INTRODUCTION
Liver ailment is one among the foremost causes for death, distressing humans of all ages [1]. New Global Burden of Disease has estimated annual mortality levels, patterns, and temporal trends in liver cirrhosis between 1980 and 2010 in 187 countries covering 99.7% of the global population, which says cirrhosis has led to millions of deaths [2]. Irrespective of advancements in current medicine, the hepatic disorder remains as a worldwide health issue. Hence research for new drugs to treat this illness is carried out. Herbal medicines are important as hepatoprotective drugs and participate as the most important in curing several ailments with minimum information of their scientific mechanisms. The usage of synthetic drugs causes adverse reactions and are also of high cost. Therefore, treating liver complications with plant-based drugs is of utmost importance now [3].
Gnetum ula belongs to the family Gnetaceae. It is a woody climber and G. ula is believed by Kodavas Karnataka, India, as a sacred plant. Gnetum ula is an important indigenous plant aided for medication. Stem extracts are used in treating jaundice [4–6] and leaf extracts are used in the treatment of liver enlargement. Stem and root used are used as antiperiodic [7]. Stem is also administered for piles, hemicranias, and piercing wounds brought on by horn thrust [8]. Rheumatism is treated with seed oil and roasted fruit [9–11]. The fruits of G. ula are edible. Additionally, the seeds yield an oil that can be applied to burns and used as medicine [12,13]. Stem yields gnetol characterized as 2, 6, 11, 13-tetrahydroxy-trans stilbene [14], 3, 4, methylenedioxy-4- methoxy -trans-stilbene [15], and gnetulin, a dimer of 3, 4, 5”- trihydroxy -3- methoxystilbene [16,17]. Based on the usage of stem extracts by tribes and medical practitioners to treat jaundice and other ailments we have selected G. ula to investigate antihepatotoxic activity by using in vivo parameters.
MATERIALS AND METHODS
Chemicals
Petroleum ether, chloroform, and ethanol were procured (Ranbaxy Fine Chemicals New Delhi, India). Carbon tetrachloride and Silymarin from Sigma-Aldrich (USA). Biochemical analysis kit was procured from ERBA Diagnostics Inc. Transasia Bio-Medicals Ltd. Other chemicals were analytical grade (Sigma-Aldrich, USA and E-Merck, Mumbai).
Plant material and extraction
Stem of G. ula was collected from Biligirirangana Hills (B.R. Hills) of Chamarajanagar district, Karnataka, India, and authenticated at the Department of AYUSH, Govt. of India Bangalore by Dr. Shiddamallayya. N. (Ref No: RRCBI-MUS-0107).
A plant specimen of 500 gm was dried and powered using a mechanical blender, defatted with petroleum ether, and extracted using ethanol using a soxhlet apparatus. The extracts of G. ula were filtered and the solvent was evaporated by using a rotary evaporator [18]. For the present study, ethanol extract was used to evaluate its hepatoprotective potential.
In vivo studies of Gnetum ula
A Wistar strain rat of both sex and female Swiss albino mice was procured from Sri Raghavendra Enterprises, Bangalore, Karnataka, India. The animals were granted by the Institutional Animal Ethics Committee (DSCBS/Ph.D./IAEC/01/14-15) and were nursed with a standard pellet diet and water ad libitum, accustomed prior to the experimentation.
Determination of acute toxicity
Acute oral toxicity studies were conducted for ethanolic extract of G. ula as per the OECD guidelines 423 [19]. Female Swiss albino mice weighing 20–30 g were used for the study. These mice were fasted for 4 hours providing only water. Later GUE was administered orally at a limited dose of 2,000 mg/kg body weight. The animals were monitored continuously for 4 hours for gross behavioral changes and finally for 48 hours and then daily for 14 days for any signs of toxicity including death.
Evaluation of Hepatoprotective activity of G. ula
Hepatoprotective activity was conducted using both the sex of Wistar albino rats (150–180 g) and were divided into five groups with six animals each. Group 1 was provided with 0.5% Carboxymethyl cellulose (CMC) (10 ml/kg body weight) functioned as normal control and Group 2 with 0.5% CMC (10 ml/kg body weight) as toxicant control. Group 3 as positive control served with the standard silymarin (200 mg/kg). Groups 4 and 5 were treated with ethanolic extracts of G. ula at doses of 100 and 200 mg/kg body weight, respectively. As mentioned, all the drugs were dispensed orally using gavage for 7 days. On the eighth day, apart from group 1, all other groups were treated with CCl4 intraperitoneally (1 ml/kg body weight in olive oil). On the ninth day, blood was collected by retro-orbital puncturing under mild anesthetic conditions (diethyl ether) followed by separation of serum by centrifugation at 2 000g for 10 minutes. Serum was preserved in vials at 4°C, and soon used for the estimation of biochemical markers. The animals were sacrificed after 24 hours of CCl4 treatment by overdosing with diethyl ether. The liver was taken off all animals, perfused with phosphate buffer, homogenized with 10% potassium chloride (KCl) solution, and centrifuged for 10 minutes at 2,000 rpm. The supernatant was used for the estimation of antioxidant assays. A portion of liver tissue was preserved in a 10% formalin solution for histopathological studies to observe gross necrosis, fatty acid changes, and inflammatory cells [20,21].
Lipid Peroxidation assay
Breaking of polyunsaturated fatty acids gives malondialdehyde and forms a pink-colored compound upon reaction with thiobarbituric acid, which is measured at 532 nm [22]. Its concentration in the test can be calculated using an extinction coefficient of 1.6 × 105 M-1cm-1.
Effect of GUE on serum biochemical parameters
Serum was subjected to biochemical investigations like serum glutamic pyruvic transaminase or ALT, serum glutamic-oxaloacetic transaminase or AST, alkaline phosphatase (ALP), total bilirubin, total protein, and albumin by kit methods as per instructions provided by the company (Erba diagnostics inc).
Effect of GUE on liver endogenous antioxidant enzymes
Superoxide dismutase activity assay (SOD)
SOD inhibits auto-oxidation of epinephrine to adrenochrome at alkaline pH [23]. SOD activity was expressed as unit/mg protein. One unit of the enzyme is defined as the amount of enzyme required to inhibit 50% of the rate of epinephrine auto-oxidation.
Catalase assay (CAT)
Catalase activity was approached by Aebi methodology [24]. The decrease in optical density at 0 time and 1 minute time is read at 240 nm. Units of catalase were expressed as the amount of enzyme that decomposes 1 mM H2O2/minute at 25°C. Catalase activity was expressed as U/mg of protein.Estimation of Glutathione (GSH)
GSH is a sulfhydryl-containing non-protein substance. DTNB (5.5’ di thio bis (2-nitrobenzoic acid) is a disulphide chromagen reduced by sulfhydryl compounds to a yellow color compound and is measured at 412 nm. This is directly proportional to the GSH concentration [25].
Histopathological studies
Liver samples were used to study the effect of GUE on the CCl4-induced rats. Phosphate phosphate-buffered 10% formaldehyde solution was used to fix the liver sample of different groups. It was then embedded in paraffin wax to cut thin sections of 5 µm thickness and stained with hematoxylin–eosin. Treated sections were observed by using a high-resolution microscope and photomicrographs were captured [26].
Statistical analysis
The results were provided as the mean ± S.E.M. of six animals that were employed in each group. One-way analysis of variance was used to do a statistical analysis of the data. Followed by Tukey’s multiple comparison test using Graph pad Prism 6.0 software at the level of p < 0.05.
RESULTS
Extraction of G. ula
Soxhlet extraction of the stem (500 gm) of G. ula with ethanol yielded 4.45%w/w. During this extraction process, plant material was defatted with petroleum ether first and extraction with ethanol led to the accumulation of highly polar molecules with an increase in the yield, which may result in good hepatoprotective activity. Ethanol extract was studied for hepatoprotective activity GUE.
In vivo studies of G. ula.
In recent years, several findings have been supported by traditional medicines in an attempt to find new drugs for liver disorders. In the present study, ethanol extract of the stem of G. ula was evaluated for hepatoprotective activity against CCl4-induced hepatotoxicity to find out the therapeutic efficacy of the extract.
Acute toxicity studies
Ethanol extract of G. ula GUE did not show symptoms of mortality or toxicity at the dose of 2,000 mg/kg body weight. Therefore, 110 (200 mg/kg) and 120 (100 mg/kg) of GUE were chosen as high dose and low dose, respectively.
Effect of GUE on hepatic biochemical markers
The hepatoprotective effect of GUE (Groups 4 and 5) is summarised in Table 1. CCl4-induced elevation of serum AST, ALT, ALP, total bilirubin, and demotion of total protein, albumin was observed in the toxicant group. GUE in comparison to group 2, reversed the condition significantly (p < 0.05) decreasing the serum AST, ALT, ALP, and total bilirubin and elevating total protein and albumin. Silymarin treated group (200 mg/kg) revealed significant protection against CCl4-induced hepatic damage. Treatment with GUE decreased the serum markers level at the dose of 100 mg/kg and 200 mg/kg only, which is backed by histological changes.
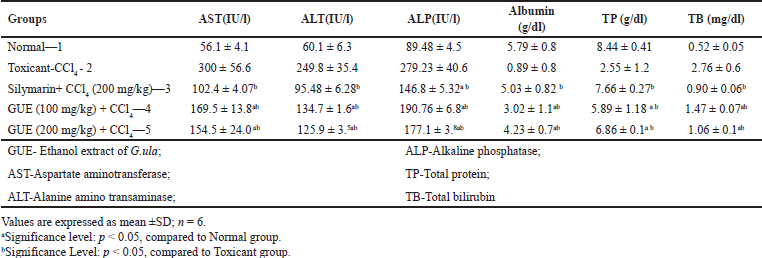 | Table 1. Biochemical parameters of ethanolic extract G.ula in CCl4 treated animals. [Click here to view] |
Effect of GUE on hepatic antioxidant markers
The antioxidant defense system in the liver (SOD, CAT, and GSH) is affected by lipid peroxidative degradation of bio membrane, the basis for hepatotoxicity [27]. CCl4 produced a significant reduction in the SOD, CAT, and GSH activities and increases in the MDA level compared to the normal group. GUE (100 mg/kg and 200 mg/kg) made a significant (p < 0.05) increase in the enzyme activity of SOD, CAT, and GSH and a decrease in MDA. Group III treated with standard silymarin also showed comparable results. Results are tabulated in Table 2.
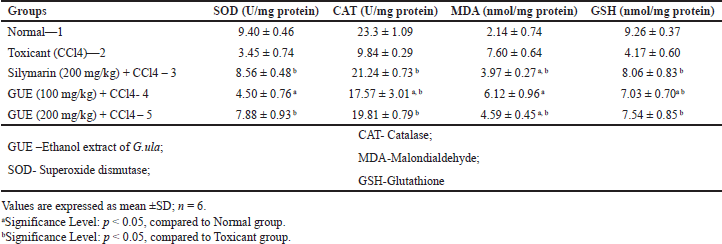 | Table 2. In vivo antioxidant assays of ethanolic extract of G. ula in CCl4 treated animals. [Click here to view] |
Histopathological studies (Fig. 3)
Normal Group of rats studied shows intact architecture of liver parenchyma (Fig. 1). Toxicant Group showed distorted architecture of liver the perivenular region shows severe necrosis (Fig. 2). The central veins and sinusoids appear distorted(Fig. 2). Standard group liver Section studied from shows undamaged architecture of liver parenchyme (Fig. 3). The perivenular region shows mild necrosis consisting of degenerative hepatocytes (Fig. 3) and mixed inflammatory cells. Few regenerative hepatocytes are also seen. Some of the veins appear congested (Fig. 3). GUE at 100 mg/kg treated group shows micro vesicular steatosis (Fig. 4) with the regeneration of hepatocytes. The perivenular region shows moderate necrosis consisting of degenerative hepatocytes (Fig. 5) and mixed inflammatory cells (Fig. 5). GUE treated group (200 mg.kg) directs the normal liver parenchyma architecture (Fig. 5). The periportal hepatocytes (Fig. 4) and midzonal hepatocytes appear unremarkable.
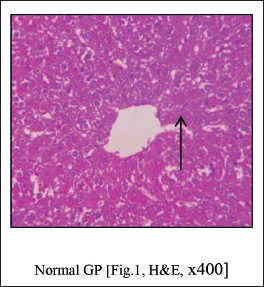 | Figure 1. Normal group shows normal architecture of liver. [Click here to view] |
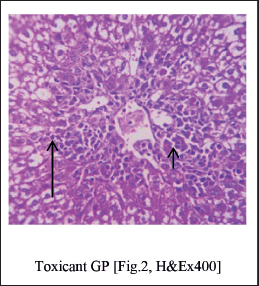 | Figure 2. Distorted architecture of liver the perivenular region shows severe necrosis [Short Arrow], the central veins and sinusoids appear distorted. [Long Arrow]. [Click here to view] |
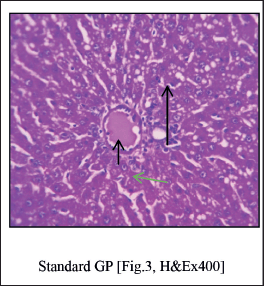 | Figure 3. Standard group liver shows undamaged architecture of liver parenchyma [Long Arrow]. The perivenular region shows mild necrosis consisting of degenerative hepatocytes [Green Arrow] and mixed inflammatory cells. Veins appear congested [Short Arrow]. [Click here to view] |
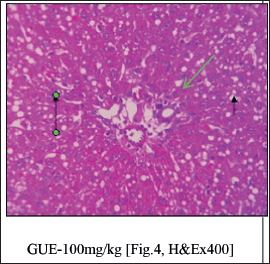 | Figure 4. GUE at 100 mg/kg treated group shows micro vesicular steatosis [Green Arrow] with regeneration of hepatocytes. The perivenular region shows moderate necrosis consisting of degenerative hepatocytes [Long Arrow] and mixed inflammatory cells [Short Arrow]. [Click here to view] |
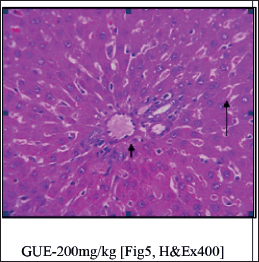 | Figure 5. Normal liver parenchyma architecture [Long Arrow]. The periportal hepatocytes [Short Arrow] and midzonal hepatocytes appear unremarkable. [Click here to view] |
DISCUSSION
Carbon tetrachloride, a well-studied hepatotoxin is used to induce liver damage in rats reported in 1936 [28], widely used and accepted by many researchers. The cytochrome P450 activates CCl4 to create the trichloromethyl radical (CCl3?), which combines with a molecule of oxygen to produce trichloromethylperoxyl radical (CCl3O2?) (Cameron and Karunaratne, 1936).CCl3O2? binds to macromolecules and causes per oxidative degradation of lipid membranes, leading to the formation of lipid peroxides like MDA causing loss of integrity of cell membrane and hepatic damage [29,30]. Stress and injury caused by CCl4 induce the activation of other cells like natural killer cells, kupffer cells, and NKT cells, progressing to liver injury by producing various inflammatory cytokines like tumor necrosis factor–Alfa, Interferon-gamma, and Interleukins-1betta [31]. However, interleukins (IL-10 and IL-6) and prostaglandins exhibit a liver-protective role [32]. AST, ALT, ALP, and total bilirubin in plasma are sensitive to liver injury [33] and cause leakage from cells due to the altered permeability of the membrane. AST is normally found in heart, liver, and kidney and in skeletal muscles. AST helps in the conversion of L-aspartate and Alpha-ketoglutarate to L-glutamate and oxaloacetate. ALT is found in liver disorders, cirrhosis, mycocardial infraction, and high levels of liver issues. It catalyzes the transamination of L-alanine and Alpa –ketoglutarate to form pyruvate and L-glutamate. Serum ALP is found in bone, liver, intestine placenta and excreted in bile. An increased level is noted in the case of hepatobiliary and bone diseases, [34]. The present investigation revealed that ethanolic extract of G. ula has therapeutic values in treating liver damage induced by CCl4 in rats. GUE attempted to recover the damage caused by increased significantly serum marker levels.
Bilirubin, metabolite from heme breakdown also affects the hepatic cell [35]. A raised level of bilirubin is found in hepatic diseases, hemolysis, and biliary excretion. GUE at 200 mg/kg b.wt dose was able to control from dropping down of bilirubin levels. Total protein concentration will be deprived in the hepatoxic condition by defective protein biosynthesis [36,37]. Hepatotoxic CCl4 causes disruption of polyribosomes on the endoplasmic reticulum, thereby bringing down the process of protein synthesis. In this study, ethanolic extract of G. ula at 200 mg/kg significantly stabilizes the membrane, repairs the hepatic tissue, protects the polyribosomes, and restores protein synthesis.
Whenever the cellular capacity of removal of reactive oxygen species diminishes then the cell gets injured [38,39]. Our results showed significant depletion of SOD, CAT, and GSH levels in rat liver cells with CCl4 induction. However, antioxidants can inhibit CCl4-induced toxicity at various levels by directly scavenging the CCl3, donating hydrogen to CCl3, and donating hydrogen CC13O2. Antioxidant enzymes include SOD, CAT, and GSH are the first line of the defense system, converting oxygen molecules its non-toxic compounds [27].
SOD eliminates superoxide radicals by turning them into H2O2, which CAT and GSH can quickly convert into water. Thus, CAT and GSH inhibit the formation of hydroxyl radicals.
MDA is one of the end products in the lipid peroxidation process. An increase in MDA levels indicates the extent of lipid peroxidation leading to tissue damage and preventing the antioxidant system from working through [40]. GUE-treated groups enhanced enzymatic activity thereby protecting the hepatocytes and diminishing the lipid peroxidation.
Since their chemical structures are interrelated, a direct connection between antioxidant activity and total phenol concentration has been identified. Therefore, in vivo antioxidant activity of the ethanolic extract of G. ula is associated with the total phenol content present in the extract. Hepatoprotective activity has been linked to the antioxidant activity of the plant extracts. Most of the naturally occurring phenolic compounds possess antioxidant activity [41,42]. Stilbenes are a group of polyphenols and many of them have been isolated from the genus Gnetum [43,44] and also from the G. ula [14,15,16]. Stilbenes are a rich source of lead phytoconstituent for newer drugs and treatments. Many of the naturally occurring stilbenes exhibit uniqueness by possessing strong antioxidant activity/radical scavenging properties. Most recently isolated stilbene is known to possess antioxidant, antimicrobial, and anti-inflammatory properties and some of them have the potential to be developed as a new drug [45]. In our present study, the stem of G. ula exhibited antihepatotoxicity and the stilbenes content of the plant might be contributing to the protection of hepatic cells, thereby bringing back to normal functioning of liver cells.
CONCLUSION
In the present study, GUE possesses a strong antioxidant activity. The antioxidant and free radical-scavenging properties of G. ula stem were found to be responsible for the plant’s hepatoprotective effects. These reports, therefore, support the tribal use of G. ula stem for liver problem treatment. For the first time, G. ula has been studied for ant hepatoprotective activity. A further study on the isolation and identification of phytochemical compounds to correlate the antioxidant and hepatoprotective activity has been obtained to support the present study.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICT OF INTEREST
The author reports no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
Ethical approvals details are given in the ‘Material and Method’ section
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Negi AS, Kumar JK, Luqman S, Shanker K, Gupta MM, Khanuja SP. Recent advances in plant hepatoprotectives: a chemical and biological profile of some important leads. Med Res Rev. 2008;28(5):746–72. CrossRef
2. Ali AM, Alan DL, Saied S, Rafael L, Ali HM, Jeff S, et al. Liver cirrhosis mortality in 187 countries between 1980 and 2010: a systematic analysis. BMC Med. 2014;12(1):145. CrossRef
3. Maity T, Maity S, Pahari N, Ranjan DK, Ganguli S. A review on hepatic diseases and development of herbal drugs for the treatment of liver complications. World J Pharm Res. 2015;5(5):677–91.
4. Vikneshwaran D, Viji M, Raja LK. Ethanomedicinal plants survey and documentation related to paliyar community. J Ethnobot Leaflets. 2008;12(1):1108–11015.
5. Thirumalian T, Elumalai EK, Viviyan TS, Senthil BK, Ernest. D. Ethnobotanical survey of folklore plants for the treatment of jaundice and snakebites in vellore. J Ethnobot Leaflets. 2010;14:529–36.
6. Mohan VR, Rajesh A, Athiperumalsami T, Sutha S. Ethnomedicinal plants of the Tirunelveli District. Tamilnadu, India. J Ethnobot Leaflets. 2008;12:79–95.
7. Pushpangadan P, Atal CK. Ethnomedical and ethnobotanical investigations among some scheduled caste communities of Travancore, Kerala. India. J Ethnopharmacol. 1986;16(1-2):175–90. CrossRef
8. Raj Kumar MH, Rajanna MD. Ex-situ conservation of climbing plants at university of agricultural sciences, Bangalore, Karnataka. Rec Res Sci Tech. 2011;3(4):18–20.
9. Basu P, Mitra B. Preliminary notes on the climbing taxa of Andaman and Nicobar Islands with special reference to their importance. J Econ Taxon Bot. 1992;16:393–9.
10. Pullaiah. T. Biodiversity of India. Paris, France: Regency publications; 1992. Pp 552.
11. Prusti AB, Behera KK. Ethnobotanical exploration of Malkangiri District of Orissa, India. J Ethnobot Leaflets. 2007;11:122–40.
12. Warrier PK, Nambiar VPK, Ramankutty C. Indian medicinal plants: a compendium of 500 species. Madras, India: Orient Longman; 1993.
13. Mondal P, Mukerjee PK. Notes on ethnobotany of Keonjhar district, Orissa. J Econ Tax Bot Addl Ser. 1992;10:7–18.
14. Zaman A, Prakash S, Wizarat. K. Isolation and structure of gnetol, a novel stilbene from Gnetum ula. Indian J Chem. 1983;22b:101–4.
15. Prakash S, Khan MA, Khan KZ. Stilbenes of Gnetum Ula. Phytochemistry. 1985;24:622–4. CrossRef
16. Siddiqui Z, Rahman S, Khan MA. Gnetulin, a dimer of 3’,4,5’ – Trihydoxy- 3- methoxy Stilbene from Gnetum ula. Tetrahedron. 1993;49:10393–6. CrossRef
17. Tandon N. Reviews on Indian medicinal Plants. Medicinal Plant Unit, Indian Council of Medical Research, New Delhi. 2013;11:1120.
18. Khandewal KR. Practical pharmacognosy. 22nd ed. Pune, India: Nirali publications; 2013.
19. OECD/OCDE. OECD Guideline for testing of chemicals. Acute Oral Toxicity-Acute Toxicity Class methods. Paris, France: OECD Publishing; 2002.
20. Chen Y, Huang B, He J, Han L, Zhan Y, Wang Y. In vitro and in vivo antioxidant effects of the ethanolic extract of Swertia chiraytia. J Ethnopharmacol. 2011;136(2):309–15. CrossRef
21. Pareek A, Godvarthi A, Issarani R, Nagori BP. Antioxidant and hepatoprotective activity of Fagonia schweinfurthii (Hadidi) extract in carbon tetrachloride induced hepatotoxicity in HepG2 cell line and rats. J Ethnopharmacol. 2013;150(3):973–81. CrossRef
22. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobartituric acid reaction Anal. Biochem. 1979;95(2):351–58. CrossRef
23. Mishra HP, Fridovich I. The role of superoxide anion in the autooxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247(10):3170–5. CrossRef
24. Aebi H. Catalase. In: Bergmeyer HV. Methods in enzymatic analysis Newyork, NY: Academic press; 1974. Vol. 2, pp 674–84. CrossRef
25. Moran MA, Depierre JW, Mannervick B. Levels of glutathione, glutathione reductase, glutathione-S-transferase activities in rat liver. Biochimica Biophysica Acta. 1979;582(1):67–8. CrossRef
26. Shenoy, KA, Somayaji SN, Bairy KL. Hepatoprotective effects of Ginkgo biloba against carbon tetrachloride induced hepatic injury in rats. Indian J Pharmacol. 2001;33(4):260–6.
27. Ames BN, Shigenenga MT, Hagen M. Oxidants, antioxidants and the degenerative diseases of aging. Proc Natl Acad Sci United States Am. 1993;90(3):7915–22. CrossRef
28. Cameron GR, Karunaratne WAE. Carbon tetrachloride cirrhosis in relation to liver regeneration. J Pathol Bacteriol. 1936;42(1):1–21. CrossRef
29. Thabrew MI, Joice PD, Rajatissa WA. Comparative study of the ef?cacy of Paetta indica and Osbeckia octandra in the treatment of liver dysfunction. Planta Med. 1987;53(3):239–41. CrossRef
30. Bishayee A, Sarkar A, Chaterjee M. The hepatoprotective activity of carrot (Daucas carota L.) against carbon tetrachloride intoxication in mouse liver. J Ethnopharmacol. 1995;49(2):69–74. CrossRef
31. Blazka ME, Wilmer JL, Holladay SD, Wilson RE, Luster MI. Role of pro-inflammatory cytokines in acetaminophen hepatoxicity. Toxicol Appl Pharmacol. 1995;133(1):43–52. CrossRef
32. Bourdi M, Masubuchi Y, Rreilly TP. Protection against acetaminophen –induced liver injury and lethality by interleukin 10, role of inducible nitric oxide synthase. Hepatology. 2002;35(2):289–98. CrossRef
33. Molander DWF, Wrolewski, JS. LA-Due. Transaminase compared with cholesterase and alkaline phosphatase an index of hepatocellular intergrity. Clin Res Proc. 1987;3:20–4.
34. Harsha Mohan. The liver, biliary tract and exocrine pancreas. In: Text book of pathology, 4th ed. New Delhi, India: Jaypee Brothers Medical Publishers LTD; 2002. Pp 447–9.
35. Victor J, Navarro MD, John R, Senior MD. Drug-related hepatotoxicity. N Engl J Med. 2006;354(7):731–39. CrossRef
36. Goel A, Dani.V, Dhawan DK. Protective effects of zinc on lipid peroxidation, antioxidation, antioxidant enzymes and hepatic histoarchitecture in chlorphrifos induced toxicity. Chem Biol Interact. 2005;156(2-3):131–40. CrossRef
37. Wu YH, Zhang XM, Hu MH, Wu XM, Zhao Y. Effect of Lagera alata on hepatocytes damage induced by carbon tetrachloride in vitro and in vivo. J Ethnopharmacol. 2009;126(1):50–6. CrossRef
38. Velioglu YS, Mazza G, Gao L, Oomah BD. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J Agric Food Chem. 1998;46:4113–7. CrossRef
39. Sevindik M, Akgul H, Pehlivan M, Selamoglu Z. Determination of therapeutic potential of Mentha longifolia ssp. longifolia. Fresen Environ Bull. 2017;26(7):4757–63.
40. Antonio A, Mario F. Muñoz, Sandro Argüelles. “Lipid Peroxidation: production, metabolism, and signaling mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal”. Oxid Med Cell Longev. 2014;2014:31. CrossRef
41. Preetham J, Kiran S, Sharath R, Sundari S, Ganapathy S, Sushma S Murthy. Pharmacognostic and preliminary phytochemical studies in Gnetum ula brongn. Int Res J Pharm. 2015;6:377–38. CrossRef
42. Pereira DM, Valentão P, José AP, Andrade PB. Phenolics: from chemistry to biology. Molecules. 2009;14(6):2202–11. CrossRef
43. Lin M, Li JB, Li SZ, Yu DQ, Liang XT. A dimeric stilbene from Gnetum parvifolium. Phytochemistry. 1992;31(2):633–8. CrossRef
44. Tanaka T, Iliya I, Ito T, Furusawa M, Nakaya KI, Iinuma M, et al. Stilbenoids in Lianas of Gnetum parvifolium. Chem Pharm Bull. 2001;49(7):858–62. CrossRef
45. Tao S, Wang XN, Lou HX. Natural stilbenes: an overview. Nat Prod Rep. 2009;26:916–35. CrossRef