INTRODUCTION
Current global pharmaceutical industry-based research is basically focused on developing new drugs with cost effectiveness having better drug delivery systems for patient compliance and effective therapeutic potential. Conventional drug delivery formulations do not have the capabilities to control drug release profiles for maintaining the appropriate concentration of drug at site-specific regions for the desired time [1,2]. Advancement of technology in the development of long and sustained drug release profile dosage forms has been improved in countless ways due to intense and vigorous research in this field to improve the physical and biopharmaceutical limitations of numerous drugs [3,4].
An osmotic controlled-release tablet is an advanced controlled-release system that has a semipermeable outer membrane with a laser-drilled small orifice that allows fluid to enter via osmosis through a semipermeable membrane [5]. The osmosis phenomenon causes osmotic pressure which is responsible for API release through the drilled small orifice opening. The coating provides protection against gastric fluids and prevents drug irritation-related issues such as ulceration, nausea, and vomiting [6]. Irrespective of dosage, the coating also provides limited release of drug through the polymer semipermeable membrane to impart a prolonged release profile of API [7,8].
Metformin hydrochloride (HCl) is a globally accepted drug for the treatment of type 2 diabetes mellitus having high solubility via a prototypical transport-mediated mechanism [9]. Metformin HCl acts by inhibiting the absorption of intestinal glucose in addition improves peripheral glucose uptake and lowers fasting plasma insulin which results in a reduction of blood glucose concentrations. It has been reported that gastrointestinal intolerance restricts its use in some subjects but its extended-release formulation can provide improved gastro intestinal tolerability and reduce dosing frequency [10,11].
In 1974, a US patent (3845770) osmotic tablet was made using laser drilling after a semi-permeable coating. Due to sophisticated laser drilling steps, the costs of production of these tablets are too high which increases treatment costs [12,13]. Drilling holes without the laser has been tried earlier but the reproducibility and accuracy of orifice within parameters could not be achieved. On the other side, the sophisticated process of laser step tablet production is costlier and time consuming. Customized tooling is another approach for developing osmotic tablets without the use of a drill [14].
With the above background, an effort was made to explore an alternative method for producing osmotic tablets without the requirement of drilling, using customized tooling. It was planned to validate the prepared tooling through established parameters and thereafter prepare an optimized formulation using metformin HCl as a suitable candidate.
The plan of the work was to develop tooling with an innovative notch in the die to create an in situ passageway for a cost-effective osmotic controlled drug delivery system, eliminating the requirement for laser drilling. In addition, the study aimed to optimize the composition of the core tablet, coating composition, and coating process parameters for osmotically controlled metformin HCl tablets.
MATERIAL AND METHODS
Materials
Metformin HCl was obtained as a gift sample from Sun Pharmaceutical Industries Limited, compression tooling purchased from Pacific Tools Pvt. Ltd., povidone (PVP) and sodium lauryl sulfate (SLS) sourced from BASF Pharma, hydroxypropyl methylcellulose, and cellulose acetate sourced from Dupont, magnesium stearate sourced from Peter Greven, polyethylene glycol (PEG) 400 sourced from Croda, acetone sourced from Thermo Fischer, and Gluconorm sustained release (SR) tablets (1 g) sourced from Lupin. Software JMP version 17 is used for the design of experiment data analysis.
Development of tooling
Design of special notch tooling for the development of metformin osmotic tablets was done with dimensions as shown in Figure 1. Two tooling samples were manufactured with different notch widths of 0.7 and 0.9 mm with 1.0 mm depth of notch, with dimensions of 21 × 10 mm, an oval shape, tapered dies, and hard chrome plating using S7 MOC. One tool with an 11 mm width, round shape, 0.5 mm notch width, and 1.0 mm notch depth was manufactured with the same material of construction. Core tablets were prepared using composition as per Table 1 and evaluated for physical attributes.
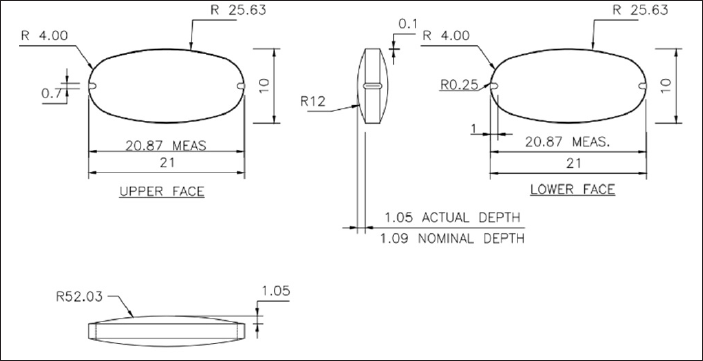 | Figure 1. Design parameters of special notch tooling for metformin osmotic system. [Click here to view] |
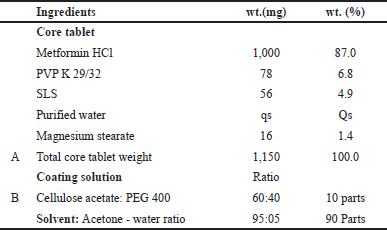 | Table 1. Composition of the tablet excipients and coating material. [Click here to view] |
Reservoir system controlled release tablets
Tablets without a special notch were prepared as per the formula (Table 1) to compare the performance of a notched osmotic system. The required quantities of PVP and SLS were dissolved in water. Sifting of the metformin was done using #36 BSS to ensure a uniform particle size and to break down any large agglomerates. Granulation was done using a solution of PVP and SLS in a fluid bed processor. After granulation, the wet mass was dried at 60°C. Subsequently, the dried granules were milled through a 0.8 mm sieve to ensure uniformity. To improve the tablet compression process, the in-process blend was lubricated with magnesium stearate, reducing friction between particles during compression. The blend was then compressed using plain 21 × 10 mm oval-shaped punches, shaping the granules into tablets. In preparation for coating, a coating solution with a 10% solid content was formulated. The tablets were spray-coated in a coating pan using this prepared coating solution. Finally, samples were collected at intervals of 5%, 10%, and 15% weight build-up. Since Metformin HCl extended release is independent of the pH of the media, hence Metformin HCl ER tablets dissolution was performed with 900 ml, pH 6.8 phosphate buffer, USP type-I/IP type –II (basket # 10) at 50 rpm.
Osmotic system with notch-controlled release tablets
Tablets with a special notch were optimized using a full factorial design to compare the performance of a notched osmotic system. Characterization of the developed tablet was carried out by evaluating blend bulk density, tablet disintegration time, tablet hardness (kp), sticking/picking, and friability. The formulation variables affecting responses with their ranges and their type are tabulated in Table 2. Experimental Run Matrix represents levels of each factor to prepare tablets with a special notch osmotic system to optimize core tablet composition, keeping magnesium stearate constant with ten batches using a full factorial design, as shown in Table 3. A similar manufacturing process as described in “Reservoir system-controlled release tablets” using specialized oval-shaped punches measuring 21 × 10 mm, featuring a notch-enabled design, was used. The tablets were spray-coated in a coating pan using a coating solution of cellulose acetate and PEG 400 with a 90:10 ratio of acetone and water (in a 95:5 ratio) as a solvent. Samples were collected to assess 2% and 3% weight build-up during the coating process.
 | Table 2. Formulation variables and response for osmotic controlled release system with notch. [Click here to view] |
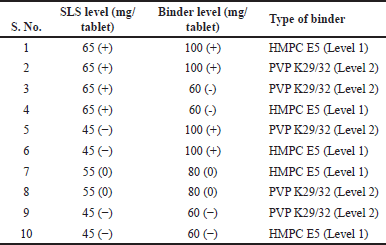 | Table 3. Design matrix of tablets with special notch osmotic system. [Click here to view |
Optimization of coating composition
After selection and optimization of the core tablet excipients, coating composition was optimized using full factorial design as shown in Table 4, and the design matrix for six trials is given in Table 5. This involved dissolving PEG in water and cellulose acetate in acetone separately. Subsequently, the PEG solution was carefully added to the cellulose acetate under constant stirring, maintaining this stirring for a duration of 45 minutes. The tablet coating process involved spray coating within a coating pan, utilizing a semi-permeable coating solution. Samples were collected at two different stages, specifically when achieving 2% and 3% weight buildup on the optimized core tablet. Then, the in vitro dissolution profile was evaluated in pH 6.8 phosphate buffer, and drug release kinetics were evaluated using the zero-order, first-order, Higuchi, and Korsmeyer–Peppas models
 | Table 4. Factors and levels for coating composition of osmotic system with notch-controlled release. [Click here to view] |
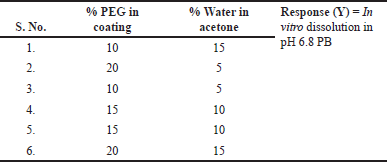 | Table 5. Design matrix of tablet coating composition with special notch osmotic system. [Click here to view] |
Optimization of coating process
The selected and optimized composition of coating and tablet core was used for optimizing the coating process using full factorial design in ten trials by evaluating bed temp (?C), spray rate (g/min), and curing time (minutes) as factors of the coating process on in vitro dissolution are given in Tables 6 and 7.
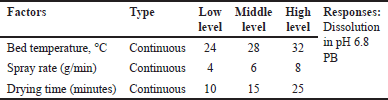 | Table 6. Factors affecting coating process of osmotic system with notch-controlled release. [Click here to view] |
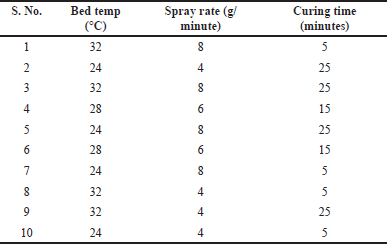 | Table 7. Design matrix of tablet coating process with special notch osmotic system. [Click here to view] |
Characterization
Characterization of blend
Bulk Density of Blend (B.D.)
100 g of blend transferred into measuring cylinder of 250 ml; volume of sample noted (V0). Bulk density (g/cc) calculated using the formula [15]:
Bulk density (BD) =100 / V0.
Characterization of core and coated tablets
Tablet friability (%)
10 core tablets were weighed and placed in a rotating drum. The drum is rotated to 100 revolutions. The sample is reweighed after 100 revolutions to find % weight loss [16].
Tablet hardness (kp)
10 core tablets were placed under a hardness tester and the average was calculated [16].
Dissolution
One coated tablet was placed in each dissolution vessel (N = 12 units), USP type I (basket # 10 mesh) containing 900 ml pH 6.8 phosphate buffer, with 50 rpm basket rotation, 10 ml samples were withdrawn at regular time intervals with replacement of media. The sample is further diluted to 100 times. Absorbance was calculated in a UV spectrophotometer at 232 nm wavelength and the concentration of metformin HCl was estimated against a standard known solution of known concentration [17].
Disintegration time (mins)
Each core tablet was placed in 6 tubes of the basket in a disintegration apparatus with water as a medium, maintained at 37°C ± 2°C. Time in minutes noted for complete disintegration of tablets [18].
Drug release kinetics and similarity factor
The optimized batch and marketed product (Gluconorm SR 1 g tablets) were further analyzed to check drug release kinetics and the best-fit release model using zero order (% drug release= Slope × time), first order (log [Fraction unreleased] = Slope × time/2.303), Higuchi model (% Drug release = Slope × [time]0.5), and Korsmeyer–Peppas equation (% Drug release = Slope × [time]n). Similarity factor (f2) for dissolution profiles was calculated between the optimized batch and the marketed product on 12 units [7].
Stability studies
Stability studies were performed according to ICH guidelines. Tablets were packed in HDPE bottle 30’s Count and charged on accelerated (40°C/75%RH/6 months) and long-term conditions (25°C/60%RH/6 months). The dissolution profile, F2 (similarity factor), and appearance of tablets were compared for initial and at 6 months stability [16].
RESULT AND DISCUSSION
Tooling optimization
Core tablets with oval tooling (20 × 10 mm) having a notch width of 0.9 and 1.0 mm notch depth show friability of 0.9% w/w, and chipping was observed at notch edges, hence not considered for further trials. This may be due to a wide notch. Core tablets with round tooling (11 mm width) having a notch diameter of 0.5 with 1.0 mm notch depth produced friction noise between punches and the tablet surface while ejecting tablets, hence not considered for further trials. Core tablets with oval tooling (20 × 10 mm) having a notch width of 0.7 with 1.0 mm notch depth show friability of 0.3% w/w without any noise while tablet ejection from the machine. Hence, this tool was finalized for further trials.
For reservoir systems
Osmotic tablets with 5% coating weight build up were able to control the release of the drug till 6 hours only; however, with 10% and 15% coat, drug release can be controlled till 12 hours as shown in Table 8. One tablet out of 6 tablets after completion of dissolution found pin holes at the edges causing leakage in the system. The reason for the leakage was the insufficient mechanical strength of the coating membrane, leading to the formation of pinholes in the coating. Also, as a result of 10% and 15% weight gain during the coating process, the total tablet weight increased.
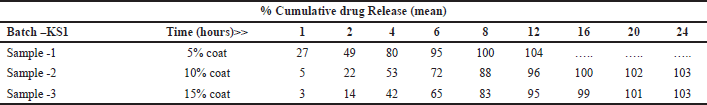 | Table 8. % Metformin release in 900 ml, pH 6.8 PB, 50 rpm (reservoir system). [Click here to view] |
For notch-controlled osmotic tablets optimization
Characterization of blend and core tablet
The bulk density of the blend was found in a range of 0.48–0.92 g/cc; Core tablet hardness ranges from 11.3 to 16.3 kp, (Table 9). Tablet DT was found between 5 minutes 50 seconds to 9 minutes 10 seconds for all trials. Batch no. CORE4 observed the sticking of tablets, no picking/sticking was observed in other experiments.
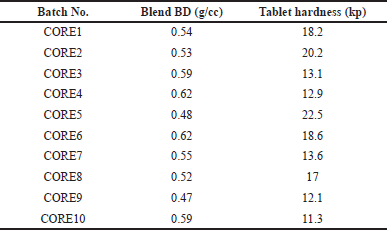 | Table 9. Characterization of blend BD and core tablet hardness. [Click here to view] |
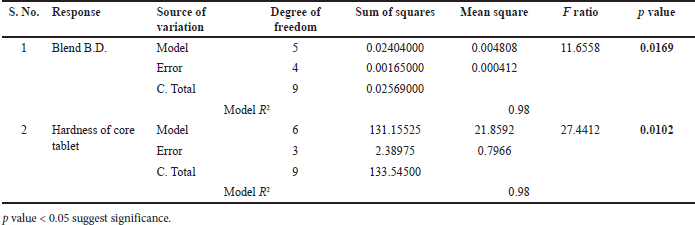 | Table 10. ANOVA summary for regression. [Click here to view] |
Regression analysis for core tablet
In response blend BD (g/cc), p value for the blend BD model was 0.0169 (<0.05) and R2 = 0.98, hence model was significant which is shown in Table 10. The type of binder was significant with p value of 0.0068; however, SLS level and binder level had no impact on blend BD. All two-factor interactions are significant given in Table 11. Pareto chart, surface profiler, and prediction profiler are shown in Figure 2A, 2C, and 2E for response: Blend BD. In response to tablet hardness (kp), the p-value was 0.0102 (<0.05) and R2 = 0.98; hence, the model was significant which is Shown in Table 10. Binder level and type of binder were significant with p value of 0.0013 and 0.0355, respectively (Table 12). Based on the Pareto chart, prediction profiler, and surface profiler as shown in 2B, 2D, and 2E, increasing the binder level will increase the hardness of core tablets. On the physical description, batches with HMPC E5 at low levels show a capping phenomenon, which may create concerns in the coating process, hence PVP K29/32 was selected as a binder with 84 mg/tablet, and SLS at 60 mg/tablet was finalized for further trails.
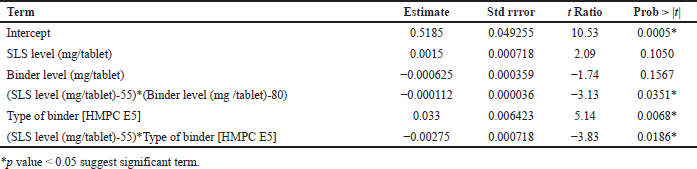 | Table 11. Parameters estimated for response blend BD (g/cc). [Click here to view] |
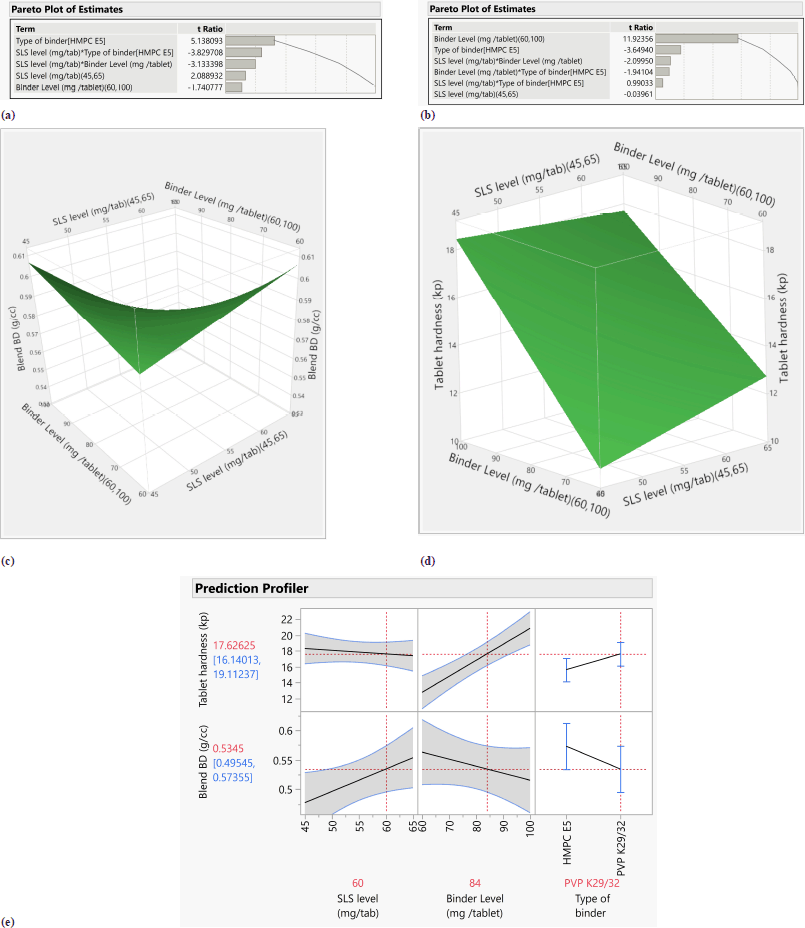 | Figure 2. (a) Pareto chart for response- blend BD (g/cc). (b) Pareto plot of response tablet hardness (kp). (c). Surface profiler for response -blend B.D. (d) Surface profiler for response hardness of tablets. (e) Prediction profiler for response. [Click here to view] |
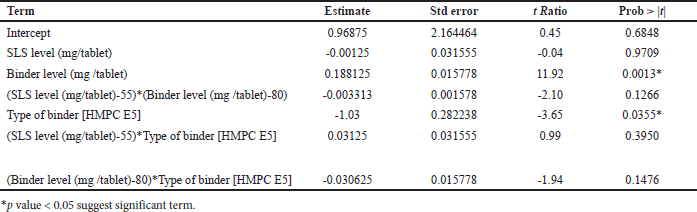 | Table 12. Parameters estimated for response tablet hardness (kp). [Click here to view] |
Characterization for coating composition
The characterization of the percentage of PEG and percentage of water in acetone were studied and the impact on in vitro dissolution data are shown in Table 13 with different coating compositions.
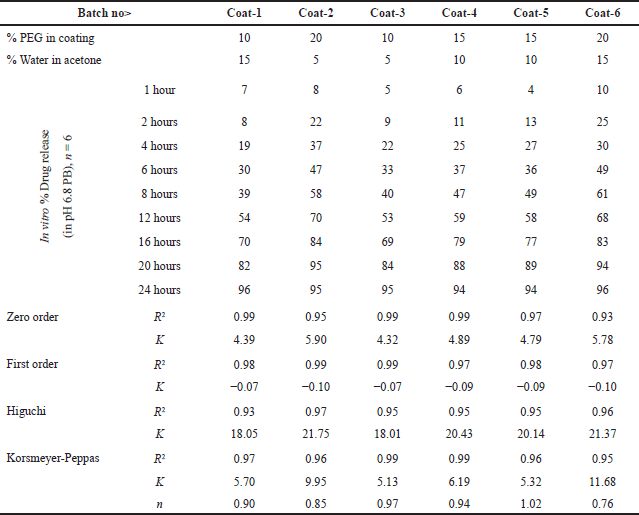 | Table 13. In vitro-dissolution data of metformin HCl in pH 6.8 phosphate buffer for analysis of coating composition with 2% weight build up and drug release kinetics. [Click here to view] |
Regression analysis for coating composition
From ANOVA Table 14, p-value and R2 value of the model were 0.0247 (<0.05) and 0.98, respectively, for dissolution at 4 hours. p-value and R2 value of the model were 0.0369 (<0.05) and 0.98, respectively, for dissolution at 16 hours, and hence both models were significant as shown in Table 14. p-value for lack of fit was 0.564 (>0.05) and 0.4359 (>0.05) for 4 and 16 hours dissolution models respectively which suggest insignificant lack of fit. % PEG in the coating was a significant factor in dissolution at 4 and 16 hours. (p value < 0.05). On increasing the % PEG in the coating membrane, dissolution was increased as per Tables 15 and 16. Based on the Pareto chart, surface profiler, and contour profiler (Fig. 3a–e), % PEG was significantly affecting drug release. Hence, % PEG at 15%w/w and water: acetone ratio of 10:90 are to be selected for further studies. In vitro dissolution data fitted in zero order, first order, Higuchi and Korsmeyer–Peppas model for all 6 trials with varying PEG levels and water/acetone ratio. R2 for zero order ranges 0.93–0.99 suggests model fitting. R2 for the first order and Higuchi model also >0.9. Release exponent (n) from Korsmeyer–Peppas equation for all batches suggests anomalous (non-fickian) transport as the value of n lies between 0.45 < n < 1.0 except one batch number Coat-5 with PEG and water/acetone ratio at a center point which suggest super Case-II transport (Table 13).
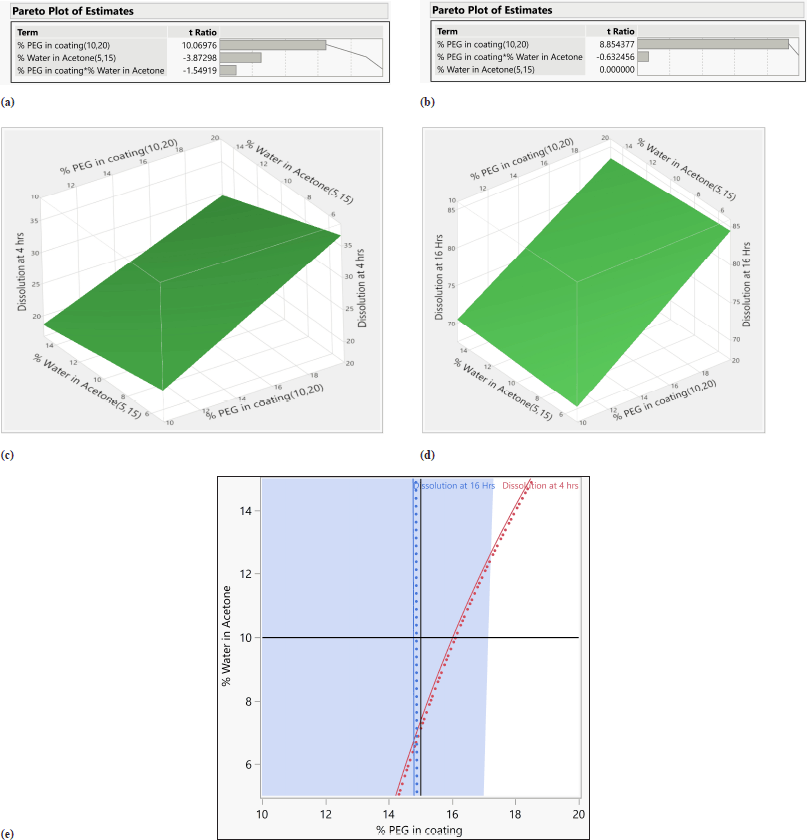 | Figure 3. (a) Pareto chart for dissolution at 4 hours. (b) Pareto chart for dissolution at 16 hours. (c) Surface profiler for dissolution at 4 hours. (d) Surface profiler for dissolution at 16 hours. (e) Contour profiler for dissolution at 4 and 16 hours. [Click here to view] |
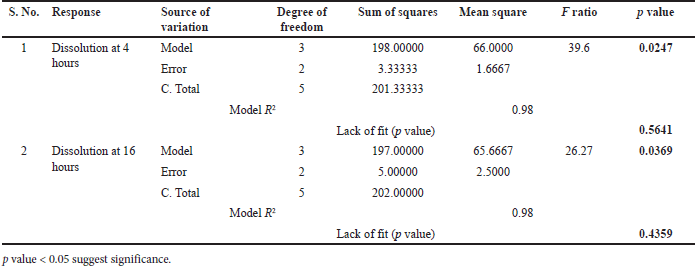 | Table 14. ANOVA summary for regression (Coating composition optimization). [Click here to view] |
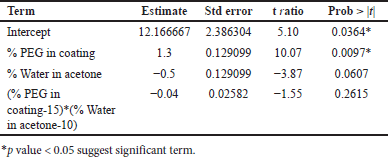 | Table 15. Parameters estimated for response tablet coating composition dissolution at 4 hours. [Click here to view] |
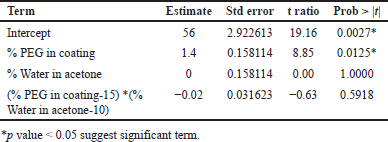 | Table 16. Parameters estimated for response tablet coating composition dissolution at 16 hours. [Click here to view] |
Characterization for the coating process
In the characterization of the coating process various parameters involved bed temp., Spray rate, curing time with their different values, and dissolution data are shown in Table 17.
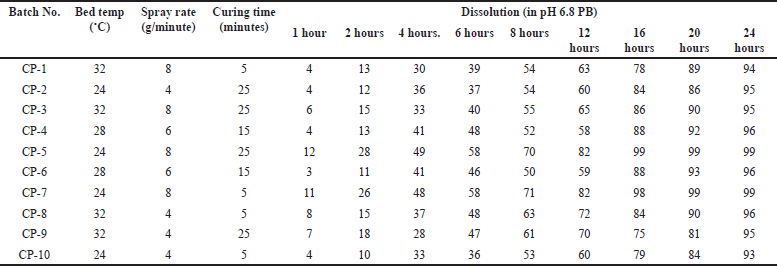 | Table 17. Dissolution data of metformin HCl in pH 6.8 phosphate buffer for analysis of coating process with 2% weight build up. [Click here to view] |
Regression analysis for coating process
From ANOVA Table 18, p-value and R2 value of the model were 0.0105 (<0.05) and 0.83, respectively, for dissolution at 4 hours. p-value and R2 value of the model were 0.0103 (<0.05) and 0.83, respectively, for dissolution at 16 hours. p-value for lack of fit was 0.1499 (>0.05) and 0.4585 (>0.05) for 4 and 16 hours dissolution models respectively which suggest an insignificant lack of fit. Bed temperature and spray rate are significantly impacting dissolution at 4 and 16 hours (p value < 0.05). The quadratic term between factors is also significant with p value < 0.05. Increasing the spray rate increases dissolution at 4 and 16 hours while increasing bed temperature reduces the dissolution at 4 and 16 hours as shown in Tables 19 and 20, respectively. Based on the Pareto chart, contour profiler, and surface profiler, spray rate of 6–8 g/min, curing time of 15 minutes, and temperature of 26°C–32°C for the coating process proposed as an optimum range (Fig. 4a–e).
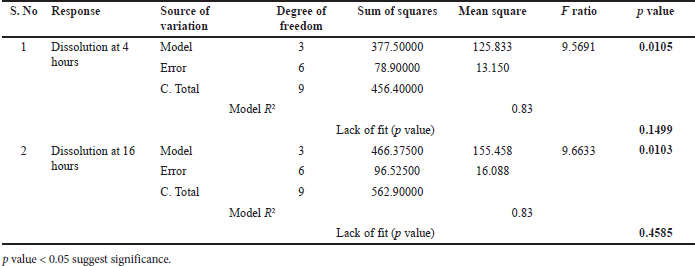 | Table 18. ANOVA summary for regression (Coating process optimization). [Click here to view] |
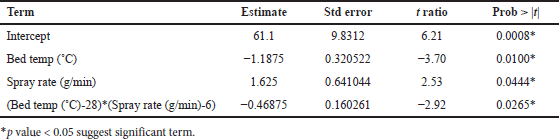 | Table 19. Parameters estimated for response tablet coating process for in vitro dissolution at 4 hours. [Click here to view] |
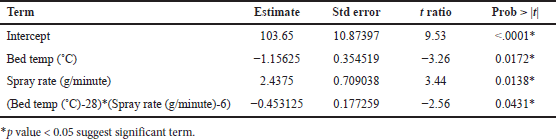 | Table 20. Parameters estimated for response tablet coating process for dissolution at 16 hours. [Click here to view] |
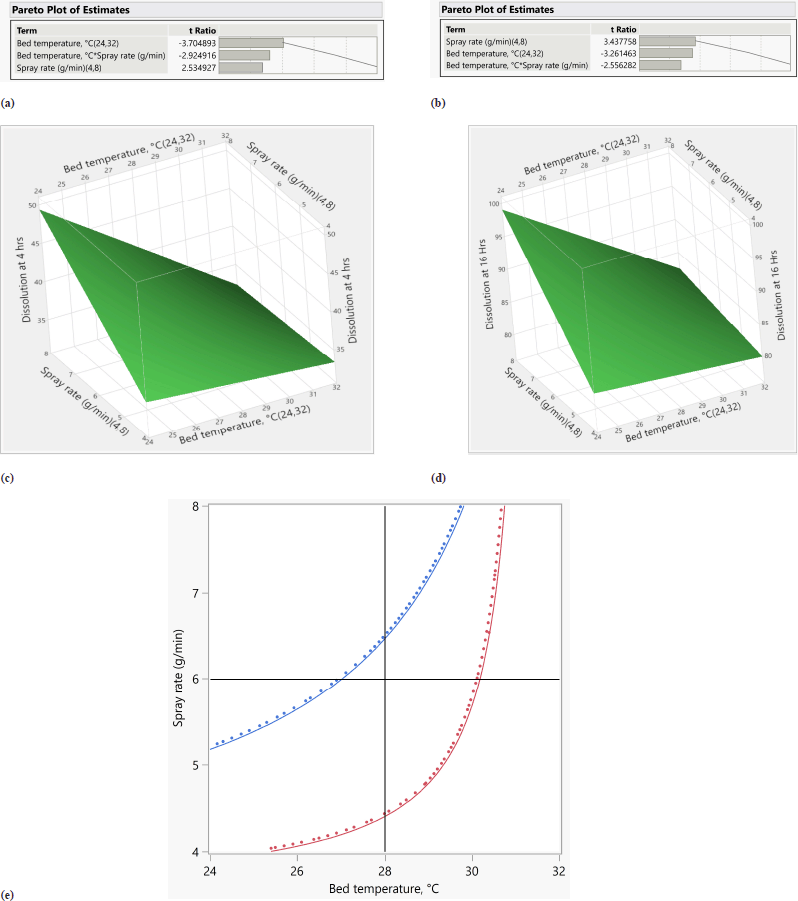 | Figure 4. (a) Pareto chart for dissolution at 4 hours. (b) Pareto chart for dissolution at 16 hours. (c) Surface profiler for dissolution at 4 hours. (d) Surface profiler for dissolution at 16 hours. (e) Contour profiler for dissolution at 4 hours and 16 hours. [Click here to view] |
Release kinetics and similarity factor for optimized batch (CP-4) and marketed product
In vitro dissolution data for optimized formulation (CP-4) and marketed product Gluconorm SR 1g tablets fitted with zero order, first order, Higuchi and Korsmeyer–Peppas model. R2 for zero order >0.9 suggests model fitting. R2 for Higuchi model also >0.9. Release exponent (n) from the Korsmeyer–Peppas equation for both products suggests super case II transport as a value of n > 1.0 (Table 21). A similarity factor was found F2 = 54 between optimized and marketed formulations (Table 21).
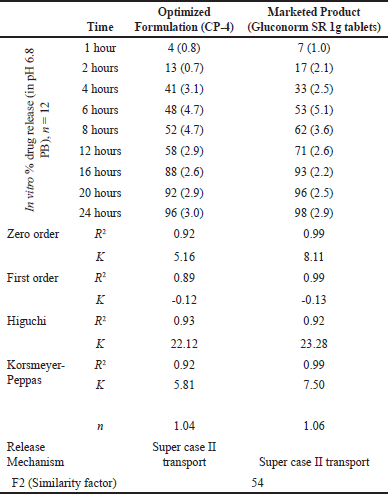 | Table 21. In vitro-dissolution data and drug release kinetics for optimized formulation vs marketed product. [Click here to view] |
Stability studies
There was no change in the appearance and dissolution profiles of the optimized batch after 6 months at accelerated and long-term conditions from initial samples. F2 values between the initial (0 month) and 6 months dissolution profile were 75 (>50) and 72 (>50), at ACC and LT conditions respectively which suggest similar profiles during stability.
DISCUSSION
Osmotic systems utilize osmotic pressure to release drug substances from the dosage form. The osmotic systems for dosage form come in existence in 1974, and since then many modifications have been made to improve this drug delivery system. The current osmotic drug delivery system utilizes a laser drilling process to make orifice in tablets which makes process costlier and time consuming. Alternate to laser drilling reservoir systems are also available in the market which involve coating with water-soluble pore former which generally increases the volume of tablets, especially in the case of high-dose drugs like Metformin HCl. To make the process efficient and cost-effective osmotically controlled delivery without laser drilling and without high volume (%) coating novel tool was developed. The current study successfully developed a tool design incorporating notches, and different depths, and widths of notches were evaluated utilizing oval-shaped modified punches. Punches measuring 21 × 10 mm with notch sizes of 0.7 mm and a depth of 1.0 mm were finalized for further optimization of dosage forms. The optimization focused on the extended-release osmotic composition of Metformin HCl. Binder level,, SLS level and type of binder (PVP and HMPC) studied using full factorial design resulting in an optimal combination of 84 mg/tablet of PVP K29/30 and 60 mg/tablet of SLS. Furthermore, the coating composition was fine-tuned, % PEG in coating and acetone to water ratio were studied using full factorial design leading to a finalized formulation consisting of 15% PEG in the coating membrane, employing an acetone to water ratio of 90:10. Refinements were also made to the coating process, spray rate, curing time, and bed temperature studied using full factorial design which provides optimized spray rate of 6–8 g/min, curing time 15 minutes, and a bed temperature range of 26°C–32°C. Optimized formulation shows zero order drug release kinetics and similarity established with marketed product. These optimization efforts significantly contribute to the efficient manufacturing of Metformin HCl extended-release tablets, characterized by improved swallowability, and minimized weight gain without the use of laser drilling processing step. This novel tooling approach proved effective in the production of Metformin HCl tablets, which are typically larger due to their high dosage, while achieving reduced weight gain compared to the traditional reservoir system. However, there is a need for a few modifications/advancements by compression machine manufacturers for such type of novel tooling to optimize the impact of force on tooling by machine rollers, and improvement of lubrication mechanism in machine, which can be explored by machine manufacturers.
CONCLUSION
In the current research, substituting laser drilling with specialized notched tools can make drug production cheaper and create tablets with reduced tablet volume. Composition and process variables optimized for tablet dissolution. Compression machine manufacturers are encouraged to explore integrating these specialized tools into their machines to capitalize on these advancements. Going ahead, collaboration between pharmaceutical manufacturers, tool manufacturers, and compression machine manufacturers will be crucial to further refine and implement these technologies. This collaborative effort can drive innovation in drug delivery systems, paving the way for more efficient and affordable osmotic tablet dosage forms globally. As the field continues to evolve, integrating notched tools into compression machines stands out as a pivotal advancement for the pharmaceutical industry.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CONFLICTS OF INTEREST
The author reports no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Ezike TC, Okpala US, Onoja UL, Nwike CP, Ezeako EC, Okpara OJ, et al. Advances in drug delivery systems: challenges and future directions. Heliyon. 2023;9(6):e17488. CrossRef
2. Baryakova TH, Pogostin BH, Langer R, McHugh KJ. Overcoming barriers to patient adherence: the case for developing innovative drug delivery systems. Nat Rev Drug Discov. 2023;22:387–409. CrossRef
3. Janczura M, Sip S, Cielecka-Piontek J. The development of innovative dosage forms of the fixed-dose combination of active pharmaceutical ingredients. Pharm. 2022;14(4):834. CrossRef
4. Quarterman JC, Geary SM, Salem AK. Evolution of drug-eluting biomedical implants for sustained drug delivery. Eur J Pharm Biopharm. 2021;159:21–35. CrossRef
5. Ogueri KS, Shamblin SL. Osmotic-controlled release oral tablets: technology and functional insights. Trends Biotechnol. 2022;40(5):606–19. CrossRef
6. Almoshari Y. Osmotic pump drug delivery systems - A comprehensive review. Pharm. 2022;15(11):1430. CrossRef
7. Wang Q, Jiao J, Cai Q, Wang Q, Zhou W. Design and evaluation of a zero-order controlled release system based on pre-hydrated constant release area prepared by compression coating technology. Pharm Dev Technol. 2021;26(10):1120–9. CrossRef
8. Laffleur F, Keckeis V. Advances in drug delivery systems: work in progress still needed? Int J Pharm. 2020;2:100050. CrossRef
9. Apostolova N, Iannantuoni F, Gruevska A, Muntane J, Rocha M, Victor VM. Mechanisms of action of metformin in type 2 diabetes: effects on mitochondria and leukocyte-endothelium interactions. Redox Biol. 2020;34:101517. CrossRef
10. Guo LX, Liu GE, Chen L, Wang HF, Guo J, Zheng XL, et al. Comparison of clinical efficacy and safety of metformin sustained-release tablet (II) (Dulening) and Metformin tablet (Glucophage) in treatment of Type 2 Diabetes Mellitus. Front Endocrinol. 2021;12:712200. CrossRef
11. Liu Y, Huyan X, Zhang Q, Qing H, Zhang Q, Wang X, et al. Effects of Food and multiple-dose administration on the pharmacokinetic properties of hr20033, a sustained-release formulation of Henagliflozin and metformin for the treatment of diabetes, in healthy Chinese volunteers. Clin Pharmacol Drug Dev. 2023;12(4):376–84. CrossRef
12. Theeuwes F, Higuchi T. Osmatic dispensing device for releasing beneficial agent. US3845770A (1973).
13. Almoshari Y. Osmotic pump drug delivery systems - A comprehensive review. Pharm. 2022;15(11):1430. CrossRef
14. Tao Y, Wang Z, Hu S, Feng Y, Yang F, Li G. Improving the quality of laser drilling by assisted process methods of static solution and mist blowing. Micromachines. 2024;15(4):515. CrossRef
15. Ohizua ER, Adeola AA, Idowu MA, Sobukola OP, Afolabi TA, Ishola RO, et al. Nutrient composition, functional, and pasting properties of unripe cooking banana, pigeon pea, and sweetpotato flour blends. Food Sci Nutr. 2017;5(3):750–62. CrossRef
16. Tzakri T, Rehenbrock L, Senekowitsch S, Rump A, Schick P, Krause J, et al. Determination of gastric water emptying in fasted and fed state conditions using a compression-coated tablet and salivary caffeine kinetics. Pharm. 2023;15(11):2584. CrossRef
17. Machado JC, Lange AD, Todeschini V, Volpato NM. Development and validation of a discriminative dissolution method for atorvastatin calcium tablets using in vivo data by LC and UV methods. AAPS PharmSciTech. 2014;15(1):189–97. CrossRef
18. Markl D, Zeitler JA. A review of disintegration mechanisms and measurement techniques. Pharm Res. 2017;34(5):890–917. CrossRef