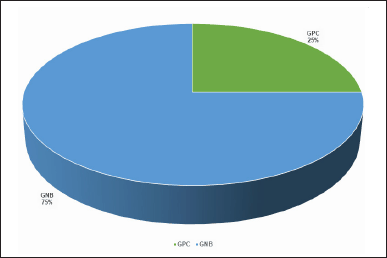 | Figure 1. Incidence of Gram-positive cocci and Gram-negative bacilli causing wound infections (n = 20). [Click here to view] |
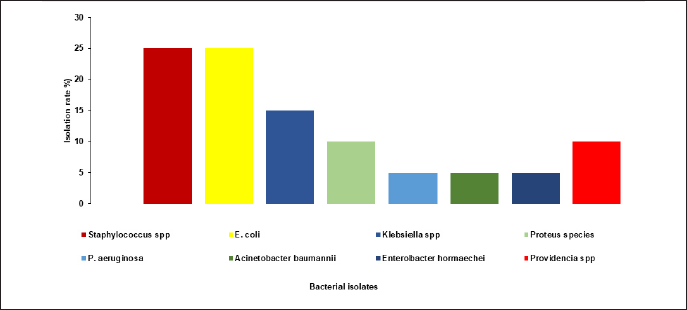 | Figure 2. Distribution of bacterial isolates from wound infection. [Click here to view] |
Antibiotic susceptibility test
The results revealed that GPC exhibited 100 % sensitivity to Amoxicillin, Bacitracin, Oxytetracycline, and Vancomycin, however, showed 80% resistance to Novobiocin, Amoxicillin, Cephalothin, and Erythromycin. Conversely, GNB exhibited high levels of resistance, including 86.7% to Ciprofloxacin, 80% to Carbericillin and Nitrofurantoin, 66.7% to Streptomycin and Tetracycline, 60% resistance to Co-Trimazine; however, they showed 73.3% sensitivity to Amikacin and 53.3% sensitivity to Kanamycin.
Staphylococcus spp. isolates exhibited 100% sensitivity to Amikacin, Bacitracin, Oxytetracycline, and Vancomycin, followed by 80% sensitivity to Novobiocin. However, they exhibited 80% resistance to Amoxicillin, Cephalothin, and Erythromycin (Table 1). The susceptibility pattern of GNB against standard antibiotics is shown in Table 2. P. aeruginosa exhibited 100% sensitivity to Amikacin, Carbericillin, Ciprofloxacin, Co-Trimazine, Streptomycin, Tetracycline, and 100% resistance to Nitrofurantoin and intermediate to Kanamycin. Proteus species were highly resistant (100%) to Ciprofloxacin, Co-Trimazine, Nitrofurantoin, and Tetracycline, followed by 50% resistance to Amikacin, Carbericillin, and Streptomycin. E. hormaechei demonstrated 100% resistance to Amikacin, Carbericillin, Ciprofloxacin, Co-Trimazine, Kanamycin, Nitrofurantoin, and Tetracycline, while showing 100% intermediate to S. A. baumannii exhibited 100% resistance to Amikacin, Carbericillin, Ciprofloxacin, Co-Trimazine, Kanamycin, Nitrofurantoin, and Streptomycin, and 100% sensitive to Tetracycline. Providencia species isolates exhibited 100% resistance to Nitrofurantoin and Tetracycline, followed by 50% resistance to Amikacin, Carbericillin, Ciprofloxacin, and Kanamycin. These strains also showed 100% sensitivity to Co-Trimazine and Streptomycin. E. coli demonstrated 100% resistance to Carbericillin and Ciprofloxacin, 80% resistance to Co-Trimazine and Tetracycline, however, exhibited 100% sensitivity to Amikacin, 80% sensitivity to Kanamycin, 60% sensitivity to Nitrofurantoin and S. Klebsiella spp. showed 100% sensitivity to Amikacin, Kanamycin, and Streptomycin, followed by 66.6% sensitivity to Co-Trimazine and Tetracycline. These strains showed 100% resistance to Carbericillin, Ciprofloxacin, and Nitrofurantoin.
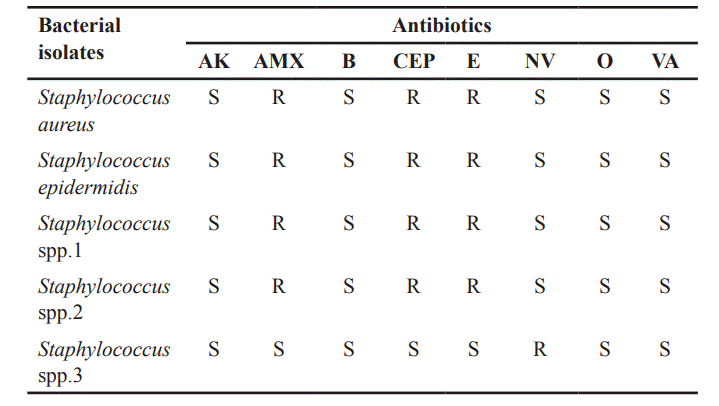 | Table 1. Susceptibility pattern of Gram-positive cocci against standard antibiotics. [Click here to view] |
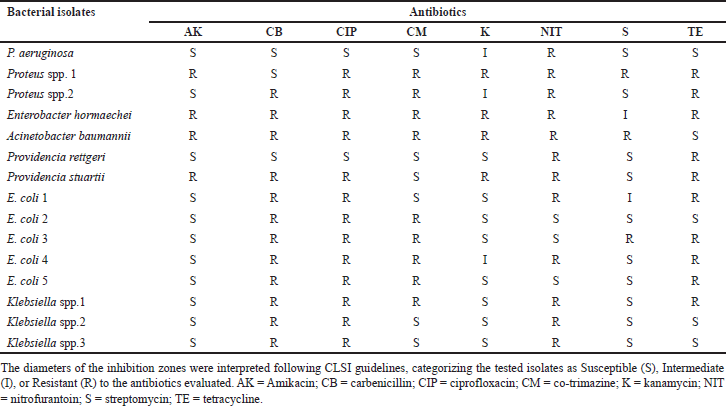 | Table 2. Susceptibility pattern of Gram-negative bacilli against standard antibiotics. [Click here to view] |
Among the 20 bacterial strains, 13 (65%) were classified as MDR, 4 (20%) as XDR, and the remaining 3 isolates (15%) were sensitive to most of the antibiotics tested. Figure 3 illustrates the prevalence of MDR and XDR among both GP and GN isolates. Among the five Staphylococcus species, four were found to be MDR. Of the two Providencia species, Providencia stuartii was XDR. A. baumannii and E. hormaechei were identified as XDR. One of the two Proteus species was found to be XDR, while the other was MDR. All the E. coli and Klebsiella species isolates were found to be MDR.
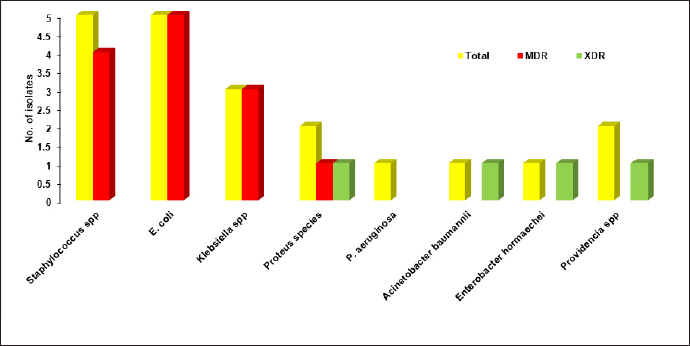 | Figure 3. Incidence of MDR and XDR of the Gram-positive and Gram-negative isolates. [Click here to view] |
Effect of C. crepidioides leaf extract against the wound isolates
Crassocephalum crepidioides leaf extract was found to be effective against all the Staphylococcus spp. isolates (17 ± 1 mm–25.83 ± 1.04 mm), E. hormaechei (11.16 ± 0.76 mm), A. baumannii (13 ± 0.5 mm), Providencia rettgeri (14 ± 1 mm), P. stuartii (15 ± 0.5 mm), two E. coli isolates (12 ± 0.5 mm and 10.83 ± 0.28 mm), and one Klebsiella spp. (11.0.5 mm) which is shown in Figure 4. On the other hand, P. aeruginosa, Proteus spp., three E. coli isolates, and two Klebsiella spp. isolates were resistant to it. It showed superior efficacy against GPC compared to GNB. Notably, it exhibited sensitivity against MDR and XDR bacterial isolates from the infected wound, thus signaling its broad-spectrum antibacterial potential.
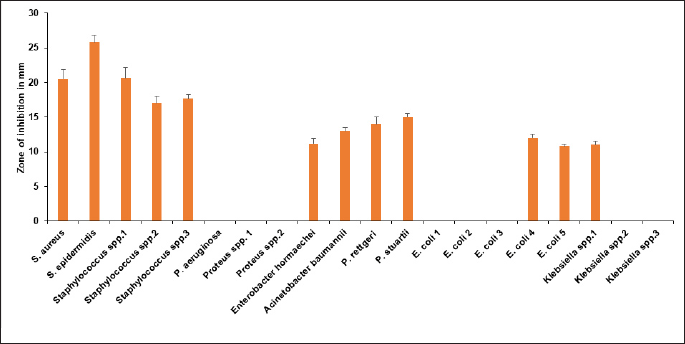 | Figure 4. Zone of inhibition in mm of C. crepidioides leaf extract against the bacterial isolates. [Click here to view] |
MIC and MBC
Crassocephalum crepidioides leaf extract exhibited antibacterial activity against 12 bacterial wound isolates at a concentration of 50 mg/ml. Therefore, the MIC of C. crepidioides leaf extract was determined utilizing the agar well diffusion method against 12 bacterial wound isolates. The MIC of C. crepidioides leaf extract was found to be between 2.5 and 5 mg/ml against all Staphylococcus spp., with the inhibition zones of 9.1 ± 1.04–13.3 ± 0.29 mm, and 5 mg/ml for P. stuartii (12.2 ± 0.29) and A. baumannii (11 ± 0.5). MIC values against P. rettgeri was 20 mg/ml with the inhibition zone of 10.8 ± 0.29 mm, 40 mg/ml for E. coli 4 (10.7 ± 0.29), E. coli 5 (9 ± 0.5), E. hormaechei (10 ± 0.5), and Klebsiella spp. 1 (10.2 ± 0.58) (Table 3). MBC of C. crepidioides leaf extract was found to be between 5 and 10 mg/ml against all Staphylococcus spp. MBC value was 10 mg/ml for P. stuartii and A. baumannii, and 40 mg/ml against P. rettgeri, E. coli 4, E. hormaechei, and Klebsiella spp.1 (Table 4).
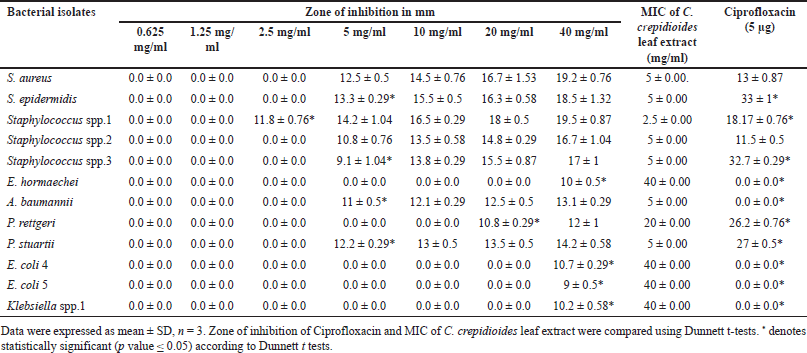 | Table 3. MIC of C. crepidioides leaf extract against the bacteria isolated from wounds. [Click here to view] |
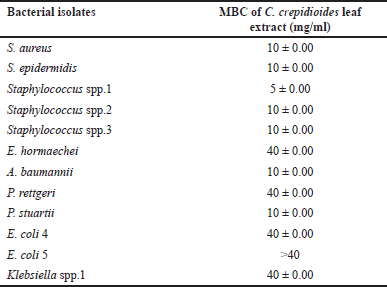 | Table 4. MBC of C. crepidioides leaf extract against the bacteria isolated from wounds. [Click here to view] |
DISCUSSION
Wound infections can prolong hospital stays and increase mortality rates by 70%–80% [29]. The administration of antibiotics is usually initiated empirically, which may help microorganisms become resistant to antibiotics [6].
A total of 20 bacterial species were isolated from 69 pus samples. The distribution of these isolates was as follows: 5 (25%) GPC and 15 (75%) GNB. The higher prevalence of GN bacteria could be linked to their elevated antibiotic resistance. These results are in agreement with previous studies [13].
Gram-negative bacteria exhibit high resistance to antibiotics, such as β-lactams and quinolones, due to their outer membrane, which acts as a barrier preventing most of the antibiotics from reaching their targets [30]. Hydrophilic antibiotics can enter through porins, while hydrophobic drugs enter via diffusion pathways. In contrast, vancomycin cannot cross the outer membrane because of its structure. Resistance can develop when the outer membrane is altered, such as through changes in porin function. However, Gram-positive bacteria lack an outer membrane and instead have a thick peptidoglycan layer, facilitating easier antibiotic penetration and resulting in minimal resistance [31, 32].
In the present investigation, Staphylococcus species and E. coli were the most common bacteria isolated from wound infection samples, consistent with previous studies [4,33–35]. The high incidence of S. aureus infections is often linked to endogenous sources or contamination of surgical instruments, as it can easily enter wounds when the skin barrier is breached [4]. The study by Atef et al. [13] also noted a high incidence of P. aeruginosa. These variations may be ascribed to factors such as the type of wound, the site of infection, and the use of prophylactic antibiotics during treatment [13]. According to WHO statistics, approximately 50% of E. coli, K. pneumoniae, S. aureus, and P. aeruginosa were resistant to most antibiotics, including cephalosporins which could be linked to misuse of antibiotics and prolonged hospital stays [36].
In our study, Staphylococcus isolates demonstrated high sensitivity to Amikacin, Bacitracin, Oxytetracycline, and Vancomycin (100%) followed by Novobiocin (80%); however, they exhibited resistance to Amoxicillin, Cephalothin, and Erythromycin (80%). These findings align with the previous studies indicating that Staphylococcus isolates are highly sensitive to Amikacin (100%) and Vancomycin (100%) [37–39]. The same organism showing notable resistance to Amoxicillin and Cephalothin demonstrates results consistent with studies conducted by Nobel et al. [40] and Ahmed et al. [41]. Over 90% of S. aureus were reported to be resistant to Amoxicillin and Ceftazidime [42]. The high sensitivity of gram-positive bacteria to Amikacin and Vancomycin could be attributed to their infrequent use, likely due to less availability, high cost, and potential toxicity [4]. Aminoglycosides represent the only class of ribosome-targeting antibiotics that exhibit bactericidal activity, due to their unique ability to cause mRNA misreading during translation [13].
Amoxicillin, a β-lactam drug was introduced in the 1970s for treating bacterial infections in humans [43]. S. aureus resistance to β-lactam primarily results from β-lactamase production, which deactivates the antibiotic and the acquisition of the mecA gene [44–46]. In addition, mutations in penicillin-binding proteins (PBPs) lead to the formation of PBP 2a, which aids in cell wall synthesis and further contributes to resistance [47].
Pseudomonas aeruginosa demonstrated 100% sensitivity to Amikacin, Carbericillin, Ciprofloxacin, Co-Trimazine, Streptomycin, and Tetracycline, while exhibiting 100% resistance to Nitrofurantoin and intermediate sensitivity to Kanamycin. Earlier investigations by Bhalchandra et al. [48] and Mama et al. [4] similarly found that P. aeruginosa strains exhibited 100% susceptibility to Ciprofloxacin.. Currently, Ciprofloxacin (oral) and gentamycin (injectable) are considered the most potent antibiotics for managing P. aeruginosa-related wound infections, outperforming other commonly used antimicrobial agents [4]. The increasing prevalence of P. aeruginosa in wound infections among hospitalized patients in developing regions raises significant concern, largely attributed to poor hygiene conditions, the use of low-quality antiseptics and low-quality medicinal solutions [49].
Proteus spp. demonstrated complete resistance (100%) to Ciprofloxacin, Co-Trimazine, Nitrofurantoin, and Tetracycline in our study, along with 50% resistance to Amikacin, CB, and Streptomycin. Previous study reported that Proteus mirabilis showed 100% resistance to Tetracycline [13]. Another study also reported that Proteus species were resistant to Tetracycline (73.9%) and sensitive to Ciprofloxacin (83%) [4].
Acinetobacter baumannii exhibited complete resistance (100%) to Amikacin, Carbericillin, Ciprofloxacin, Co-Trimazine, Kanamycin, Nitrofurantoin, and Streptomycin, while showing 100% sensitivity to Tetracycline. These findings align with the previous study which showed 70.6% resistance of A. baumannii to Amikacin [50]. In contrast, Puca et al. reported that A. baumannii was highly sensitive to Amikacin, with a sensitivity rate of 96.7% [6]. Sheeba et al. observed the highest percentage of resistant strains among A. baumannii isolates [51].
It was found that E. coli exhibited high resistance (100%) to Carbericillin and Ciprofloxacin, followed by Co-Trimazine and Tetracycline (80%), while exhibiting 100% sensitivity to Amikacin, 80% to Kanamycin, and 60% to Nitrofurantoin and Streptomycin. The resistance of E. coli to Ciprofloxacin and Tetracycline aligns with findings from other studies [4,13,39,52]. It was reported that Amikacin was highly effective against E. coli [53, 54].
Klebsiella species exhibited 100% sensitivity to Amikacin, Kanamycin, and Streptomycin, followed by 66.6% sensitivity to Co-Trimazine and Tetracycline; however, 100% resistant to Carbericillin, Ciprofloxacin, and Nitrofurantoin. These findings contrast with those reported by Atef et al. [13] and Gomatheswari and Jeyamurugan [53].
Among the 20 bacterial strains isolated in the present study, 13(65 %) were classified as MDR and 4 (20%) were XDR. Of the 17 MDR and XDR isolates, 4 (24 %) were gram-positive bacteria, while 13 (76 %) were Gram-negative bacteria. The elevated resistance observed in these isolates may be attributed to factors such as self-medication, limited diagnostic services, and lack of proper antibiotic guidelines [4]. The global spread of antimicrobial resistance is driven by antibiotic misuse, with mobile genetic elements and horizontal gene transfer playing a significant role [55]. Most pathogens isolated from infected wounds demonstrated resistance to commercial antibiotics (Tables 1 and 2).
The C. crepidioides leaf extract demonstrated varying levels of antibacterial activity against bacterial isolates from the infected wound. It showed superior efficacy against GPC compared to GNB. Notably, it exhibited sensitivity against Staphylococcus spp. isolates (16.16 ± 0.76 mm–25.83 ± 1.04 mm), E. hormaechei (11.16 ± 0.76 mm), A. baumannii (13 ± 0.5 mm), P. rettgeri (14 ± 1 mm), P. stuartii (15 ± 0.5 mm), two E. coli isolates (12 ± 0.5 mm and 10.83 ± 0.28 mm), and one Klebsiella spp. (11.0.5 mm), with a MIC between 2.5 and 40 mg/ml. The previous study done by Omotayo et al. [56] confirmed the strong antibacterial activity of C. crepidioides leaf extract against S. aureus (18 mm), E. coli (16 mm), and K. pneumoniae (21 mm) at 150 mg/ml. Another study also reported that C. crepidioides leaf extract was found to be sensitive against S. aureus and P. aeruginosa [57]. The use of plant extract to treat wound infections is supported by Atef et al. [13] who reported the effectiveness of aqueous extract of Moringa oleifera against Staphylococcus spp. (13–30 mm), E. coli (13–19 mm), Klebsiella spp. (12–26 mm), P. aeruginosa (14–20), and Proteus mirabilis (15 mm) at 500 mg/ml isolated from the wound infections. Aqueous extract of Alchornea cordifolia had strong antibacterial activity against the MDR S. aureus isolated from post-operative wound infections with an inhibition size of 21.4 mm [22].
According to previous studies, C. crepidioides leaf extract contains bioactive constituents, including phenol, tannin, flavonoids, terpenoids, and saponin [17, 56]. The possible antibacterial mechanisms of some secondary metabolites can be described as follows: Tannins can disrupt the structural integrity of bacterial cell walls and membranes, resulting in cellular damage and death. They can interfere with bacterial enzymes, impairing essential metabolic processes and limiting bacterial growth [58]. Furthermore, Tannins may inhibit microbial growth by interfering the cell wall synthesis, and cell envelope transport proteins [59]. Flavonoids can disrupt microbial cell membranes, inhibit energy metabolism, and interfere with nucleic acid synthesis [60]. They can interact with extracellular proteins in the bacterial cell wall, forming complex bonds that weaken its structure, eventually leading to cell wall breakdown and cell lysis [61]. Alkaloids, a diverse group of compounds, exhibit antimicrobial effects by inhibiting enzyme activity and other cellular mechanisms [62]. Saponins have been reported to exhibit antibacterial activity, likely due to their ability to form complexes with extracellular proteins, soluble proteins, and bacterial cell wall [14].
The differences in sensitivity observed among the tested pathogens may be due to the intrinsic tolerance of the microorganisms, as well as the specific types and combinations of phytochemicals present in the crude extract. The structural differences in bacterial cell walls also play a role; Gram-positive bacteria have a single-layered cell wall, whereas Gram-negative bacteria possess a more complex multi-layered cell wall. This structural complexity in Gram-negative bacteria may impede the penetration of active compounds, contributing to the differences in inhibition zones [63].
The present study highlights the potential of C. crepidioides leaf extract as effective antibacterial agents against the MDR and XDR organisms causing wound infection, emphasizing the significant role of plant extracts in treating bacterial wound infections, thereby preventing the delay of the wound healing process. These findings provide a robust scientific foundation for the ethnopharmacological use of C. crepidioides in the management of wound infections and underscore the potential of plant-derived extracts as effective antibacterial agents in clinical settings.
LIMITATIONS OF THE STUDY
This study did not include the isolation of anaerobic bacteria and fungi, as such procedures are not routinely performed in our laboratory. In addition, the small sample size represents another limitation of the study as the analysis was performed during a short period of time. Other herbal extracts with established antibacterial properties will be incorporated in future studies as control treatments to further contextualize the antibacterial efficacy of C. crepidioides. The wound-healing effect of C. crepidioides leaf extract on in vitro and in vivo condition needs to be done.
CONCLUSION
In the current research, 20 pure bacterial species were isolated from 69 wound samples, of which 13 (65%) were classified as MDR and 4 (20%) as XDR. The most common bacterial isolates were Staphylococcus species and E. coli followed by Klebsiella spp., Proteus spp., P. aeruginosa, A. baumannii, E. hormaechei, and Providencia species. Most pathogens isolated from infected wounds exhibited resistance to commercial antibiotics. Given the rapid emergence of antibiotic resistance, the use of herbal products as a novel, non-toxic, and environmentally sustainable alternative for managing virulent diseases is essential. C. crepidioides plant is traditionally used for minor wounds. In vitro antibacterial activity assay revealed that C. crepidioides leaf extract produced an inhibitory effect against MDR and XDR bacterial wound isolates. These findings provide a robust scientific foundation for the ethnopharmacological use of C. crepidioides in the management of wound infections and underscore the potential of plant-derived extracts as effective antibacterial agents in clinical settings. Further investigation on the wound-healing effect of C. crepidioides on in vitro and in vivo conditions is recommended.
AUTHOR CONTRIBUTIONS
All the authors made significant contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/ guidelines.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors reports no financial or any other conflicts of interest in this work.
ETHICS STATEMENT
Ethical approvals are given in the ‘Material and Method’ section.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Maillard JY, Kampf G, Cooper R. Antimicrobial stewardship of antiseptics that are pertinent to wounds: the need for a united approach. JAC Antimicrob Resist. 2021;3(1):dlab027. [Crossref]
2. Tasnim A, Alam MS, Yusuf MA, Khan FA, Ferdose J, Sultana M. Prevalence of multidrug resistant (MDR) Proteus spp. in burn wound infection of a Tertiary Care Hospital, Rajshahi. Int J Infect Dis Ther. 2021;6(2):65–8. [Crossref]
3. Tom IM, Ibrahim MM, Umoru AM, Umar JB, Bukar MA, Haruna AB, et al. Infection of wounds by potential bacterial pathogens and their resistogram. Open Access Library J. 2019;6:1–13. [Crossref]
4. Mama M, Abdissa A, Sewunet T. Antimicrobial susceptibility pattern of bacterial isolates from wound infection and their sensitivity to alternative topical agents at Jimma University Specialized Hospital, South-West Ethiopia. Ann Clin Microbiol Antimicrob. 2014;13(1):14. [Crossref]
5. Pallavali RR, Degati VL, Lomada D, Reddy MC, Durbaka, VRP. Isolation and in vitro evaluation of bacteriophages against MDR bacterial isolates from septic wound infections. PLoS ONE. 2017;12(7):179–245. [Crossref]
6. Puca V, Marulli RZ, Grande R, Vitale I, Niro A, Molinaro G, et al. Microbial species isolated from infected wounds and antimicrobial resistance analysis: data emerging from a three-years retrospective study. Antibiotics (Basel). 2021;10(10):1162. [Crossref]
7. Taati Moghadam M, Khoshbayan A, Chegini Z, Farahani I, Shariati A. Bacteriophages, a new therapeutic solution for inhibiting multidrug-resistant bacteria causing wound infection: lesson from animal models and clinical trials. Drug Des Dev Ther. 2020;14:1867–83. [Crossref]
8. Sandoz H. An overview of the prevention and management of wound infection. Nurs Stand. 2022;37(10):75–82. [Crossref]
9. Williams M. Wound infections: an overview. Br J Community Nurs. 2021;26(6):S22–5. [Crossref]
10. Roselinelfeyinwa E. Microbial assessment of wound infection among patients in hospitals within Enugu metropolis. IOSR J Pharm Biol Sci. 2023;18(2):18–23. [Crossref]
11. Salam MdS, Al-Amin MdY, Salam MT, Pawar JS, Akhter N, Rabaan AA, et al. Antimicrobial resistance: a growing serious threat for global public health. Healthcare (Basel). 2023;11(13):1946. [Crossref]
12. World Health Organization. WHO global report on traditional and complementary medicine 2019. World Health Organization. Available from: [Crossref]>
13. Atef NM, Shanab SM, Negm SI, Abbas YA. Evaluation of antimicrobial activity of some plant extracts against antibiotic susceptible and resistant bacterial strains causing wound infection. Bull Natl Res Cent. 2019;43:144. [Crossref]
14. Mummed B, Abraha A, Feyera T, Nigusse A, Assefa S. In vitro antibacterial activity of selected medicinal plants in the traditional treatment of skin and wound infections in Eastern Ethiopia. Biomed Res Int. 2018;2018:1862401. [Crossref]
15. Abdallah EM, Alhatlani BY, Menezes RdeP, Martins CHGM. Back to nature: medicinal plants as promising drugs in the post-antibiotic era. Plants. 2023;12(17):3077. [Crossref]
16. Das A. Evaluation of the effect of Crassocephalum crepidioides on the wound healing in Albino rats. Int J Adv Res. 2022;10:402. [Crossref]
17. Devi YA, Gnanasekaran P, Devi HJ. Antibacterial, antioxidant and cytotoxicity assessment of Crassocephalum crepidioides leaf extract. J Pure Appl Microbiol. 2024;18(4):2528–38. [Crossref]
18. Adedayo BC, Oyeleye SI, Ejakpovi II, and Oboh G. Effects of hot water treatment on the radicals scavenging, lipid peroxidation, and α-amylase and α-glucosidase inhibitory abilities of Crassocephalum crepidioides leaves. Nutrafoods. 2015;14:217–25. [Crossref]
19. Akinpelu BA, Godwin A, Gbadegesin T, Ajakaye N, Omotosho SE, Azeez SO, et al. Comparative studies on anti-inflammatory, antioxidant and antimutagenic activities of Crassocephalum crepidioides (Bent) leaf cold and hot water extracts. Asian Food Sci J. 2019;9(1):1–12. [Crossref]
20. Abbas A, Naqvi SAR, Rasool MH, Noureen A, Mubarik MS, Tareen RB. Phytochemical analysis, antioxidant and antimicrobial screening of seriphidium oliverianum plant extracts. Dose Response. 2021;19(1):15593258211004739. [Crossref]
21. Ahmed EF, Rasmi AH, Darwish AA, Gad GFM. Prevalence and resistance profile of bacteria isolated from wound infections among a group of patients in upper Egypt: a descriptive cross-sectional study. BMC Res Notes. 2023;16:106. [Crossref]
22. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. 33rd ed. CLSI supplement M100. 2023. Available from: [Crossref]
23. Owusu E, Ahorlu MM, Afutu E, Akumwena A, Asare GA. Antimicrobial activity of selected medicinal plants from a Sub-Saharan African Country against bacterial pathogens from post-operative wound infections. Med Sci. 2021;9(2):23. [Crossref]
24. Basak S, Singh P, Rajurkar M. Multidrug resistant and extensively drug resistant bacteria: a study. J Pathogens. 2016;2016(1):4065603. [Crossref]
25. Almuhayawi MS, Alruhaili MH, Gattan HS, Alharbi MT, Nagshabandi M, Al Jaouni S, et al. Staphylococcus aureus induced wound infections which antimicrobial resistance, methicillin- and vancomycin-resistant: assessment of emergence and cross-sectional study. Infect Drug Resist. 2023;16:5335–46. [Crossref]
26. Klink MJ, Laloo N, Taka AL, Pakade VE, Monapathi ME, Modise JS. Synthesis, characterisation and antimicrobial activity of zinc oxide nanoparticles against selected waterborne bacterial and yeast pathogens. Molecules. 2022;27:3532. [Crossref]
27. Gonelimali FD, Lin J, Miao W, Xuan J, Charles F, Chen M, Hatab SR. Antimicrobial properties and mechanism of action of some plant extracts against food pathogens and spoilage microorganisms. Front Microbiol. 2018;9:1639. [Crossref]
28. Mostafa AA, Al-Askar AA, Almaary KS, Dawoud TM, Sholkamv EN, Bakri MM. Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Bakri Saudi J Biol Sci. 2018;25(2):361–6. [Crossref]
29. Rai S, Yadav UN, Pant ND, Yakha JK, Tripathi PP, Poudel A, et al. Bacteriological profile and antimicrobial susceptibility patterns of bacteria isolated from pus/wound swab samples from children attending a tertiary care hospital in Kathmandu, Nepal. Int J Microbiol. 2017;2017:2529085. [Crossref]
30. Breijyeh Z, Jubeh B, Karaman R. Resistance of gram-negative bacteria to current antibacterial agents and approaches to resolve it. Molecules. 2020;25(6):1340. [Crossref]
31. Miller SI. Antibiotic resistance and regulation of the gram-negative bacterial outer membrane barrier by host innate immune molecules. mBio. 2016;7(5):e01541–16. [Crossref]
32. Datta P, Gupta V. Next-generation strategy for treating drug resistant bacteria: antibiotic hybrids. Indian J Med Res. 2019;149:97–106. [Crossref]
33. Alam MM, Islam MN, Hawlader MDH, Ahmed S, Wahab A, Islam M, et al. Prevalence of multidrug resistance bacterial isolates from infected wound patients in Dhaka, Bangladesh: a cross-sectional study. Int J Surg Open. 2021;28:56–62. [Crossref]
34. Sultana SH, Mawla N, Kawser SH, Akhtar N, Ali MK. Current microbial isolates from wound swab and their susceptibility pattern in a Private Medical College Hospital in Dhaka city. Delta Med Coll J. 2015;3(1):25–30. [Crossref]
35. Mohammed A, Seid ME, Gebrecherkos T, Tiruneh M, Moges F. Bacterial isolates and their antimicrobial susceptibility patterns of wound infections among inpatients and outpatients attending the University of Gondar Referral Hospital, Northwest Ethiopia. Int J Microbiol. 2017;2017:8953829. [Crossref]
36. WHO. Antimicrobial resistance global report on surveillance. 2014. Available from: [Crossref]
37. Gelaw A, Gebreselassie S, Tiruneh M, Yifru S, Matiwos E. Isolation of bacterial pathogens from patients with postoperative surgical site infections and possible sources of infections at University of Gondar Hospital, Northwest Ethiopia. J Environ Occup Sci. 2014;3(2):1. [Crossref]
38. Gautam R, Acharya A, Nepal HP, Shrestha S. Antibiotic susceptibility pattern of bacterial isolates from wound infection in Chitwan Medical College Teaching Hospital, Chitwan, Nepal. IJBAR. 2013;4(4):248–52. [Crossref]
39. Manikandan C, Amsath A. Antibiotic susceptibility of bacterial strains isolated from wound infection patients in Pattukkottai, Tamil Nadu, India. Int J Curr Microbiol App Sci. 2013;2(6):195–203. Available from: [Crossref]
40. Nobel FA, Islam S, Babu G, Akter S, Jebin RA, Sarker TC, et al. Isolation of multidrug resistance bacteria from the patients with wound infection and their antibiotics susceptibility patterns: a cross-sectional study. Ann Med Surg. 2022;84:104895. [Crossref]
41. Ahmed SMA, Saleh AA, Nigar I, Khan RR, Ahmed S, Sattar ANI, et al. Retrospective study of bacterial profile in wound swab and their susceptibility pattern in a Tertiary Care Hospital in Dhaka, Bangladesh. Arch Microbiol Immunol. 2024;8(3):383–9. [Crossref]
42. Morehead MS, Scarbrough C. Emergence of global antibiotic resistance. Prim Care Clin Off Pract. 2018;45(3):467–84. [Crossref]
43. Sutherland R, Croydon EA, Rolinson GN. Amoxicillin: a new semi-synthetic penicillin. Br Med J. 1972;3(5817):13–6. [Crossref]
44. Keshri V, Panda A, Levasseur A, Rolain JM, Pontarotti P, Raoult D. Phylogenomic analysis of β- lactamase in archaea and bacteria enables the identification of putative new members. Genome Biol Evol. 2018;10(4):1106–14. [Crossref]
45. Katayama Y, Zhang HZ, Hong D, Chambers HF. Jumping the barrier to β-lactam resistance in Staphylococcus aureus. J Bacteriol. 2003;185(18):5465–72. [Crossref]
46. Yao Q, Gao L, Xu T, Chen Y, Yang X, Han M, et al. Amoxicillin administration regimen and resistance mechanisms of Staphylococcus aureus established in tissue cage infection model. Front Microbiol. 2019;10:1638. [Crossref]
47. Loeffler A, Pfeiffer DU, Lloyd DH, Smith H, Saoresmagalhaes R, Lindsay JA. Methicillin-resistant Staphylococcus aureus carriage in UK veterinary staff and owners of infected pets: new risk groups. J Hosp Infect. 2010;74(3):282–288. [Crossref]
48. Bhalchandra HM, Naik DS, Verma KP. Aerobic bacterial profile of wound infections and its sensitivity pattern at Tertiary Care Hospital. Int J Curr Microbiol App Sci. 2018;7(6):1668–79. [Crossref]
49. Goswami N, Trivedi HR, Goswami APP. Antibiotic sensitivity profile of bacterial pathogens in postoperative wound infections at a tertiary care hospital in Gujarat, India. J Pharmacol Pharmacother. 2011;2(3):158–64. [Crossref]
50. Li L, Dai JX, Xu L, Chen ZH, Li XY, Liu M, et al. Antimicrobial resistance and pathogen distribution in hospitalized burn patients: a multicenter study in Southeast China. Medicine (Baltimore). 2018;97(34):e11977. [Crossref]
51. Sheeba PM, Prathyusha K, Anila MA. Antibiotic susceptibility trends in bacterial isolates from wound infections. MIR J. 2024;11(1):1–9. [Crossref]
52. Yakha JK, Sharma AR, Dahal N, Lekhak B, Banjara MR. Antibiotic susceptibility pattern of bacterial isolates causing wound infection among the patients visiting B & B hospital. Nepal J Sci Technol. 2015;15(2):91–6. [Crossref]
53. Gomatheswari SN, Jeyamurugan T. Bacteriological profile and the antibiotic susceptibility pattern of microorganisms isolated from pus/wound swab isolates in patients attending a Tertiary Care Hospital in South India. Int J Curr Microbial App Sci. 2017;6(10):1405–13. [Crossref]
54. Saha AK, Nandi S, Dhar P. Spectrum of microbial isolates from wound infections in patients admitted in a Tertiary Care Hospital, Kolkata. MGM J Med Sci. 2017;4(1):10–8. [Crossref]
55. Vikesland P, Garner E, Gupta S, Kang S, Maile-Moskowitz A, Ni Zhu. Differential drivers of antimicrobial resistance across the world. Acc Chem Res. 2019;52(4):916–24. [Crossref]
56. Omotayo MA, Avungbeto O, Sokefun OO, Eleyowo OO. Antibacterial activity of Crassocephalum crepidioides (Fireweed) and Chromolaena odorata (Siam weed) hot aqueous leaf extract. Int J Pharm Bio Sci. 2015;5(2):114–22. Available from: [Crossref]
57. Baral R, Karki A, Karki S, Neupane B, Ko?rala P, Baral S, et al. Phytochemical screening, free radical scavenging, and in vitro anti-bacterial activity studies of various extracts of selected medicinal plants of Nepal. Curr Perspect Med Aroma Plant. 2021;4(1):22–35. [Crossref]
58. Huang J, Zaynab M, Sharif Y, Khan J, Al-Yahyai R, Sadder M, et al. Tannins as antimicrobial agents: understanding toxic effects on pathogens. Toxicon. 2024;247:107812. [Crossref]
59. Begashaw B, Mishra B, Tsegaw A, Shewamene Z. Methanol leaves extract Hibiscus micranthus Linn exhibited antibacterial and wound healing activities. BMC Complement Altern Med. 2017;17(1):337. [Crossref]
60. Gupta PD, Birdi TJ. Development of botanicals to combat antibiotic resistance. J Ayurveda Integr Med. 2017;8(4):266–75. [Crossref]
61. Wirahmi N, Masrijal CDP, Amri F, Ikhsan, Triyansyah MI. Formulation and antibacterial activity of natural disinfectant combination of Psidium guajava and piper betle leaf infusion against Staphylococcus aureus. Proceedings of the 2nd International Conference on Contemporary Science and Clinical Pharmacy 2021 (ICCSCP 2021). Atlantis Pressp. 91–7. [Crossref]
62. Othman L, Sleiman A, Abdel-Massih RM. Antimicrobial activity of polyphenols and alkaloids in middle eastern plants. Front Microbiol. 2019;10:911. [Crossref]
63. Saxena D, Maitra R, Bormon R, Czekanska M, Meiers J, Titz J, et al. Tackling the outer membrane: facilitating compound entry into Gram-negative bacterial pathogens. Antimicrob Resist. 2023;1:17. [Crossref]